
Clinical study on risk factors related to postoperative recurrence or metastasis of rectal cancer: a retrospective cohort study
Introduction
Rectal cancer is a life-threatening malignancy, the incidence and mortality of which are gradually increasing. According to the statistics of the International Agency for Research on Cancer (IARC), the number of newly diagnosed global rectal cancer patients in 2020 was 1,331,600, accounting for 10% of the total number of new cancers and ranking third in the incidence of malignant tumors (1,2). At present, rectal cancer is mainly treated by surgery, combined with chemotherapy, radiotherapy, and molecular targeted therapy after surgery. However, studies have shown the postoperative recurrence or metastasis rate of stage I–III rectal cancer is still about 30%. Once recurrence or metastasis occurs, the five-year survival rate is less than 5%, and the average survival time is only about 7 months (3-5). Even under the premise of total mesenterectomy (TME) and neoadjuvant chemotherapy, rectal cancer still has a recurrence rate of 5.6–17.6% (6-8). Therefore, postoperative recurrence or metastasis resulting in death remain a significant clinical problem and have a serious impact on the quality of life and lifetime of patients. Relevant risk factors related to postoperative recurrence or metastasis of rectal cancer have been proposed. However, some studies have shown that local recurrence or liver metastasis after rectal cancer surgery is associated with tumor location, while others have shown that tumor location is only associated with postoperative pelvic wall recurrence, but not with rectal cancer metastasis or other sites of metastasis (9,10). In addition, the research methods, inclusion criteria and research perspectives of these studies are different, resulting in numerous risk factors for postoperative recurrence or metastasis of rectal cancer, but no specificity. Five years of rectal cancer data were analyzed, and closed follow-up of the patients. Postoperative recurrence or metastasis of rectal cancer patients for the single factor analysis and multifactor variables, and prospective validation would proceed in a follow-up study. This could establish high specificity risk factors for rectal cancer of postoperative recurrence or metastasis, which was for clinical treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-942/rc).
Methods
Participants and trial design
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of The Fifth People’s Hospital of Shanghai, Fudan University (No. 2021-237) and individual consent for this retrospective analysis was waived.
The clinical records of 321 patients who underwent rectal cancer surgery in the hospital between 2016.1–2020.12 were retrospectively collected, and the number of patients determined the sample size. According to the principle of TME, laparoscopic or open surgery was performed directly after evaluation of cT1-cT2 rectal cancer. For cT3 and some cT4 rectal cancer patients, preoperative evaluation was followed by neoadjuvant radiotherapy or chemotherapy, followed by re-evaluation 6–8 weeks later, when surgery was performed. Postoperative adjuvant chemotherapy and follow-up were routinely performed according to NCCN guidelines. Patients were followed up in hospital or as outpatients every 3 months during the first 2 years, every 6 months in the third year, and once a year in the fourth year. Follow-up included routine blood and biochemical examinations, tumor markers, and imaging examinations, such as lung CT, abdominal enhanced CT or enhanced MRI, and colonoscopy. The inclusion criteria were: Preoperative pathological diagnosis of ct1-cT3 and part of cT4 rectal malignant tumor; and preoperative CT/MRI examination showed no distant metastasis. The exclusion criteria were: Rectal cancer patients with distant metastasis confirmed before surgery; patients who had palliative surgery or could not tolerate surgery; patients with multiple tumors or other tumors; and familial adenomatous polyposis. A total of 185 rectal cancer patients were included for statistical analysis, and the screening procedures are shown in Figure 1.
Intervention and study setting
Patients were followed up in our hospital and through outpatient records, telephone follow-up, and other ways, to record whether recurrence or metastasis had occurred after surgery, and the survival status. The cut-off of the research was recurrence, metastasis, or death. Recurrence refers to the occurrence of malignant tumors related to the primary tumor after rectal cancer surgery, including local recurrence of anastomosis, and recurrence of pelvic and perineum tissues and organs around the surgical site. Metastasis refers to organ metastasis outside the surgical site, such as the lung, liver, or other sites. Recurrence or metastasis was confirmed by lung CT, enhanced CT or MRI, and other imaging examinations during the patient’s visit, combined with surgery, puncture, biopsy or cytopathology. According to the data reported in relevant research literatures at home and abroad, as well as the clinical experience and observation results of our hospital, the following relevant clinical risk factors were included. Tumor distance to the anal margin (preoperative colonoscopy localization, measurement of tumor distance to the anal margin, digital examination of rectum, and intraoperative judgment), preoperative bowel preparation (emptying colon contents preoperative by oral polyethylene glycol electrolyte), preoperative antibiotics (three days of oral cephalosporins antibiotics and metronidazole were given), involvement of the mesorectal fascia (MRF) (preoperative pelvic CT or MRI evaluation of the rectal fascia around the infiltrating tumor), operation type (laparoscopic or open surgery), operation time, intraoperative perfusion chemotherapy (platinum chemotherapy drugs 50 mg/m2 for preoperative irrigation), intraoperative blood transfusion, sphincter-preservation, colostomy, left colic artery preservation, postoperative complications (bleeding, infection, intestinal obstruction, deep vein thrombosis, pulmonary infection, abdominal infection, or wound infection), anastomosis leakage, postoperative retained catheter time, hospital stay, tumor size, distance of distal tumor incisional margin (the tumor was cut and measured after surgery), T staging of tumor, nerve, and/or vascular invasion, circumferential resection margin (CRM), total number of lymph nodes, number of metastasis lymph nodes, Dukes staging, and other relevant factors.
Statistical analysis
As for the lost follow-up and censored data, it was deleted because there were only 2 cases of lost follow-up data, which had little significant impact on this study. In addition, we conducted stratified statistical and comparative studies on the offset caused by different follow-up times. For measurement data conforming to normal distribution, the independent sample t test was used for comparison between groups, and χ2 test or Fisher’s exact probability method was used for comparison of count data. Univariate and multiple logistic regression were used for the risk factors analyzed. Cox regression and Kaplan-Meier method were used for establishing survival curves. A univariate COX regression model was applied followed by multivariate COX regression model in backward stepwise (Wald) that was used to provide an estimate of the hazard ratio (HR) and its confidence interval (CI) for investigating the association between the survival time of patients and one or more predictor variables. P values were two-sided, and P<0.05 were considered statistically significant. Statistical analyses were performed using SPSS 26.0 (IBM Corporation, Armonk, NY, USA).
Results
Of the initial 321 patients counted, 12 had missing data, 37 had distant metastasis before operation, 23 had advanced tumors that could not be treated surgically, four had familial polyposis, eight had concomitant other tumors, and 21 underwent transanal minimally invasive surgery, leaving 216 patients. These were then divided into two groups: Group A as a recurrence or metastasis group, and group B as a non-recurrence or metastasis group. In group A, nine patients were treated in other hospitals after operation and three did not receive regular postoperative adjuvant treatment and follow-up, while in group B, 11 patients did not receive regular postoperative adjuvant treatment and follow-up, six were treated in other hospitals after operation, and two died after surgery. This left a total of 185 patients included in the final study. All patients were followed up between 20 to 60 months and the median time was 45 months. Their age ranged from 33 to 94 years, with a mean of 66.3 years, and included 125 males (67.6%) and 60 females (32.4%). There were 130 patients in Group A, with an average age of 65.36 years, including 87 males (66.9%) and 43 females (33.1%), and group B contained 55 patients, with an average age of 68.53 years, including 38 males (69.1%) and 17 females (30.9%). Recurrence or metastasis occurred in 55 patients, accounting for 29.7%, with an average time of 10.8 months. Detailed data and other indicators are shown in Table 1.
Table 1
| Factors of research | No recurrence or metastasis, n (%) | Recurrence or metastasis, n (%) | β | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Sex | |||||
| Male | 87 (66.9) | 38 (69.1) | −0.10 | 0.9 (0.46–1.78) | 0.773 |
| Female | 43 (33.1) | 17 (30.9) | |||
| Age(years) | |||||
| ≤50 | 14 (10.8) | 1 (1.8) | 1.87 | 6.5 (0.83–50.8) | 0.074 |
| 51–60 | 25 (19.2) | 8 (14.5) | −0.33 | 0.7 (0.30–1.70) | 0.448 |
| 61–70 | 48 (36.9) | 23 (41.8) | 0.21 | 1.2 (0.64–2.33) | 0.532 |
| 71–80 | 30 (23.1) | 16 (29.1) | 0.31 | 1.4 (0.67–2.78) | 0.388 |
| >80 | 13 (10.0) | 7 (12.7) | 0.27 | 1.3 (0.49–3.49) | 0.586 |
| BMI (kg/m2) | |||||
| <18.5 | 9 (7.0) | 5 (9.1) | −0.29 | 0.7 (0.24–2.33) | 0.611 |
| 18.5–24.9 | 93 (71.5) | 34 (61.8) | −0.44 | 0.6 (0.33–1.25) | 0.194 |
| 25–29.9 | 26 (20.0) | 14 (25.5) | 0.31 | 1.4 (0.65–2.87) | 0.411 |
| ≥30 | 2 (1.5) | 2 (3.6) | 0.88 | 2.4 (0.33–17.59) | 0.384 |
| Medical history | |||||
| Diabetes | 15 (11.5) | 7 (12.7) | 0.11 | 1.1 (0.43–2.92) | 0.819 |
| Cardiovascular disease | 59 (45.4) | 21 (38.2) | −0.30 | 0.7 (0.39–1.42) | 0.367 |
| Pulmonary disease | 3 (2.3) | 0 (0.0) | −20.37 | 0.0 (0.00–0.00) | 0.999 |
| Other major disease | 9 (7.0) | 3 (5.5) | −0.13 | 0.9 (0.22–3.45) | 0.854 |
| Distance from anal verge (cm) | |||||
| ≤5 | 46 (35.4) | 22 (40.0) | −0.20 | 0.8 (0.43–1.57) | 0.552 |
| 5–10 | 54 (41.5) | 18 (32.7) | −0.38 | 0.7 (0.35–1.33) | 0.262 |
| >10 | 30 (23.1) | 15 (27.3) | 0.22 | 1.3 (0.61–2.57) | 0.544 |
| Preoperation bowel preparation | |||||
| Yes | 125 (96.2) | 51 (92.7) | −0.67 | 0.5 (0.13–1.98) | 0.330 |
| No | 5 (3.8) | 4 (7.3) | |||
| Preoperative antibiotic | |||||
| Yes | 75 (57.7) | 14 (25.5) | 0.76 | 2.1 (1.07–4.32) | 0.032 |
| No | 55 (42.3) | 41 (74.5) | |||
| Involvement of mesorectal fascia | |||||
| Yes | 73 (56.2) | 47 (85.5) | 1.52 | 5.6 (2.01–10.48) | 0.000 |
| No | 57 (43.8) | 8 (14.5) | |||
| Operation type | |||||
| Laparoscopic | 106 (81.5) | 43 (78.2) | 0.21 | 1.2 (0.57–2.68) | 0.059 |
| Open | 24 (18.5) | 12 (21.8) | |||
| Operation time (mean) | 213.4 | 246.4 | 0.01 | 1.0 (1.00–1.01) | 0.015 |
| Intraoperative chemotherapy | |||||
| Yes | 37 (28.5) | 17 (30.9) | 0.12 | 1.1 (0.57–2.24) | 0.738 |
| No | 93 (71.5) | 38 (69.1) | |||
| Intraoperative blood transfusion | |||||
| Yes | 17 (13.1) | 40 (72.7) | 0.91 | 2.5 (1.14–5.45) | 0.022 |
| No | 113 (86.9) | 15 (27.3) | |||
| Sphincter preservation | |||||
| Yes | 117 (90.0) | 45 (81.8) | −0.69 | 0.5 (0.21–1.22) | 0.128 |
| No | 13 (10.0) | 10 (18.2) | |||
| Ileostomy | |||||
| Yes | 64 (49.2) | 37 (67.3) | 0.75 | 2.1 (1.10–4.10) | 0.026 |
| No | 66 (50.8) | 18 (32.7) | |||
| Left colonic artery preservation | |||||
| Yes | 59 (45.4) | 24 (43.6) | −0.07 | 0.9 (0.49–1.76) | 0.827 |
| No | 71 (54.6) | 31 (56.4) | |||
| Anastomotic leakage | |||||
| Yes | 4 (3.1) | 4 (7.3) | 0.90 | 2.5 (0.59–10.26) | 0.213 |
| No | 126 (96.9) | 51 (92.7) | |||
| Postoperative complications | |||||
| Yes | 14 (10.8) | 6 (10.9) | 0.01 | 1.0 (0.37–2.79) | 0.978 |
| No | 116 (89.2) | 49 (89.1) | |||
| Postoperative catheter retention time (mean) | 7.1 | 8.5 | 0.08 | 1.1 (1.00–1.16) | 0.051 |
| Length of stay (mean) | 25.3 | 26.8 | 0.01 | 1.0 (0.98–1.04) | 0.455 |
| Tumor volume (mean) | 22.52 | 24.37 | 0.00 | 1.0 (0.99–1.01) | 0.722 |
| Distal margin distance (mean) | 2.04 | 2.25 | 0.08 | 1.1 (0.89–1.33) | 0.428 |
| T stage of tumor | |||||
| T1 | 5 (3.8) | 1 (1.8) | 0.77 | 2.2 (0.25–18.93) | 0.487 |
| T2 | 13 (10.0) | 0 (0.0) | −20.45 | 0.0 (0.00–0.00) | 0.999 |
| T3 | 29 (22.3) | 6 (10.9) | −0.85 | 0.4 (0.17–1.10) | 0.076 |
| T4 | 83 (63.8) | 48 (87.3) | 1.36 | 3.9 (1.63–9.27) | 0.002 |
| Nerve or vascular invasion | |||||
| Yes | 50 (38.5) | 39 (70.9) | 1.36 | 3.9 (1.97–7.70) | 0.000 |
| No | 80 (61.5) | 16 (29.1) | |||
| Nerve and vascular invasion | |||||
| Yes | 20 (15.4) | 27 (49.1) | 0.83 | 2.3 (1.61–3.29) | 0.000 |
| No | 110 (84.6) | 28 (50.9) | |||
| Nerve invasion | |||||
| Yes | 11 (8.5) | 9 (16.4) | 0.75 | 2.1 (0.82–5.44) | 0.120 |
| No | 119 (91.5) | 46 (83.6) | |||
| Vascular invasion | |||||
| Yes | 19 (14.6) | 3 (5.5) | −1.09 | 0.3 (0.10–1.19) | 0.091 |
| No | 111 (85.4) | 52 (94.5) | |||
| CRM | |||||
| Positive | 3 (2.3) | 6 (10.9) | 1.65 | 5.2 (1.25–21.54) | 0.024 |
| Negative | 127 (97.7) | 49 (89.1) | |||
| Total lymph nodes (mean) | 17.33 | 15.8 | −0.02 | 1.0 (0.94–1.02) | 0.267 |
| No. of lymph node metastasis (mean) | 1.15 | 4 | 0.23 | 1.3 (1.12–1.41) | 0.000 |
| Dukes stage | |||||
| Dukes A | 38 (29.2) | 3 (5.5) | −1.97 | 0.1 (0.04–0.48) | 0.002 |
| Dukes B | 52 (40.0) | 12 (21.8) | −0.87 | 0.4 (0.20–0.87) | 0.019 |
| Dukes C | 39 (30.0) | 37 (67.3) | 1.57 | 4.8 (2.44–9.44) | 0.000 |
| Dukes D | 1 (0.8) | 3 (5.5) | 2.01 | 7.4 (0.76–73.20) | 0.085 |
OR, odds ratio; CI, confidence interval; BMI, body mass index; CRM, circumferential resection margin.
Univariate logistic regression analysis showed the risk factors associated with recurrence or metastasis were involvement of the MRF (OR =5.6, 95% CI: 2.01–10.48, P=0.000), without preoperative antibiotics (OR =2.1, 95% CI: 1.07–4.32, P=0.032), nerve and vascular invasion (OR =2.30, 95% CI: 1.61–3.29, P=0.000), nerve or vascular invasion (OR =3.90, 95% CI: 1.97–7.70, P=0.000), ileostomy (OR =5.6, 95% CI: 2.01–10.48, P=0.000), operative time (OR =1.0, 95% CI: 1.00–1.01, P=0.015), intraoperative blood transfusion (OR =2.5, 95% CI: 1.14–5.45, P=0.022), tumor T stage (OR =2.6, 95% CI: 1.34–4.91, P=0.005), positive CRM (OR =5.2, 95% CI: 1.25–21.54, P=0.024), number of metastatic lymph nodes (OR =1.25, 95% CI: 1.12–1.41, P=0.000), and Dukes stage (OR =3.7, 95% CI: 2.19–6.16, P=0.000), as shown in Table 1. According to the area under ROC curve analysis of the number of metastatic lymph nodes, the critical value was calculated as 1.5, being the number of metastatic lymph nodes found in pathological specimens was more than 1.5, which was a risk factor for postoperative recurrence or metastasis. Multivariate logistic regression analysis of statistically significant risk factors in univariate analysis showed MRF involvement (OR =2.9, 95% CI: 1.16–7.29, P=0.023), nerve and vascular invasion (OR =1.7, 95% CI: 1.08–2.59, P=0.022), intraoperative blood transfusion (OR =3.7, 95% CI: 1.45–9.40, P=0.006), and Dukes staging (OR =2.3, 95% CI: 1.26–4.35, P=0.007) were independent risk factors for postoperative recurrence or metastasis of rectal cancer with statistical significance, as shown in Table 2.
Table 2
| Factors of research | No recurrence or metastasis, n (%) | Recurrence or metastasis, n (%) | β | OR (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Involvement of mesorectal fascia | ||||||
| Yes | 73 (56.2) | 47 (85.5) | 1.07 | 2.9 (1.16–7.29) | 0.023 | |
| No | 57 (43.8) | 8 (14.5) | ||||
| Nerve and vascular invasion | ||||||
| Yes | 20 (15.4) | 27 (49.1) | 0.51 | 1.7 (1.08–2.59) | 0.022 | |
| No | 110 (84.6) | 28 (50.9) | ||||
| Intraoperative blood transfusion | ||||||
| Yes | 17 (13.1) | 40 (72.7) | 1.31 | 3.7 (1.45–9.40) | 0.006 | |
| No | 113 (86.9) | 15 (27.3) | ||||
| Dukes stage | ||||||
| Dukes A | 38 (29.2) | 3 (5.5) | 0.85 | 2.3 (1.26–4.35) | 0.007 | |
| Dukes B | 52 (40.0) | 12 (21.8) | ||||
| Dukes C | 39 (30.0) | 37 (67.3) | ||||
| Dukes D | 1 (0.8) | 3 (5.5) | ||||
OR, odds ratio; CI, confidence interval.
Among the 55 patients with recurrence or metastasis, three with postoperative recurrence involved the pelvic floor. Postoperative metastasis occurred in 52 patients, and included 27 (49.1%) with liver metastasis, 11 (20%) with lung metastasis, while others such as sacrococcygeal and peritoneal metastasis occurred in 14 patients (25.45%). The mean time of liver metastasis was 7.2 months, lung metastasis was 8.6 months, and other sites metastasis was 18.1 months. Among patients with T stage, recurrence, or metastasis, 1 patient (1.8%) was T1 stage, none were T2 stage, 6 (10.9%) were T3 stage, and 48 (87.3%) were T4 stage. Dukes stages included 3 patients (5.5%) in stage A, 12 (21.8%) in stage B, 37 cases (67.3%) in stage C, and 3 patients (5.5%) in stage D. Univariate logistic regression analysis showed that in patients with liver metastasis low rectal cancer (OR =0.3, 95% CI: 0.12–0.95, P=0.040), nerve and vascular invasion (OR =1.8, 95% CI: 1.21–2.81, P=0.005), nerve or vascular invasion (OR =2.5, 95% CI: 1.04–5.79, P=0.041), tumor T stage (OR =4.7, 95% CI: 1.22–18.18, P=0.025), lymph node metastasis (OR =1.1, 95% CI: 1.03–1.24, P=0.007), Dukes stage (OR =3.7, 95% CI: 1.83–7.30, P=0.000), and MRF involvement (OR =17.7, 95% CI: 2.34–133.77, P=0.005) were risk factors. Multivariate logistic regression analysis showed MRF involvement (OR =11.5, 95% CI: 1.49–88.79, P=0.019) and Dukes stage (OR =3.0, 95% CI: 1.46–6.26, P=0.003) were independent risk factors for liver metastasis, as shown in Table 3. For patients with lung metastasis, univariate logistic regression analysis showed nerve and vascular invasion (OR =2.8, 95% CI: 1.39–5.65, P=0.004), intraoperative nonperfusion chemotherapy (OR =4.0, 95% CI: 1.07–14.68, P=0.039), ileostomy (OR =8.1, 95% CI: 1.01–65.46, P=0.049), lymph node metastasis (OR =1.2, 95% CI: 1.05–1.30, P=0.005), and Dukes stage (OR =3.9, 95% CI: 1.33–11.65, P=0.013) were risk factors, and multivariate logistic regression analysis showed nerve and vascular invasion (OR =2.4, 95% CI: 1.19–5.00, P=0.015) was an independent risk factor, as shown in Table 4.
Table 3
| Logistic regression | No Liver metastasis, n (%) | Liver metastasis, n (%) | β | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Univariate logistic regression | |||||
| Distance from anal verge (cm) | |||||
| ≤5 | 63 (39.9) | 5 (18.5) | −0.07 | 0.3 (0.12–0.95) | 0.040 |
| >5 | 95 (60.1) | 22 (81.5) | |||
| Nerve and vascular invasion | |||||
| Yes | 34 (21.5) | 13 (48.1) | 0.61 | 1.8 (1.21–2.81) | 0.005 |
| No | 124 (78.5) | 14 (51.9) | |||
| Nerve or vascular invasion | |||||
| Yes | 71 (44.9) | 18 (66.7) | 0.89 | 2.5 (1.04–5.79) | 0.041 |
| No | 87 (55.1) | 9 (33.3) | |||
| T stage of tumor | |||||
| T3 | 33 (20.9) | 2 (7.4) | −1.19 | 0.3 (0.07–1.35) | 0.116 |
| T4 | 106 (67.1) | 25 (92.6) | 1.18 | 6.1 (1.40–26.88) | 0.016 |
| No. of lymph node metastasis | |||||
| >0.5 | 60 (38.0) | 21 (77.8) | 0.12 | 1.1 (1.03–1.24) | 0.007 |
| ≤0.5 | 98 (62.0) | 6 (22.2) | |||
| Dukes stage | |||||
| Dukes A | 40 (25.3) | 1 (3.7) | −2.18 | 0.1 (0.02–0.86) | 0.036 |
| Dukes B | 59 (37.3) | 5 (18.5) | −0.96 | 0.4 (0.14–1.06) | 0.065 |
| Dukes C | 57 (36.1) | 19 (70.4) | 1.44 | 4.2 (1.73–10.22) | 0.002 |
| Dukes D | 2 (1.3) | 2 (7.4) | 1.83 | 6.2 (0.84–46.34) | 0.073 |
| Involvement of mesorectal fascia | |||||
| Yes | 94 (59.5) | 26 (96.3) | 2.87 | 17.7 (2.34–133.8) | 0.005 |
| No | 64 (40.5) | 1 (3.7) | |||
| Multiple logistic regression | |||||
| Involvement of mesorectal fascia | |||||
| Yes | 94 (59.5) | 26 (96.3) | 2.4 | 11.5 (1.49–88.79) | 0.019 |
| No | 64 (40.5) | 1 (3.7) | |||
| Dukes stage | |||||
| Dukes A | 40 (25.3) | 1 (3.7) | 1.1 | 3.0 (1.46–6.26) | 0.003 |
| Dukes B | 59 (37.3) | 5 (18.5) | |||
| Dukes C | 57 (36.1) | 19 (70.4) | |||
| Dukes D | 2 (1.3) | 2 (7.4) | |||
OR, odds ratio; CI, confidence interval; T stage, tumor stage.
Table 4
| Factors of research | No pulmonary metastasis, n (%) | Pulmonary metastasis, n (%) | β | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Univariate logistic regression | |||||
| Nerve and vascular invasion | |||||
| Yes | 40 (23.0) | 7 (63.6) | 1.03 | 2.8 (1.39–5.65) | 0.004 |
| No | 134 (77.0) | 4 (36.4) | |||
| Intraoperative chemotherapy | |||||
| Yes | 48 (27.6) | 6 (54.5) | 1.38 | 3.9 (1.07–14.68) | 0.039 |
| No | 126 (72.4) | 5 (45.5) | |||
| Ileostomy | |||||
| Yes | 92 (52.9) | 9 (81.8) | 2.09 | 8.1 (1.01–65.46) | 0.049 |
| No | 82 (47.1) | 2 (18.2) | |||
| No. of lymph node metastasis | |||||
| ≤2.5 | 137 (78.7) | 2 (18.2) | 0.16 | 1.2 (1.05–1.31) | 0.005 |
| >2.5 | 37 (21.3) | 9 (81.8) | |||
| Dukes stage | |||||
| Dukes A | 40 (23.0) | 1 (9.1) | −0.98 | 0.4 (0.05–3.05) | 0.359 |
| Dukes B | 64 (36.8) | 0 (0.0) | −18.80 | 0.0 (0.00–0.00) | 0.997 |
| Dukes C | 67 (38.5) | 9 (81.8) | 1.84 | 6.3 (1.30–30.53) | 0.022 |
| Dukes D | 3 (1.7) | 1 (9.1) | 1.85 | 6.4 (0.60–67.49) | 0.124 |
| Multiple logistic regression | |||||
| Nerve and vascular invasion | |||||
| Yes | 40 (23.0) | 7 (63.6) | 1.6 | 2.4 (1.19–5.00) | 0.015 |
| No | 134 (77.0) | 4 (36.4) | |||
OR, odds ratio; CI, confidence interval.
The median follow-up time was 45 months, and 28 (15.1%) of the 185 patients died. Univariate logistic regression analysis showed recurrence or metastasis (OR =22.3, 95% CI: 6.26–79.77, P=0.000), liver metastasis (OR =15.4, 95% CI: 5.59–42.26, P=0.000), MRF involvement (OR =3.9, 95% CI: 1.11–13.68, P=0.034), intraoperative blood transfusion (OR =3.3, 95% CI: 1.26–8.74, P=0.016), no left colon artery preservation (OR =0.3, 95% CI: 0.11–0.93, P=0.033), total number of lymph nodes (OR =0.9, 95% CI: 0.88–0.99, P=0.028), and Dukes stage (OR =3.5, 95% CI: 1.67–7.35, P=0.001) were risk factors for death, as shown in Table 5. Multivariate logistic regression analysis showed recurrence or metastasis (OR =7.6, 95% CI: 1.59–36.59, P=0.011), liver metastasis (OR =4.7, 95% CI: 1.17–18.57, P=0.029), total number of lymph nodes (OR =0.9, 95% CI: 0.78–0.98, P=0.016) and left colon artery without preservation (OR =0.2, 95% CI: 0.06–0.74, P=0.016) were independent risk factors for death, as shown in Table 6. In addition, the ROC curve analysis showed involvement of ≤6 lymph nodes was a risk factor for death.
Table 5
| Factors of research | No death, n (%) | Death, n (%) | β | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Recurrence or metastasis | |||||
| Yes | 30 (19.1) | 25 (89.3) | 3.11 | 22.3 (6.26–79.77) | 0 |
| No | 127 (80.9) | 3 (10.7) | |||
| Pulmonary metastasis | |||||
| Yes | 8 (5.1) | 3 (10.7) | −0.21 | 0.8 (0.10–6.76) | 0.85 |
| No | 149 (94.9) | 25 (89.3) | |||
| Liver metastasis | |||||
| Yes | 11 (7.0) | 16 (57.1) | 2.73 | 15.4 (5.59–42.26) | 0 |
| No | 146 (93.0) | 12 (42.9) | |||
| Involvement of mesorectal fascia | |||||
| Yes | 96 (61.1) | 24 (85.7) | 1.36 | 3.9 (1.11–13.68) | 0.034 |
| No | 61 (38.9) | 4 (14.3) | |||
| Intraoperative blood transfusion | |||||
| Yes | 22 (14.0) | 10 (35.7) | 1.2 | 3.3 (1.25–8.74) | 0.016 |
| No | 135 (86.0) | 18 (64.3) | |||
| Sphincter preservation | |||||
| Yes | 140 (89.2) | 22 (78.6) | −0.86 | 0.4 (0.14–1.28) | 0.128 |
| No | 17 (10.8) | 6 (21.4) | |||
| Left colonic artery preservation | |||||
| Yes | 76 (48.4) | 7 (25.0) | −1.14 | 0.3 (0.11–0.93) | 0.033 |
| No | 81 (51.6) | 21 (75.0) | |||
| T stage of tumor | |||||
| T1 | 6 (3.8) | 0 (0.0) | −19.24 | 0.0 (0.00–0.00) | 0.999 |
| T2 | 12 (7.6) | 1 (3.6) | −0.51 | 0.6 (0.07–4.85) | 0.631 |
| T3 | 31 (19.7) | 4 (14.3) | −0.93 | 0.4 (0.09–1.77) | 0.224 |
| T4 | 108 (68.8) | 23 (82.1) | 1.06 | 2.9 (0.82–10.19) | 0.1 |
| CRM | |||||
| Positive | 6 (3.8) | 3 (10.7) | 1.42 | 4.1 (0.95–17.89) | 0.058 |
| Negative | 151 (96.2) | 25 (89.3) | |||
| Total lymph nodes (mean) | 17.5 | 13.5 | −0.07 | 0.9 (0.88–0.99) | 0.028 |
| No. of lymph node metastasis (mean) | 1.9 | 2.5 | 0.05 | 1.1 (0.95–1.17) | 0.312 |
| Dukes stage | |||||
| Dukes A | 40 (25.5) | 1 (3.6) | −19.49 | 0.0 (0.00–0.00) | 0.998 |
| Dukes B | 58 (36.9) | 6 (21.4) | −0.39 | 0.7 (0.25–1.83) | 0.444 |
| Dukes C | 57 (36.3) | 19 (67.9) | 1.05 | 2.9 (1.13–7.19) | 0.026 |
| Dukes D | 2 (1.3) | 2 (7.1) | 2.09 | 8.1 (1.07–60.34) | 0.042 |
OR, odds ratio; CI, confidence interval; CRM, circumferential resection margin.
Table 6
| Factors of research | No death, n (%) | Death, n (%) | β | OR (95% CI) | P value |
|---|---|---|---|---|---|
| Recurrence or metastasis | |||||
| Yes | 30 (19.1) | 25 (89.3) | −2.18 | 0.1 (0.03–0.46) | 0.002 |
| No | 127 (80.9) | 3 (10.7) | |||
| Liver metastasis | |||||
| Yes | 11 (7.0) | 16 (57.1) | −1.54 | 0.2 (0.08–0.61) | 0.004 |
| No | 146 (93.0) | 12 (42.9) | |||
| Dukes D | 2 (1.3) | 2 (7.1) | −2.10 | 0.1 (0.02–0.80) | 0.028 |
| Intraoperative blood transfusion | |||||
| Yes | 22 (14.0) | 10 (35.7) | −0.99 | 0.4 (0.15–0.94) | 0.036 |
| No | 135 (86.0) | 18 (64.3) | |||
| Left colonic artery preservation | |||||
| Yes | 76 (48.4) | 7 (25.0) | 1.82 | 6.1 (1.80–20.95) | 0.004 |
| No | 81 (51.6) | 21 (75.0) | |||
| Total lymph nodes (mean) | 17.5 | 13.5 | −0.11 | 0.9 (0.82–0.99) | 0.024 |
OR, odds ratio; CI, confidence interval.
During the follow-up, multiple factors were further stratified and analyzed, and among these, the recurrence or metastasis rate of low rectal cancer was 32.3%, middle rectal cancer was 25%, and high rectal cancer was 33.3%, and the rate of T1, T2, T3, and T4 was 16.7%, 0, 17.1%, and 36.6%, respectively. Dukes A stage was 7.3%, Dukes B stage 18.8%, Dukes C stage 48.7%, and Dukes D stage was 75%.
Multivariate Cox regression was used to analyze the survival of postoperative death from rectal cancer, and showed intraoperative blood transfusion (HR =0.4, 95% CI: 0.15–0.94, P=0.036), recurrence or metastasis (HR =0.1, 95% CI: 0.03–0.46, P=0.002), liver metastasis (HR =0.2, 95% CI: 0.08–0.61, P=0.004), left colic artery preservation (HR =6.1, 95% CI: 1.80–20.95, P=0.004), number of lymph nodes (HR =0.9, 95% CI: 0.82–0.99, P=0.024) and Dukes D stage (HR =0.1, 95% CI: 0.02–0.80, P=0.028) were statistically significant, and were independent risk factors affecting patient death, as shown in Figure 2.
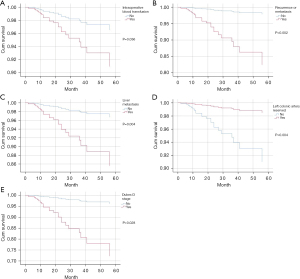
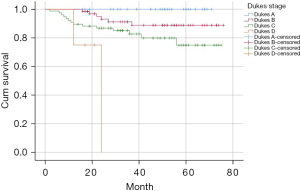
Kaplan-Meier was used to analyze the survival curve of postoperative death, and the results showed MRF involvement, recurrence or metastasis, liver metastasis, positive CRM, intraoperative blood transfusion, unreserved left colon artery, and Dukes stage were statistically significant risk factors, and the survival curve is shown in Figure 3. The Dukes stages were stratified, and the 2-year and 3-year survival rates were 92% and 78.1% for Dukes A, 78.1% and 60.9% for Dukes B, 78.9% and 46.1% for Dukes C, and 0% for Dukes D. The 2-year and 3-year survival rates were 65.5% and 34.5% in patients with recurrence or metastasis, 86.2% and 64.6% in patients with non-recurrence or metastasis, 48.1% and 29.6% in patients with liver metastasis, and 85.4% and 62% in patients with non-liver metastasis.
Discussion
Rectal cancer is comprehensive treated by surgery, and both TME (total mesorectum excision) standards and neoadjuvant chemoradiation have greatly improved the quality of surgery and the cure rate, bringing a significant improvement in the quality of life of patients. However, the rectal cancer postoperative survival rate remains variable, mainly because of recurrence or metastasis. At the time of initial diagnosis, about 25% of stage IV patients have liver metastasis and about 50% have metastasis to other sites (11,12). For locally advanced or advanced rectal cancer, distant metastasis is the most common cause of death, and the lung is the most likely site, followed by the liver (13,14). However, if local recurrence occurs without surgery, the 5-year overall survival is about 4% (15-20).
Studies on postoperative recurrence or metastasis of rectal cancer exist, but varying inclusion criteria, methods, and research sites mean a unified conclusion has not been reached. A study on factors related to the local recurrence in 497 patients with T3 rectal cancer with tumor located within 12 cm of the anal margin by laparoscopic surgery without preoperative chemoradiotherapy in four Asian countries found gender, tumor location, lymph node metastasis, and tumor perforation were independent factors for local recurrence (21). In our study, recurrence or metastasis was found in 29.7% of patients after surgery. There are many risk factors associated with recurrence or metastasis, such as MRF involvement, no preoperative antibiotics use, nerve and/or vascular invasion, stoma, operative time, intraoperative blood transfusion, T stage of tumor, positive CRM, number of metastatic lymph nodes, and Dukes stage of tumor. Multivariate logistic regression analysis showed MRF involvement (P=0.023), nerve and vascular invasion (P=0.022), intraoperative blood transfusion (P=0.006), and Dukes stage (P=0.007) were independent risk factors for postoperative recurrence or metastasis.
MRF involvement, diagnosed by preoperative MRI, is a risk factor for poor prognosis in rectal cancer. In a univariate analysis, poorly differentiated tumors, tumors larger than 5cm, and MRF involvement were associated with poorer 3-year disease-free survival and overall survival, and patients with early distant metastasis were more likely to develop MRF involvement than those without distant metastasis (P=0.002) (22), which is consistent with our research conclusions. Therefore, preoperative MRI evaluation of rectal cancer is crucial. However, there is no consensus on whether preoperative radiotherapy and chemotherapy reduce the rate of postoperative recurrence or metastasis if the MRF is involved, although this may suggest a more rigorous treatment and follow-up plan be implemented.
Multifactorial analysis showed venous invasion was an independent adverse prognostic factor and was associated with an increased risk of liver metastases. In a study specifically focused on pT1 and superficial pT2 rectal cancer, the involvement of small vessels at any tumor site was significantly correlated with regional nodular metastasis (23,24). Lee et al. (25) found lymphatic vascular invasion was an independent risk factor for disease-free survival after radical resection of colorectal cancer, and postoperative recurrence or metastasis were more likely. Additionally, a meta-analysis showed lymphatic vascular invasion was an independent risk factor for lymph node metastasis (26). Lymphatic vascular invasion plays an important role in the prognosis of rectal cancer, and its value can be fully utilized clinically to provide a better surgical plan and follow-up treatment. Intraoperative blood transfusion affects postoperative recurrence or metastasis of rectal cancer, and the possible reason is that during surgery, due to factors such as late tumor stage, tumor invasion of peripheral blood vessels, and low tumor location, the operation time is long and the injury is large, leading to more intraoperative blood loss.
Postoperative pathological staging has long been considered the most powerful prognostic indicator of rectal cancer and often determines the selectivity of systemic adjuvant therapy. In pT3 tumors, the degree of extraneous invasion has been reported to be an important prognostic feature regardless of regional lymph node metastasis. Many studies suggest extramural infiltration of more than 5mm may have a serious adverse effect on prognosis (27,28). The pathologic assessment of serous tumor invasion is only relevant to the upper rectum, which importantly, is frontally covered by visceral peritoneum. For pT4 tumors penetrating the visceral peritoneum, the median survival time after surgical resection was significantly reduced compared with patients without serous membrane involvement. Kim et al. (29) conducted a retrospective study on 714 patients with locally advanced rectal cancer who received TME surgery after preoperative neoadjuvant chemotherapy. Among them, 139 patients (19.5%) relapsed, and of these, 49 recurred within 1 year after operation, and 90 patients recurred over 1 year after. Multivariate analysis showed that positive ypN stage and grade 3 or above tumor stage were statistically significant and were independent risk factors for postoperative local recurrence. Therefore, tumor stage is one of the important factors affecting the prognosis of rectal cancer.
Liver metastasis of colorectal cancer is a common clinical problem. According to statistics, about 25% of patients have liver metastasis when first diagnosed, about 50% have liver metastasis during the whole course of disease, and the liver is the only metastatic site in 20–30% of patients. In a study on the correlation of preoperative liver metastasis in rectal cancer, univariate analysis showed the level of carcinoembryonic antigen, N stage, MRF invasion, and mesenteric vascular lesion (MVL) grade were correlated with liver metastasis (P<0.05), and multivariate analysis showed rectal MVL grading and MRF invasion were independent factors (30-32). The characteristics of rectal reflux blood circulation are also closely related to the liver. The capillaries in the mucosal layer of the rectal wall pass through the submucosa and muscle layer and enter the subserous membrane. If the tumor infiltrates to this point, tumor cells could enter the blood circulation and enter the liver.
The lung is a common site of postoperative lung metastasis. The influence of pelvic lymph node size before and after radiotherapy and chemotherapy on postoperative lung metastasis was studied, and the results showed the cumulative lung metastasis rate in 5 years was 15.2%. Mean lateral lymph nodes were larger in patients with lung metastasis than in those without and multivariate analysis showed lung metastasis correlated with lateral lymph node size, tumor T stage, and tumor location after radiotherapy and chemotherapy (33). Tumor location, lymph node metastasis, tumor stage, and positive CRM were independent risk factors for lung metastasis in a study of patients with postoperative lung metastasis for rectal cancer. Therefore, in view of the risk factors related to postoperative lung metastasis of rectal cancer, a more intensive monitoring program is required timely intervention, and treatment (34). Although there are many studies on the risk factors related to lung metastasis, there is still no clear specific risk factor, and the metastasis mechanism remains to be further studied.
There are many other risk factors associated with postoperative recurrence or metastasis of rectal cancer. For example, studies have found that a distal resection margin less than 0.9 cm and lymph node dissection less than 14 are independent risk factors for postoperative distant metastasis of middle and low rectal cancer (35). A prospective study of three Italian medical institutions showed that age less than 63 years, CEA greater than 3 ng/dL, and tumor location below 5 cm in the anal margin were adverse risk factors for early postoperative recurrence or metastasis of rectal cancer (36). Local recurrence after R0 resection is not uncommon. There is a correlation between the pathological features of rectal cancer and postoperative recurrence or metastasis. It was found that venous invasion of primary lesions and tumor progression pattern (bulge type, invasion type, mixed type) were independent predictors of local recurrence (37). It is still controversial whether neoadjuvant radiotherapy should be performed before surgery, and new Dutch guidelines reject radiotherapy in low-risk patients. There were 407 cases of primary rectal cancer surgery without synchronous metastasis, including 225 under the old guidelines and 182 under the new guidelines. The new group had lower tumor stage and lymph node stage, and no differences in pathological tumor stage were found. There was no significant difference in 1-year local recurrence rate and mortality (38). Therefore, radiotherapy does not have a high clinical value for patients with low-risk rectal cancer.
The effectiveness of different surveillance regimens for recurrence or metastasis after radical rectal cancer surgery has not been well established. One study using different surveillance protocols for assessment found the proportion of recurrences detected during regular follow-up was, on average, earlier than during additional follow-up (39). Therefore, for rectal cancer patients with high risk factors, individualized follow-up programs should be established to better conduct early intervention and treatment, and effectively improve the prognosis of patients.
Limitations
This was a retrospective clinical study with limited research conditions and limited quantity of patients. In the statistical process of factors related to postoperative recurrence or metastasis of rectal cancer, some factors could not be included due to a lack of data.
Conclusions
The results of multivariate analysis showed MRF involvement, nerve and vascular invasion, intraoperative blood transfusion, and Dukes stage were independent risk factors for postoperative recurrence or metastasis of rectal cancer. MRF involvement and Dukes stage were independent risk factors for liver metastasis, and nerve and vascular invasion were independent risk factor for lung metastasis. These risk factors can be combined to establish a risk prediction model, to provide a more personalized and effective follow-up and treatment plan for each patient with rectal cancer.
Acknowledgments
Funding: This study was supported by the Great Discipline Construction Project from The Fifth People’s Hospital of Shanghai, Fudan University (No. 2020WYZDZK02).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-942/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-942/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-942/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Ethics Committee of The Fifth People's Hospital of Shanghai, Fudan University (
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- van der Stok EP, Spaander MCW, Grünhagen DJ, et al. Surveillance after curative treatment for colorectal cancer. Nat Rev Clin Oncol 2017;14:297-315. [Crossref] [PubMed]
- Choi MS, Huh JW, Shin JK, et al. Prognostic Factors and Treatment of Recurrence after Local Excision of Rectal Cancer. Yonsei Med J 2021;62:1107-16. [Crossref] [PubMed]
- Morohashi H, Sakamoto Y, Miura T, et al. Effective dissection for rectal cancer with lateral lymph node metastasis based on prognostic factors and recurrence type. Int J Colorectal Dis 2021;36:1251-61. [Crossref] [PubMed]
- Oki E, Murata A, Yoshida K, et al. A randomized phase III trial comparing S-1 versus UFT as adjuvant chemotherapy for stage II/III rectal cancer (JFMC35-C1: ACTS-RC). Ann Oncol 2016;27:1266-72. [Crossref] [PubMed]
- Tanaka A, Uehara K, Aiba T, et al. The role of surgery for locally recurrent and second recurrent rectal cancer with metastatic disease. Surg Oncol 2020;35:328-35. [Crossref] [PubMed]
- Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696-701. [Crossref] [PubMed]
- Jankowski M, Las-Jankowska M, Rutkowski A, et al. Clinical Reality and Treatment for Local Recurrence of Rectal Cancer: A Single-Center Retrospective Study. Medicina (Kaunas) 2021;57:286. [Crossref] [PubMed]
- Liška V, Emingr M, Skála M, et al. Liver metastases from colon and rectal cancer in terms of differences in their clinical parameters. Rozhl Chir 2016;95:69-77. [PubMed]
- Trakarnsanga A, Ithimakin S, Weiser MR. Treatment of locally advanced rectal cancer: controversies and questions. World J Gastroenterol 2012;18:5521-32. [Crossref] [PubMed]
- Ghiasloo M, Pavlenko D, Verhaeghe M, et al. Surgical treatment of stage IV colorectal cancer with synchronous liver metastases: A systematic review and network meta-analysis. Eur J Surg Oncol 2020;46:1203-13. [Crossref] [PubMed]
- Masaki T, Matsuoka H, Kishiki T, et al. Site-specific risk factors for local recurrence after rectal cancer surgery. Surg Oncol 2021;37:101540. [Crossref] [PubMed]
- Ikoma N, You YN, Bednarski BK, et al. Impact of Recurrence and Salvage Surgery on Survival After Multidisciplinary Treatment of Rectal Cancer. J Clin Oncol 2017;35:2631-8. [Crossref] [PubMed]
- Baig M, Sayyed R, Nasim S, et al. Effect of rectal washout on local recurrence of rectal cancer in the era of total mesorectal excision: Meta-analysis. Surgeon 2021;19:351-5. [Crossref] [PubMed]
- Peeters KC, Marijnen CA, Nagtegaal ID, et al. The TME trial after a median follow-up of 6 years: increased local control but no survival benefit in irradiated patients with resectable rectal carcinoma. Ann Surg 2007;246:693-701. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Hagemans JAW, van Rees JM, Alberda WJ, et al. Locally recurrent rectal cancer; long-term outcome of curative surgical and non-surgical treatment of 447 consecutive patients in a tertiary referral centre. Eur J Surg Oncol 2020;46:448-54. [Crossref] [PubMed]
- Johnstone P, Okonta L, Aitken K, et al. A multicentre retrospective review of SABR reirradiation in rectal cancer recurrence. Radiother Oncol 2021;162:1-6. [Crossref] [PubMed]
- Staal FCR, van der Reijd DJ, Taghavi M, et al. Radiomics for the Prediction of Treatment Outcome and Survival in Patients With Colorectal Cancer: A Systematic Review. Clin Colorectal Cancer 2021;20:52-71. [Crossref] [PubMed]
- Matsuyama T, Yamauchi S, Masuda T, et al. Treatment and subsequent prognosis in locally recurrent rectal cancer: a multicenter retrospective study of 498 patients. Int J Colorectal Dis 2021;36:1243-50. [Crossref] [PubMed]
- Lee SI, Kim SH, Wang HM, et al. Local recurrence after laparoscopic resection of T3 rectal cancer without preoperative chemoradiation and a risk group analysis: an Asian collaborative study. J Gastrointest Surg 2008;12:933-8. [Crossref] [PubMed]
- Park H. Predictive factors for early distant metastasis after neoadjuvant chemoradiotherapy in locally advanced rectal cancer. World J Gastrointest Oncol 2021;13:252-64. [Crossref] [PubMed]
- Harrison JC, Dean PJ, el-Zeky F, et al. From Dukes through Jass: pathological prognostic indicators in rectal cancer. Hum Pathol 1994;25:498-505. [Crossref] [PubMed]
- Goldstein NS, Hart J. Histologic features associated with lymph node metastasis in stage T1 and superficial T2 rectal adenocarcinomas in abdominoperineal resection specimens. Identifying a subset of patients for whom treatment with adjuvant therapy or completion abdominoperineal resection should be considered after local excision. Am J Clin Pathol 1999;111:51-8. [Crossref] [PubMed]
- Lee JH, Lee JL, Park IJ, et al. Identification of Recurrence-Predictive Indicators in Stage I Colorectal Cancer. World J Surg 2017;41:1126-33. [Crossref] [PubMed]
- Bosch SL, Teerenstra S, de Wilt JH, et al. Predicting lymph node metastasis in pT1 colorectal cancer: a systematic review of risk factors providing rationale for therapy decisions. Endoscopy 2013;45:827-34. [Crossref] [PubMed]
- Timmerman C, Taveras LR, Huerta S. Clinical and molecular diagnosis of pathologic complete response in rectal cancer: an update. Expert Rev Mol Diagn 2018;18:887-96. [Crossref] [PubMed]
- Haggstrom L, Kang S, Winn R, et al. Factors influencing recurrence of stage I-III rectal cancer in regional Australia. ANZ J Surg 2020;90:2490-5. [Crossref] [PubMed]
- Kim HG, Kim HS, Yang SY, et al. Early recurrence after neoadjuvant chemoradiation therapy for locally advanced rectal cancer: Characteristics and risk factors. Asian J Surg 2021;44:298-302. [Crossref] [PubMed]
- Kang KA, Jang KM, Kim SH, et al. Risk factor assessment to predict the likelihood of a diagnosis of metastasis for indeterminate hepatic lesions found at computed tomography in patients with rectal cancer. Clin Radiol 2017;72:473-81. [Crossref] [PubMed]
- Chandra R, Karalis JD, Liu C, et al. The Colorectal Cancer Tumor Microenvironment and Its Impact on Liver and Lung Metastasis. Cancers (Basel) 2021;13:6206. [Crossref] [PubMed]
- Takei S, Homma Y, Matsuyama R, et al. Hepatectomy for liver metastasis from rectal cancer in a patient with mitochondrial disease. BMJ Case Rep 2021;14:238653. [Crossref] [PubMed]
- Shiratori H, Kawai K, Hata K, et al. Correlations between the Recurrence Patterns and Sizes of Lateral Pelvic Lymph Nodes before and after Chemoradiotherapy in Patients with Lower Rectal Cancer. Oncology 2019;96:33-43. [Crossref] [PubMed]
- Pan HD, Zhao G, An Q, et al. Pulmonary metastasis in rectal cancer: a retrospective study of clinicopathological characteristics of 404 patients in Chinese cohort. BMJ Open 2018;8:e019614. [Crossref] [PubMed]
- Lai IL, You JF, Chern YJ, et al. The risk factors of local recurrence and distant metastasis on pT1/T2N0 mid-low rectal cancer after total mesorectal excision. World J Surg Oncol 2021;19:116. [Crossref] [PubMed]
- Restivo A, Delrio P, Deidda S, et al. Predictors of Early Distant Relapse in Rectal Cancer Patients Submitted to Preoperative Chemoradiotherapy. Oncol Res Treat 2020;43:146-52. [Crossref] [PubMed]
- Uemura M, Ikeda M, Yamamoto H, et al. Clinicopathological assessment of locally recurrent rectal cancer and relation to local re-recurrence. Ann Surg Oncol 2011;18:1015-22. [Crossref] [PubMed]
- Tersteeg JJC, van Esch LM, Gobardhan PD, et al. Early local recurrence and one-year mortality of rectal cancer after restricting the neoadjuvant therapy regime. Eur J Surg Oncol 2019;45:597-605. [Crossref] [PubMed]
- Räsänen M, Carpelan-Holmström M, Mustonen H, et al. Pattern of rectal cancer recurrence after curative surgery. Int J Colorectal Dis 2015;30:775-85. [Crossref] [PubMed]



