
Application value of continuous seromuscular layer sutures in the reinforcement of esophagojejunostomy in total gastrectomy for gastric cancer: a retrospective comparative cohort study
Highlight box
Key findings
• Seromuscular layer suturing of the esophagojejunostomy may reduce the incidence of postoperative anastomotic leakage and pleural effusion.
What is known and what is new?
• With the rapid development of instrument anastomosis, the anastomosis process becomes more and more convenient. However, related complications such as anastomotic leakage still occur.
• We combined the characteristics of instrument anastomosis and hand-sewn sutures. After the instrument anastomosis was completed, we sutured the seromuscular layer of anastomosis continuously using knot-free barbed sutures.
What is the implication, and what should change now?
• This suture method is feasible and may provide a new option to increase surgical safety.
Introduction
Gastric cancer is one of the most common malignant tumors of the gastrointestinal tract, and its morbidity and mortality rates are high (1-4). Radical surgical resection is the primary treatment for early and advanced gastric cancer (5-8). Digestive tract reconstruction, especially esophagojejunostomy, plays an essential role in the surgery (9). With the development of surgical techniques, the safety of the surgery has gradually improved; however, surgery-related complications occur occasionally. The emergence of these complications significantly increases the suffering of patients, prolongs hospitalization time, increases hospitalization costs, and delays subsequent antitumor therapy (10,11). A common complication after total gastrectomy is esophagojejunostomy leakage, which is closely related to a variety of factors (6,12). The main influencing factor is the clinician’s intraoperative operation, especially the suturing and reinforcement of the anastomosis (12-14). Thus, the incidence of postoperative complications needs to be reduced and the safety of the surgery enhanced.
In open total gastrectomy, the circular stapler is used for esophagojejunostomy. As the circular stapler is safe and easy to operate, it can achieve surgical margins and satisfactory anastomosis (15,16). However, the stapler also has a number of disadvantages, including that it is expensive, and prone to causing anastomotic stenosis. Even with a 3-row stapler, there may be bleeding from the anastomotic stoma. Further, the incidence of anastomotic stenosis has been reported to be correlated with the use of circular staplers (17,18).
Hand-sewn suturing is a basic operation technique of surgery, and it is mostly used for laparoscopic radical gastrectomy. It enables the surgeon to suture according to the specific situation of the anastomosis. The surgeon can also reduce the tension in the sutures based on their own experience. Hand-sewn suturing changes from double layer anastomosis to single layer anastomosis and then to seromuscular layer anastomosis. Double layer anastomosis may lead to blood supply disorder, and prone to anastomotic stenosis. Compared with double-layer anastomosis, single-layer anastomosis has fewer sutures and less foreign body reaction at the anastomosis, but the sutures on the mucosa are prone to intestinal inflammation and affect healing. The seromuscular layer anastomosis requires only the serous layer, the muscular layer and the submucosa to be connected, without compressing the submucosal vessels and with slight damage to the blood supply to the intestinal wall. However, either type of hand-sewn suturing prolongs the operative time, and does not reduce the anastomotic-related complications. Thus, both mechanical stapled anastomosis and hand-sewn sutures have certain disadvantages.
Our center combined the characteristics of mechanical stapled anastomosis and hand-sewn sutures. After the esophagojejunostomy was completed using a circular stapler, the seromuscular layer was continuously sutured with knot-free barbed sutures at the anastomosis. This study aimed to further explore and compare the safety and short-term efficacy of simple anastomosis and seromuscular layer continuous sutures. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1068/rc).
Methods
General information
This study adopted a retrospective cohort method. To be eligible for inclusion in the study, the patients had to meet the following inclusion criteria: (I) have been hospitalized for the first time at The Third Department of Surgery, The Fourth Hospital of Hebei Medical University, from April 2019 to May 2020 and be aged 18–80 years; (II) have adenocarcinoma, which was pathologically confirmed by preoperative gastroscopic biopsy, and a tumor located in the upper part of the stomach or at the esophagogastric junction; (III) have no distant metastasis as observed in the preoperative imaging examination. (IV) have not received any neoadjuvant therapy before surgery; and (V) have no complicated infectious diseases and have not received any blood transfusions before surgery. Patients were excluded from the study if they met any of the following exclusion criteria: (I) were aged >80 years; (II) had liver cirrhosis or diabetes; (III) had advanced adenocarcinoma that was obviously invading the perigastric organs or tissue; and/or (IV) had positive margins that were confirmed pathologically during the operation.
The tumor-node-metastasis (TNM) staging system of the American Joint Committee on Cancer (AJCC) (8th edition) was used for the pathological staging of the patients. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (No. 2021KS011). Individual consent for this retrospective analysis was waived.
Based on the above inclusion and exclusion criteria, the data of 192 patients with gastric cancer who underwent open radical total gastrectomy and Roux-en-Y esophagojejunostomy were collected. Based on whether the seromuscular layer suture was used at the anastomosis, the patients were divided into group A (the simple anastomosis group, n=76) and group B (the seromuscular layer suture group, n=116). There were 76 patients in group A, of whom 58 were male and 18 were female. The patients in group A had a mean age of 63.57±2.79 years, and a mean BMI of 23.88±2.95 kg/m2. There were 116 patients in group B, of whom 95 were male and 21 were female. The patients had a mean age of 63.47±3.61 years, and a mean BMI of 24.00±2.65 kg/m2. There were no significant differences between the 2 groups in terms of age, gender, BMI, Eastern Cooperative Oncology Group (ECOG) score, tumor size, or pathological tumor-node-metastasis (pTNM) (P>0.05). Thus, the 2 groups of patients were comparable (see Table 1 for further details).
Table 1
| Baseline data | Group A (n=76) | Group B (n=116) | Statistics | P value |
|---|---|---|---|---|
| Age, years | 63.57±2.79 | 63.47±3.61 | t=0.216 | 0.829 |
| Gender | χ2=0.883 | 0.347 | ||
| Male | 58 (76.32) | 95 (81.90) | ||
| Female | 18 (23.68) | 21 (18.10) | ||
| ECOG score | χ2=0.278 | 0.598 | ||
| 0 | 62 (81.58) | 91 (78.45) | ||
| 1 | 14 (18.42) | 25 (21.55) | ||
| Tumor size | χ2=0.104 | 0.747 | ||
| <5 cm | 56 (73.68) | 83 (71.55) | ||
| ≥5 cm | 20 (26.32) | 33 (28.45) | ||
| pTNM stage | χ2=0.03 | 0.984 | ||
| I | 6 | 9 | ||
| II | 22 | 35 | ||
| III | 48 | 72 | ||
| BMI, kg/m2 | 23.88±2.95 | 24.00±2.65 | t=0.303 | 0.762 |
| Follow-up time, months | 16 | 12 |
Data are presented as n, n (%) or mean ± standard deviation. Group A: the simple anastomosis group; Group B: the seromuscular layer suture group. ECOG score: Quality of life scores [Zubrod /ECOG/WHO (ZPS)]. ECOG, Eastern Cooperative Oncology Group; BMI, body mass index; pTNM, pathological tumor-node-metastasis.
Patients
All the patients underwent preoperative abdominal contrast-enhanced competed tomography examinations. No cases of distant metastasis were found, and gastroscopic biopsies were conducted to confirm the diagnosis of adenocarcinoma. Before the operation, the chief surgeon explained the advantages and disadvantages of the 2 methods to the patients and their families. Seromuscular layer suture may increase the safety of anastomosis and reduce the incidence of anastomotic complications, but it will prolong the operation time and increase the operation cost. On the contrary, simple anastomosis has shorter operation time and lower operation cost. The patients, their families, and the surgeon jointly decided if the seromuscular layer suture would be applied in esophagusjejunostomy anastomosis during the operation. The preoperative preparation for the 2 groups was the same.
Operation procedures for patients
All the operation procedures were performed by specialized gastrointestinal surgeons with more than 10 years of experience. The 2 groups of patients underwent tracheal intubation under general anesthesia. An abdominal exploration and the exfoliated cytology were performed first. After completing the D2 lymph node dissection and total gastric dissection, the duodenum was severed with a linear stapler. The esophagus was dissociated 2–3 cm above the pre-cut line, and the bilateral vagus nerves were severed. After clamping the esophagus with purse-string forceps, a temporary purse string was placed. After the esophagus was cut with an electrosurgical knife, an anvil seat was placed, and the purse string was tightened and knotted (Figure 1A). The mesentery was incised at a distance of 15 cm from the Trevor ligament, and the jejunum was severed with a linear stapler. After the mesenteric vascular arch was ligated, the distal jejunum was inserted into the main body of the circular stapler, until it protruded on the opposite side of the mesangial border (Figure 1B). Next, the anvil seat and the stapler were connected and tightened for 15 seconds to complete the end-to-side anastomosis of the esophagus and jejunum (Figure 1C,1D), and the linear stapler was used to close the jejunum stump. At this point, the esophagojejunostomy was completed.
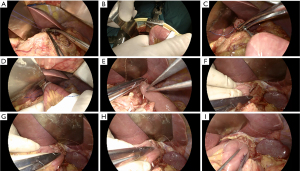
In group B, surgeons sutured the seromuscular layer of anastomosis continuously using knot-free barbed sutures. In this process, the needle distance was 4–5 mm, and the margin was 3 mm to avoid full-thickness sutures (Figure 1E-1H). The operation ensured that the anastomotic stoma had no tension, distortion, and good blood supply (Figure 1I).
The jejunojejunostomy was performed at about 40 cm below the esophagojejunostomy. The feeding tube was placed 15 cm distal to the jejuno-jejunostomy, and the gastric tube was placed 5 cm below the esophagojejunostomy. Finally, the anastomotic stoma was carefully observed to ensure that there was no leakage and no active bleeding in the operative field. Abdominal drainage tubes were placed under the esophagojejunostomy and in the spleen fossa.
Observation indicators
The intraoperative observation indicators were operation time and intraoperative blood loss. The postoperative observation indicators were time to liquid diet, time to first exhaust, and length of postoperative hospital stay. The postoperative complications included anastomotic leakage, anastomotic bleeding, anastomotic stenosis, and pleural effusion.
Evaluation criteria and definitions
The postoperative complications included any adverse conditions requiring conservative treatment or surgical intervention after surgery. Anastomotic leakage was diagnosed if the patient had intestinal contents flowing out of the drainage tube after surgery, or blue liquid was drained out after the oral administration of methylene blue, or contrast agent leakage was observed in the upper gastrointestinal angiography. Anastomotic stenosis included postoperative symptoms (e.g., difficulty eating, vomiting, or swallowing). An obstruction was diagnosed by angiography or gastroscopy. All the postoperative complications were comprehensively examined based on auxiliary examinations, blood indicators, symptoms, and signs. The operation time was calculated as the time from the opening of the abdomen to the closing of the abdomen (as noted on the anesthesia record sheet). The intraoperative blood loss was calculated based on the amount of blood in the suction bag minus the amount of irrigation. The time to liquid diet was determined based on the time recorded in the doctor’s order sheet. The time to first exhaust was based on the medical records.
Postoperative management and follow-up
The 2 groups’ postoperative nutritional support, and symptomatic intervention were the same. In both groups, following the patients’ smoot recovery, gastroenterography was checked 1 week after operation. In the gastroenterography process, the patients repeatedly adjusted their body positions to the upright, supine, and lateral positions. It is convenient for doctors to carefully observe anastomotic-related complications, especially anastomotic stenosis and anastomotic leakage. After discharge, the patients were followed up for anastomotic-related complications. The patients underwent electronic gastroscopy to check for anastomotic stenosis 6 months after the operation.
Statistical analysis
SPSS (version 26.0) software was used for the statistical analysis. The measurement data are expressed as the (), and the T-test was used for comparisons between groups. The count data are expressed as the rate (%), and the χ2 test or Fisher’s exact test was used for comparisons. A P value <0.05 was considered statistically significant.
Results
Comparison of surgical data
All the 192 patients successfully underwent total radical gastrectomy, and there were no perioperative deaths. In group A, the mean operation time was 231.93±26.35 min, and the mean intraoperative blood loss was 61.09±8.04 mL. In group B, the mean operation time was 237.04±21.91 min, and the mean intraoperative blood loss was 62.51±6.50 mL. In relation to the intraoperative situation, there were no significant differences between the 2 groups (P>0.05). In relation to the postoperative conditions, in terms of the time to first exhaust and the length of postoperative hospital stay there were no significant differences between the 2 groups (P>0.05); however, group B had a significantly earlier time to liquid diet than group A (4.23±0.76 vs. 4.57±0.58 days, P<0.001) (see Table 2 for details).
Table 2
| Surgical data | Group A (n=76) | Group B (n=116) | Statistics | P value |
|---|---|---|---|---|
| Operation time, min | 231.93±26.35 | 237.04±21.91 | t=–1.457 | 0.147 |
| Intraoperative blood loss, mL | 61.09±8.04 | 62.51±6.50 | t=–1.285 | 0.163 |
| Time to liquid diet, days | 4.57±0.58 | 4.23±0.76 | t=3.55 | <0.001 |
| Time to first exhaust, days | 4.11±0.80 | 3.99±1.02 | t=0.883 | 0.378 |
| Postoperative hospital stay, days | 7.00±1.13 | 6.98±1.06 | t=0.112 | 0.911 |
Data are presented as mean ± standard deviation. Group A: the simple anastomosis group; Group B: the seromuscular layer suture group.
Comparison of the incidence of complications between the 2 groups
In group A, there were 7 (9.21%) cases of anastomotic leakage and 25 (32.89%) cases of pleural effusion. In group B, there were 2 (1.72%) cases of anastomotic leakage and 18 (15.52%) cases of pleural effusion. The incidence of postoperative anastomotic leakage in group B (1.72%) was lower than that in group A (9.21%), and the difference was statistically significant (P=0.03). The incidence of pleural effusion in group B (15.52%) was lower than that in group A (32.89%), and the difference was statistically significant (P=0.005). No anastomotic stenosis or anastomotic bleeding was observed in the 2 groups during the follow-up period (see Table 3 for details). In this study, the median follow-up time was 16 months for patients in group A and 12 months for patients in group B (see Table 1 for further details).
Table 3
| Complication | Group A (n=76) | Group B (n=116) | Statistics | P value |
|---|---|---|---|---|
| Anastomotic leakage | 7 (9.21) | 2 (1.72) | – | 0.040 |
| Anastomotic bleeding | 0 | 0 | – | – |
| Anastomotic stenosis | 0 | 0 | – | – |
| Pleural effusion | 25 (32.89) | 18 (15.52) | χ2=7.98 | 0.005 |
Data are presented as n (%). Group A: the simple anastomosis group; Group B: the seromuscular layer suture group.
Discussion
As mature surgical procedures, radical total gastrectomy and Roux-en-Y esophagojejunostomy are widely used in clinical practice (19). With the continuous optimization of the surgical technique and the improvement of the anastomosis method, the overall complication rate continues to gradually decrease. The success or failure of total gastrectomy lies in the safety and effectiveness of the operation, and the focus of the operation is the reconstruction of the digestive tract. Anastomotic-related complications include anastomotic leakage, anastomotic bleeding, and anastomotic stenosis (20). Anastomotic leakage is one of the most severe complications, with an incidence rate of 1–11.5% (12). It seriously affects the postoperative recovery and short-term and long-term curative effect of patients and is even related to the survival of tumor patients. Thus, it is imperative to find and actively implement ways to reduce anastomotic leakage.
Clinicians have engaged in extensive work to reduce complications after digestive tract reconstruction. Previous scholars have proposed a variety of esophagojejunostomy methods. The main techniques include reverse puncture, the Orvil and purse-string suture method using circular stapler or the esophagojejunostomy (overlap) method, and the functional end-to-end anastomosis method using linear stapler (16,21-23). With the continuous updating of stapler technology, the safety of mechanical stapled anastomosis has improved, and complications related to anastomosis have been reduced compared to those associated with hand-sewn anastomosis (24). Under the stapler method, 2 or 3 staggered staples are generally implanted into the tissue to achieve the double or triple cross suturing of the tissue. The suturing is tight, and leakage is reduced to a large extent. The staples of the stapler are also arranged neatly. The spacing and tightness of the stitching can be effectively controlled through its scale. Conversely, hand-sewn suturing is challenging to execute. The staples can also effectively promote tissue healing. When the patient’s general condition is good, and the operation is not complex, the patient can recover relatively smoothly. However, if the patient has unfavorable factors, such as an advanced age, anemia, malnutrition, diabetes, high anastomotic tension, and unexpected tension (such as hiccups), the risk of surgery increases.
Compared to a stapler, it is difficult to uniformize the density and depth of hand-sewn digestive-tract suture; however, hand-sewn sutures have a broader range of applications. For example, vascular anastomosis, pancreas-jejunum anastomosis, and sometimes thinner bile duct anastomosis require hand-sewn sutures, and a stapler cannot be used for these anastomoses (25). In addition, hand-sewn sutures are also flexible and reliable (26). The surgeon can appropriately change the amount of sutured tissue and the spacing and tension of the sutures according to their own experience and skills. Hand-sewn sutures can avoid large blood vessels, hemostatic sutures can be applied to the bleeding point, and the non-smooth tissue surface can be embedded to reduce postoperative adhesions (27). Additionally, the sutures used in hand-sewn suturing are constantly developing and progressing. The surgical sutures currently used have good compatibility and flexibility, and some varieties have absorbability and antibacterial properties. The application of barbed sutures in laparoscopic gastroduodenal ulcer perforation repair is safe and effective. Barbed sutures can shorten the suture and operation time, reduce the difficulty of laparoscopic suturing, and shorten the learning curve (28-31).
Successful anastomosis and subsequent healing depend on several factors, including the tension between the 2 connected parts of the gastrointestinal tract, the healthy blood supply to the surrounding tissue, and the strength of the final anastomosis. Thus, we combined the characteristics of instrument anastomosis and hand-sewn sutures. After the instrument anastomosis was completed, we sutured the seromuscular layer of anastomosis continuously using knot-free barbed sutures, as shown in Figure 2. In relation to the postoperative situation, we found that the time to liquid diet of group B patients was earlier than that of group A patients. Thus, group B was more stable than group A in the early postoperative period and patients in group A could eat earlier and more safely. Patients in group B also had a lower incidence of anastomotic leakage than those in group A, which may be related to the seromuscular layer suture closing the pinhole of the stapler. Thus, tissue inflammatory response was alleviated, tissue healing was accelerated, and the occurrence of anastomotic leakage was reduced.
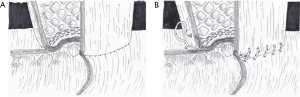
There was no significant difference in anastomotic stenosis between group B and group A; thus, that continuous suture did not increase the incidence of anastomotic stenosis. Additionally, there was no anastomotic bleeding in either group, which may be related to the application of instrument anastomosis and the selection of the correct stapler, or to the small sample size of this study. The continuous suturing of the seromuscular layer reduces anastomotic exudation, reduces stimulation to the diaphragm and lower posterior mediastinum, and further reduces the occurrence of pleural effusion. Thus, the incidence of pleural effusion in group B (15.52%) was lower than in group A (32.89%), and the difference was statistically significant. Seromuscular layer sutures can compress the hemostasis, reduce the occurrence of anastomotic leakage, and do not increase the occurrence of anastomotic stenosis. The operation time was prolonged, but the safety of the operation was improved, and the patients were discharged smoothly with few complications.
The present study had a few limitations. First, this study was retrospective, and it had a small sample size. Second, there was selection bias in this study. Third, the surgical methods were all open-surgery methods. Fourth, this study included few postoperative observation indicators. Thus, a multicenter, prospective, randomized controlled trial should be conducted in the future, and the surgical methods included in the laparoscopic surgery and the postoperative follow-up time should be appropriately extended.
Conclusions
Seromuscular layer suturing of the esophagojejunostomy can allow patients to drink water and eat safely in the early postoperative stage and may reduce the incidence of postoperative anastomotic leakage and pleural effusion. This suture method is feasible and may provide a new option to increase surgical safety.
Acknowledgments
Funding: This work was supported by the Cultivating Outstanding Talents Project of Hebei Provincial Government Fund (No. 2019012), the Hebei Public Health Committee County-Level Public Hospitals Suitable Health Technology Promotion and Storage Project (No. 2019024), and the Hebei University Science and Technology Research Project (No. ZD2019139).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1068/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1068/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-1068/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of The Fourth Hospital of Hebei Medical University (No. 2021KS011). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin 2021;71:264-79. [Crossref] [PubMed]
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26-38. [Crossref] [PubMed]
- Balakrishnan M, George R, Sharma A, et al. Changing Trends in Stomach Cancer Throughout the World. Curr Gastroenterol Rep 2017;19:36. [Crossref] [PubMed]
- Wang S, Lin S, Wang H, et al. Reconstruction methods after radical proximal gastrectomy: A systematic review. Medicine (Baltimore) 2018;97:e0121. [Crossref] [PubMed]
- Barchi LC, Ramos MFKP, Pereira MA, et al. Esophagojejunal anastomotic fistula: a major issue after radical total gastrectomy. Updates Surg 2019;71:429-38. [Crossref] [PubMed]
- Hu Y, Zaydfudim VM. Quality of Life After Curative Resection for Gastric Cancer: Survey Metrics and Implications of Surgical Technique. J Surg Res 2020;251:168-79. [Crossref] [PubMed]
- Ramos MFKP, Pereira MA, Dias AR, et al. Laparoscopic gastrectomy for early and advanced gastric cancer in a western center: a propensity score-matched analysis. Updates Surg 2021;73:1867-77. [Crossref] [PubMed]
- Weber MC, Berlet M, Novotny A, Friess H, Reim D. Reconstruction following gastrectomy. Chirurg 2021;92:506-14. [Crossref] [PubMed]
- Yuan P, Wu Z, Li Z, et al. Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 2019;19:833. [Crossref] [PubMed]
- Tokunaga M, Tanizawa Y, Bando E, et al. Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 2013;20:1575-83. [Crossref] [PubMed]
- Makuuchi R, Irino T, Tanizawa Y, et al. Esophagojejunal anastomotic leakage following gastrectomy for gastric cancer. Surg Today 2019;49:187-96. [Crossref] [PubMed]
- Oshi M, Kunisaki C, Miyamoto H, et al. Risk Factors for Anastomotic Leakage of Esophagojejunostomy after Laparoscopy-Assisted Total Gastrectomy for Gastric Cancer. Dig Surg 2018;35:28-34. [Crossref] [PubMed]
- Wiggins T, Majid MS, Markar SR, et al. Benefits of barbed suture utilisation in gastrointestinal anastomosis: a systematic review and meta-analysis. Ann R Coll Surg Engl 2020;102:153-9. [Crossref] [PubMed]
- Sugiyama M, Oki E, Ogaki K, et al. Clinical Outcomes of Esophagojejunostomy in Totally Laparoscopic Total Gastrectomy: A Multicenter Study. Surg Laparosc Endosc Percutan Tech 2017;27:e87-91. [Crossref] [PubMed]
- Nomura E, Kayano H, Seki T, et al. Preventive procedure for stenosis after esophagojejunostomy using a circular stapler and transorally inserted anvil (OrVil™) following laparoscopic proximal gastrectomy and total gastrectomy involving reduction of anastomotic tension. BMC Surg 2021;21:47. [Crossref] [PubMed]
- Umemura A, Koeda K, Sasaki A, et al. Totally laparoscopic total gastrectomy for gastric cancer: literature review and comparison of the procedure of esophagojejunostomy. Asian J Surg 2015;38:102-12. [Crossref] [PubMed]
- Wang Z, Liu X, Cheng Q, et al. Digestive tract reconstruction of laparoscopic total gastrectomy for gastric cancer: a comparison of the intracorporeal overlap, intracorporeal hand-sewn anastomosis, and extracorporeal anastomosis. J Gastrointest Oncol 2021;12:1031-41. [Crossref] [PubMed]
- Niihara M, Hiki N, Hosoda K, et al. Improved anastomotic technique for esophagojejunal anastomosis using circular stapler. Langenbecks Arch Surg 2022;407:353-6. [Crossref] [PubMed]
- Park JH, Jeong SH, Lee YJ, et al. Safety and efficacy of post-anastomotic intraoperative endoscopy to avoid early anastomotic complications during gastrectomy for gastric cancer. Surg Endosc 2020;34:5312-9. [Crossref] [PubMed]
- Wang Y, Liu Z, Shan F, et al. Short-term outcomes after totally laparoscopic total gastrectomy with esophagojejunostomy constructed by π-shaped method versus overlap method. J Surg Oncol 2021;124:1329-37. [Crossref] [PubMed]
- Yang HK, Hyung WJ, Han SU, et al. Comparison of surgical outcomes among different methods of esophagojejunostomy in laparoscopic total gastrectomy for clinical stage I proximal gastric cancer: results of a single-arm multicenter phase II clinical trial in Korea, KLASS 03. Surg Endosc 2021;35:1156-63. [Crossref] [PubMed]
- Miura S, Kanaya S, Hosogi H, et al. Esophagojejunostomy With Linear Staplers in Laparoscopic Total Gastrectomy: Experience With 168 Cases in 5 Consecutive Years. Surg Laparosc Endosc Percutan Tech 2017;27:e101-7. [Crossref] [PubMed]
- Wang H, Ge W, Liu C, et al. Design and performance evaluation of a powered stapler for gastrointestinal anastomosis. Minim Invasive Ther Allied Technol 2022;31:595-602. [Crossref] [PubMed]
- Tan YP, Lim C, Junnarkar SP, et al. 3D Laparoscopic common bile duct exploration with primary repair by absorbable barbed suture is safe and feasible. J Clin Transl Res 2021;7:473-8. [PubMed]
- Xu X, Huang C, Mou Y, et al. Intra-corporeal hand-sewn esophagojejunostomy is a safe and feasible procedure for totally laparoscopic total gastrectomy: short-term outcomes in 100 consecutive patients. Surg Endosc 2018;32:2689-95. [Crossref] [PubMed]
- Sano A, Ojima H, Ogawa A, et al. Four stay-sutures method: a simplified hand-sewn purse-string suture in laparoscopic circular-stapled esophagojejunostomy. Surg Today 2020;50:314-9. [Crossref] [PubMed]
- Bautista T, Shabbir A, Rao J, et al. Enterotomy closure using knotless and barbed suture in laparoscopic upper gastrointestinal surgeries. Surg Endosc 2016;30:1699-703. [Crossref] [PubMed]
- Tsukada T, Kaji M, Kinoshita J, et al. Use of Barbed Sutures in Laparoscopic Gastrointestinal Single-Layer Sutures. JSLS 2016;20:e2016. [Crossref] [PubMed]
- Velotti N, Manigrasso M, Di Lauro K, et al. Barbed suture in gastro-intestinal surgery: A review with a meta-analysis. Surgeon 2022;20:115-22. [Crossref] [PubMed]
- Manigrasso M, Velotti N, Calculli F, et al. Barbed Suture and Gastrointestinal Surgery. A Retrospective Analysis. Open Med (Wars) 2019;14:503-8. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


