
A phase II randomized double blinded trial evaluating the efficacy of curcumin with pre-operative chemoradiation for rectal cancer
Introduction
The standard of care treatment for locally advanced rectal cancer (LARC) was established as preoperative chemoradiation therapy (CRT) followed by surgical resection in 2004 by the German rectal cancer trial, which proved the benefit of preoperative vs. postoperative CRT in terms of improved local control (LC), sphincter preservation, and decreased toxicity (1). During this trial, patients received radiation therapy (RT) concurrent with 5-fluorouracil (5-FU), used as a radiation sensitizer, followed by surgical resection.
Since that time, one thrust of research has been to alter the sequence of treatments to preoperative chemotherapy and CRT before surgery (total neoadjuvant therapy). This approach addresses the concern that postoperative adjuvant chemotherapy has failed to reduce the incidence of distant metastases that is over twice the incidence of local and regional metastases. Preoperative chemotherapy affords the opportunity to target occult micrometastatic disease early, allows tumor downstaging, facilitates greater compliance with planned therapy, and permits early assessment of chemosensitivity. Early results suggest that this is indeed associated with improved overall outcomes, but the exact sequencing of neoadjuvant chemotherapy and CRT remains unclear (2-4).
A parallel research thrust seeks to improve the efficacy of CRT using radiation sensitizers like 5-FU to improve the current roughly 20% pathologic complete response (pCR) rate. Improved pCR rates are associated with longer progression-free survival (PFS) and overall survival (OS) (5-7). Response to pre-operative CRT may improve the chances of sphincter-preserving surgery (8,9), and a pCR to CRT may permit selected patients to undergo less extensive resections and potentially organ-preserving strategies, therefore further minimizing toxicity (10).
Another strategy has been to explore the convergence of these approaches where total neoadjuvant therapy is coupled with radiation sensitization. This approach is being tested currently in a multi-arm cooperative group study with no clear improvement thus far (11). Many have evaluated the substitution or addition of other systemic agents to the preoperative regimen (12-14), but none has shown a clear advantage over the regimen with 5-FU alone, especially without increased toxicity (15). While most strategies to improve radiation response rates with concurrent systemic agents have focused on the inhibition of inherent or constitutive pro-survival pathway overexpression within tumor cells (16,17) redundant signaling pathways hinder success (18). Alternatively, many have identified the broad spectrum blockade of inducible pathways that drive pro-survival and anti-apoptotic signals as a promising method of improving tumor response to treatment.
Curcumin, a polyphenol and major component of the spice turmeric, has been implemented for anti-inflammatory medicinal purposes in India for centuries (19). More recently, this agent has been recognized for its potential as an anti-neoplastic or chemopreventive agent (20-22). It inhibits angiogenesis, induces apoptosis or cell cycle arrest, and causes regression of tumors in preclinical models (23-26). While safety has been established in multiple phase I trials, its benefit in terms of tumor response has not been established in colorectal cancer trials. The poor bioavailability of curcumin creates challenges for therapeutic use (27); however, this obstacle could possibly be circumvented when targeting gastrointestinal tract malignancies.
We hypothesized that the addition of curcumin to the preoperative therapy regimen could improve outcomes when given with capecitabine (a 5-FU pro-drug) and RT. Therefore, we conducted a phase II randomized double-blinded study to evaluate the efficacy in terms of pCR rates of adding curcumin to preoperative CRT for LARC patients. We analyzed tumor downstaging, local regional/distant failure, and survival rates. To further explore the pharmacology of curcumin and validate observed effects, we also determined the serum and rectal tumor tissue levels to correlate with clinical response. We present the following article in accordance with the CONSORT reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-259/rc).
Methods
Patient eligibility and study design
From 2008–2010, 22 patients with LARC (T3/T4 or T2 and node positive disease) treated at our institution were enrolled in this study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the MD Anderson Cancer Center institutional review board (protocol 2006-0644) (clinicaltrials.gov number NCT00745134). Informed consent was taken from all individual participants. Inclusion and exclusion criteria are shown in Table 1. The primary endpoint was pCR rate following definitive CRT with/without curcumin. The research staff at MD Anderson Cancer Center enrolled participants. Patients, care providers, and the research team, including those assessing outcomes and toxicity, were blinded to treatment assignment. Patients were randomized to control and experimental treatment arms in a 1:2 ratio, with a planned enrollment of 15 placebo patients and 30 curcumin patients. Randomization was performed using a website created by the Department of Biostatistics and used by pharmacists. For patients randomized to curcumin, a two-stage design was employed with a significance level of 10% and 80% power (16 patients were to be treated in the first stage, and 14 were to be treated in second stage only if there were 3 or more patients with pCR in first stage). The null pCR rate was 18%, and the target pCR rate was 36% or more. There was a 0.43 probability of stopping the study after the first stage if the pCR rate was 18% and a 0.04 probability of stopping the study early if the pCR rate 36%. Secondary endpoints included patient downstaging at the time of surgery, LC, PFS, and OS. We also planned to determine curcumin serum and tissue levels. There were no significant changes to trial methods after commencement. The trial was stopped early because there was only one patient with pCR among the first 15 patients randomized to the curcumin arm.
Table 1
| Eligibility criteria |
| Clinical stage T3,4 N0,1,2 or T2N1,2 adenocarcinoma of the rectum |
| Staged using endorectal ultrasound, pelvic CT or MRI, and physical examination |
| Histology confirmed by the Department of Pathology at MD Anderson Cancer Center |
| No distant metastatic disease in the liver, peritoneum, lungs, or paraaortic lymph nodes |
| Performance status (Karnofsky scale) of 70% or greater |
| 18 years of age or greater |
| ANC >1,200 cells/mm3, platelets >100,000/mm3, total serum bilirubin <2 mg/dL, BUN <30 mg/dL, creatinine <1.5 mg/dL or creatinine clearance >50 cc/min (estimated by Cockcroft-Gault equation) |
| Signed informed consent for investigational nature of study and voluntary participation |
| No use of additional herbal supplements during study |
| For women: amenorrhea for ≥12 months or use of reliable contraception (continued 30 days from last study drug administration) |
| For men: use of reliable contraception during study |
| Exclusion criteria |
| Prior complete course up to 5 Gy of radiotherapy to the pelvis |
| Pregnant or lactating woman (women/men of childbearing potential not using a reliable contraceptive method) |
| Treatment for other carcinomas within last 5 years, except cured non-melanoma skin and treated in-situ cervical cancer |
| Patients with uncontrolled intercurrent illness including, but not limited to, ongoing or requiring IV antibiotics, cardiac disease NYHA class III or IV, unstable angina pectoris, unstable cardiac arrythmia or tachycardia (heart rate >100 beats/minute), or psychiatric illness/ social situations limiting compliance with study |
| Other serious uncontrolled medical conditions that might compromise study participation |
| Major surgery within 4 weeks of the start of study treatment |
| Prior unanticipated severe reaction to fluoropyrimidine therapy or known hypersensitivity to 5-FU, capecitabine, or curcumin |
| Concurrent use of therapeutic Coumadin |
| Concurrent use of cimetidine, allopurinol, or aluminium hydroxide and magnesium hydroxide-containing antacids |
| Sorivudine and brivudine use within 4 weeks of the start of study treatment |
CT, computed tomography; MRI, magnetic resonance imaging; ANC, absolute neutrophil count; BUN, blood urea nitrogen; 5-FU, 5-fluorouracil.
Systemic therapy
Capecitabine was given orally twice daily 12 hours apart on the days of RT at 825 mg/m2. Curcumin or placebo was given at 4 gm twice daily [dose based on prior studies (28,29)] during RT and for 6 weeks after completion of RT (for prosurvival signaling suppression until time of surgery). Curcumin was consumed approximately 1 hour before RT. Curcumin C3 complex and placebo were obtained from Sabinsa Corporation, Piscataway, NJ, USA.
RT technique
The primary tumor and involved lymph nodes, determined by pre-operative computed tomography (CT) imaging [5 patients also had magnetic resonance imaging (MRI)] and/or colonoscopy, as well as the perirectal, presacral, and internal iliac ± external iliac nodes, were treated to 45 Gy in 25 fractions using standard rectal fields. An additional 5.4 Gy in 3 fractions was given as a boost to the primary tumor and involved lymph nodes with 2–3 cm margin including the presacral space and sacrum.
Evaluation of plasma/tissue curcumin levels
Biopsies for tissue curcumin levels and patient plasma samples were obtained during CRT week 2. Plasma levels were assessed pre- (1 hour prior) and post- (1 hour after) curcumin/placebo administration. Please see the Appendix 1 for full methods of plasma and tissue sample analysis.
Toxicity assessment
Toxicity was assessed at weekly on-treatment visits at MD Anderson Cancer Center using the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0.
Patient follow-up and outcomes assessment
Patients were contacted by telephone approximately 1 month after completion of CRT and 6 weeks of maintenance therapy to evaluate toxicity. Patients then underwent surgery, usually within 1–3 weeks from this toxicity check. Pathologic response was determined by specialized gastrointestinal pathologists at our institution using the following tumor regression grading system: 1= pCR, 2= near pCR, 3= partial response, 4= no response, 5= progression.
Adjuvant chemotherapy after surgery and follow-up evaluations (including history and physical, endoscopy, and laboratory studies) were recommended at the discretion of the treating medical oncologist and generally performed at MD Anderson Cancer Center. Recurrence was defined as clinical or imaging findings consistent with disease recurrence, typically confirmed by biopsy or surgical resection. The trial was stopped early because there was only one patient with pCR among the first 15 patients randomized to the curcumin arm; otherwise, there were no changes to trial outcomes after commencement. Retrospective clinical chart review was performed until 2021 to update outcomes.
Statistical analysis
We compared the rate of pCR between treatment arms with Fisher’s exact test. We estimated OS and PFS with the Kaplan-Meier method. OS was calculated from start of CRT to date of death, censored at last follow-up. PFS was calculated from start of CRT to date of disease progression or death, censored at last endoscopy/imaging evaluation. We used the methods of Fine and Gray (30) to estimate the cumulative incidence of time to local regional failure (TTLRF) and time to distant failure (TTDF) from start of CRT to date of failure as documented by endoscopy/imaging, with death as a competing event. Patients were censored at last follow-up. Comparisons of treatment arm patient/tumor characteristics (e.g., age, T stage) and treatment outcomes (e.g., pCR) were performed using Fisher’s exact test and the Wilcoxon signed rank test as appropriate. One patient who did not undergo surgical resection was removed from analyses related to pathologic response outcomes; otherwise, there were no patient losses or exclusions.
Results
Patient and clinical tumor characteristics are shown in Table 2. Of 22 randomized patients, 15 patients received curcumin and 7 patients received placebo (Figure 1). All patients received the intended treatment with few modifications as noted below. All patients were analyzed with no losses or exclusions after randomization. Median age was 60 years with curcumin patients older than placebo patients (69 vs. 50 years, median, P=0.02). Majority of patients were male (59%). Most patients had clinical T3 (77%) and N1 (68%) disease presentations. Tumors were similar size in the curcumin (4 cm) and placebo (5 cm) groups with a median distance from the anal verge of 3 and 6.5 cm, respectively (P=0.17). Median extent of involvement of lumen circumference was 50% for the entire cohort, with tumors ranging from 15% to 100% involvement of lumen circumference. All tumors were moderately differentiated (grade 2).
Table 2
| Characteristics | All patients (n=22) | Curcumin (n=15) | Placebo (n=7) | P |
|---|---|---|---|---|
| Age (years), median [range] | 60 [28–75] | 69 [28–75] | 50 [45–64] | 0.02 |
| Sex, n [%] | 0.65 | |||
| Male | 13 [59] | 8 [55] | 5 [71] | |
| Female | 9 [41] | 7 [47] | 2 [29] | |
| Clinical T stage, n [%] | 1.00 | |||
| 2 | 4 [18] | 3 [20] | 1 [14] | |
| 3 | 17 [77] | 11 [73] | 6 [86] | |
| 4 | 1 [5] | 1 [7] | 0 [0] | |
| Clinical N stage, n [%] | 0.56 | |||
| 0 | 6 [27] | 3 [20] | 3 [43] | |
| 1 | 15 [68] | 11 [73] | 4 [57] | |
| 2 | 1 [5] | 1 [7] | 0 | |
| Tumor size (cm), median [range] | 4.5 [2–8]† | 4 [2–8] | 5 [4–8] | 0.39 |
| Distance from anal verge (cm), median [range] | 4 [1–10] | 3 [1–9] | 6.5 [2–10] | 0.17 |
| Tumor circumference (%), median [range] | 50 [15–100]† | 50 [15–100] | 55 [40–75] | 0.21 |
| Pretreatment CEA (ng/mL), median [range] | 1.6 [1.0–8.1] | 1.3 [1–5.4] | 2.4 [1.0–8.1] | 0.62 |
| Tumor differentiation, n [%] | 1.00 | |||
| Well | 0 [0] | 0 [0] | 0 [0] | |
| Moderate | 22 [100] | 15 [100] | 7 [100] | |
| Poor | 0 [0] | 0 [0] | 0 [0] |
†, one patient’s tumor found in polyp—size and circumference not evaluable. T, tumor; N, nodal; CEA, carcinoembryonic antigen.
All patients received pre-operative CRT with capecitabine. Patients were treated with 3D conformal RT except for one patient who received intensity modulated RT (IMRT). All but two patients received 50.4 Gy in 28 fractions. One patient had clinically involved iliac lymph nodes which were treated to 63 Gy in 28 fractions due to questionable resectability; the primary rectal tumor received 50.4 Gy in 28 fractions. One patient’s treatment was stopped early due to hospitalization and treatment for Clostridium difficile (C. diff.) colitis; she received 43.2 Gy in 24 fractions.
Two patients had dose reductions of capecitabine during treatment for hand-foot syndrome and paresthesias. Two patients had dose reductions of curcumin during CRT, one reduced dose to 2–3 tabs twice daily due to inability to tolerate 4 tabs twice daily and another refused curcumin for the last 8 days of CRT due to diarrhea and dehydration later attributed to C. diff. Four patients discontinued post-CRT maintenance curcumin and one reduced the dose to 3 tabs twice daily.
Toxicities separated by grade are shown in Table 3. The most common grade 1 toxicities experienced by patients during CRT were anorexia (45%), diarrhea (59%), fatigue (86%), nausea (64%), and pain (59%). Nine patients experienced grade 2 radiation dermatitis (41%). One patient experienced a grade 3 toxicity. Near the end of treatment, this patient was diagnosed with C. diff. colitis, which required hospitalization and administration of intravenous antibiotics.
Table 3
| Toxicity | Toxicity grade, n [%] | ||
|---|---|---|---|
| I | II | III | |
| Acne | 1 [5] | – | – |
| Allergic rhinitis | 1 [5] | – | – |
| Anorexia | 10 [45] | – | – |
| Diarrhea | 13 [59] | 2 [9] | – |
| Dizziness | 1 [5] | – | – |
| Dysuria | 4 [18] | 1 [5] | – |
| Elevated liver function tests | 1 [5] | – | – |
| Fatigue | 19 [86] | – | – |
| Hand foot syndrome | 4 [18] | 1 [5] | – |
| Hemorrhoids | 1 [5] | – | – |
| Hypotension | 1 [5] | – | – |
| Hypomagnesemia | 1 [5] | – | – |
| Insomnia | 1 [5] | 1 [5] | – |
| Infection | 1 [5] | – | 1 [5] |
| Nausea | 14 [64] | – | – |
| Neuropathy | 1 [5] | – | – |
| Pain | 13 [59] | 3 [14] | – |
| Radiation dermatitis | 7 [32] | 9 [41] | – |
| Rash | 1 [5] | – | – |
| Rectal bleeding | 1 [5] | – | – |
| Testicular swelling | 1 [5] | – | – |
| Urinary frequency | 6 [27] | – | – |
| Vomiting | 1 [5] | – | – |
Patients underwent surgical resection at median 7.7 weeks after completed CRT as recommended by their surgical oncologist: low anterior resection (n=10), coloanal anastomosis (n=4), abdominoperineal resection (n=5), transanal excision (n=1), and total pelvic exenteration (n=1). Only one patient did not undergo surgery as he was found on first follow up imaging to have small pulmonary nodules increasing in size. He received systemic chemotherapy.
Pathologic response rates (reported in Table 4) included a pCR for 2 of 6 placebo patients and 1 of 15 curcumin patients (P=0.18). Tumor downstaging occurred in 7 of 15 curcumin patients and 4 of 6 placebo patients (P=0.64). There were no significant differences between curcumin and placebo patients in terms of pathologic tumor stage (P=0.34) or tumor regression grade (P=0.44). All patients who underwent surgery received adjuvant systemic therapy.
Table 4
| Characteristics | All patients (n=21)† | Curcumin (n=15) | Placebo (n=6)† | P |
|---|---|---|---|---|
| Pathologic T stage, n [%] | 0.34 | |||
| 0 | 1 [5] | 1 [7] | 0 [0] | |
| 1 | 4 [19] | 2 [13] | 2 [33] | |
| 2 | 6 [29] | 5 [33] | 1 [17] | |
| 3 | 7 [33] | 6 [40] | 1 [17] | |
| 4 | 3 [14] | 1 [7] | 2 [33] | |
| Tumor downstaging, n [%] | ||||
| Yes | 11 [52] | 7 [47] | 4 [66] | 0.64 |
| No | 10 [48] | 8 [53] | 2 [33] | |
| pCR, n [%] | ||||
| Yes | 3 [14] | 1 [7] | 2 [33] | 0.18 |
| No | 18 [86] | 14 [93] | 4 [66] | |
| Tumor regression grade‡, n [%] | 0.44 | |||
| 1 | 3 [14] | 1 [7] | 2 [33] | |
| 2 | 3 [14] | 2 [13] | 1 [17] | |
| 3 | 5 [24] | 4 [27] | 1 [17] | |
| 4 | 10 [48] | 8 [53] | 2 [33] | |
| 5 | 0 [0] | 0 [0] | 0 [0] |
†, one patient did not undergo surgery; ‡, tumor regression grade (1= pCR, 2= near pCR, 3= partial response, 4= no response, 5= progression). T, tumor; pCR, pathologic complete response.
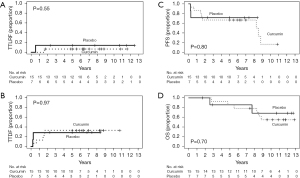
Median follow-up for all patients was 9.2 years. Figure 2 depicts TTLRF, TTDF, PFS, and OS. Five-year cumulative incidence of local regional failure was 14.3% [95% confidence interval (CI): 0 to 42%] and 6.7% (95% CI: 0 to 19.7%) and 5-year cumulative incidence of distant failure was 28.6% (95% CI: 0 to 65.0%) and 33.3% (95% CI: 8.5% to 58.2%) for placebo and curcumin groups, respectively. Five-year PFS was 71.4% (95% CI: 44.7% to 100%) (placebo) and 66.7% (95% CI: 46.6% to 95.3%) (curcumin) and 5-year OS was 85.7% (95% CI: 63.3% to 100%) (placebo) and 85.7% (95% CI: 69.2% to 100%) (curcumin).
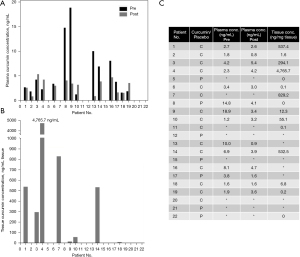
Plasma and tissue concentrations of curcumin are presented in Figure 3. Plasma concentrations for patients receiving curcumin varied widely both in terms of absolute plasma levels 1 hour after administration and the relative change when compared to baseline (Figure 3A,3C). The median serum curcumin concentrations before (3.04 ng/mL; range, 1.24–18.88 ng/mL) and 1 hour after (3.32 ng/mL; range, 0.84–5.36 ng/mL) curcumin intake did not differ significantly (P=0.33). Serum curcumin concentrations increased in some curcumin patients (n=4), while they decreased or remained constant 1 hour after administration in others (n=8) (range, as percentage of baseline: 8.8–258.1%). Twelve curcumin patients had analyzable tissue biopsies with a median curcumin concentration of 33.7 ng/mg tissue (range, 0.1–4,765.7 ng/mg) (Figure 3B,3C). Three placebo patients had analyzable tissue biopsies with a median curcumin concentration of 0.0 ng/mg tissue (range, 0.0–0.0 ng/mg). There was no association between tissue curcumin concentration and TTLRF (P=0.23) or pCR (P=0.17).
Discussion
In this phase II randomized study, we did not observe a benefit from the addition of curcumin to long course preoperative CRT for LARC patients. Unfortunately, we again demonstrate that the poor bioavailability of curcumin remains a challenge that prevents the true assessment of any potential benefit of this agent.
There is considerable interest in improving the current standard of care regimen for LARC patients. Many studies have reported an association between increased pathologic response to CRT and improved survival outcomes. Furthermore, a significant increase in pCR rates may afford the patient a non-operative treatment option with watchful waiting or a limited surgical procedure like a wide local excision. By omitting the more extensive resections involved in total mesorectal excision, this subset of patients may avoid the morbidity of surgery and potentially have a better quality of life. To improve outcomes and possibly decrease morbidity, alternative concurrent chemotherapeutic agents such as oxaliplatin have been investigated in many trials. Although some of these have resulted in improved pCR rates, this was accompanied by increased grade 3–4 toxicity. A recent meta-analysis concluded that the benefit of adding oxaliplatin to 5-FU-based neoadjuvant CRT with adjuvant chemotherapy for this population remains controversial (31). As noted previously, the evaluation of promising radiosensitizers in the total neoadjuvant therapy setting has been explored in a large cooperative group setting but the agents tested to date (veliparib, pembrolizumab) have not improved pathologic response rates significantly (11,32,33). Novel compounds with the potential to increase the pathologic response of rectal tumors during CRT without added toxicity are still needed.
Curcumin, a component of the spice turmeric, was initially popularized for its potential role in the prevention of gastrointestinal tract malignancies (22). However, there is also considerable interest in curcumin as a novel anti-neoplastic agent, based on the numerous mechanisms by which it has been shown to overcome uncontrolled cell growth (21,25,26,34). It is likely that curcumin provides a multi-faceted blockade of pathways that drive pro-survival signals, as it has been proven to interrupt proliferative pathways involving epidermal growth factor receptor (35,36), multiple tyrosine kinases (35,36), peroxisome-proliferator-activated receptors (37), and many others. It also has immunomodulatory effects mediated via activation of host macrophages and natural killer cells and modulation of lymphocyte-mediated function (38,39).
Tumors with constitutive expression of pro-survival signaling are well-characterized as more resistant to RT, and these pathways have been targets for radiosensitization strategies. Some radioresistance pathways are inducible, transiently upregulated in response to sublethal doses of radiation. Preclinical studies have demonstrated that the NF-κB pathway constitutes one such pro-survival, anti-apoptotic signal that can be transiently activated to protect cells from radiation-induced apoptosis and that curcumin suppressed this radiation-induced NF-κB activation via several mechanisms (40). As with other promising preclinical findings with curcumin for cancer therapy, including combination treatments with cytotoxic chemotherapy, targeted therapy, and immune checkpoint inhibitors (41), therapeutic success in clinical studies has been limited. The safety of curcumin has been established through multiple phase I trials (28,42). A phase I study at the University of Leicester in England included 15 subjects with advanced colorectal cancers who orally consumed curcumin doses up to 3.6 gm daily for up to 4 months. There was no dose-limiting toxicity observed. Curcumin consumed at 3.6 gm levels generated detectable levels of the parent compound and conjugates in plasma and urine, demonstrating bioavailability outside the gastrointestinal tract and caused inhibition of prostaglandin E2 production in blood leukocytes (measured ex vivo) (43,44). A recent systematic review that examined the clinical effect of curcumin in enhancing cancer therapy concluded that curcumin reduces the toxicities of chemotherapy and RT; some included studies reported increased OS duration and decreased tumor marker levels (45). A phase IIa study of metastatic colorectal cancer patients found that curcumin was a safe and tolerable addition to the FOLFOX (5-FU, leucovorin, and oxaliplatin) chemotherapy regimen; however, there was no significant difference in survival outcomes (46).
Unfortunately, a number of clinical studies have now demonstrated the poor and unpredictable bioavailability of this agent, with erratic and sometimes undetectable plasma levels after curcumin administration to humans (19). In vitro cell-based studies have shown that the concentration of curcumin needed to exert effects is in the 5 to 50 µM range (47). In our study, plasma concentrations ranged from 1.2–18.9 ng/mL, equivalent to 0.003–0.05 µM, far below the expected efficacious concentration range. In addition to these plasma studies, tissue concentrations of curcumin after oral administration have also been reported (29). Twelve patients received oral curcumin at 0.45, 1.8, or 3.6 g per day for 7 days prior to surgery. Concentrations of curcumin in normal and malignant tissue for patients consuming 3.6 g per day were 12.7±5.7 and 7.7±1.8 nmol/g, respectively. With these concentrations, the authors detected significantly lower levels of the oxidative DNA adduct M1G, suggesting that 3.6 g curcumin achieves efficacious levels in the colorectum. Despite the twice daily dosing schedule used in our study, we observed tissue concentrations ranging from 0.1–4,765.7 ng/mg tissue (equivalent to 0.04–1,753.8 nmol/g). Six of the 12 analyzable tissue biopsies had curcumin levels below the levels others have postulated to be sufficient for biological efficacy (47).
The cause for curcumin’s unpredictable pharmacokinetics and documented poor bioavailability is multifactorial and includes low water solubility, inefficient absorption, and rapid metabolism (48). Alternative promising delivery mechanisms, including liposomes, nanoparticles, phospholipid formulations, and synthetic analogs have been developed (41). Studies have also shown increased efficacy of curcumin derivatives, which furthers interest in the development of curcumin analogs with improved bioavailability and potency (49). Various lesion-oriented delivery methods have also been explored (50).
Among the strengths of the study was the strong translational component that enabled a careful analysis; however, this study also had limitations. The small number of participants prohibited robust statistical analyses. With these small numbers, there was no observed benefit to the addition of curcumin to preoperative CRT with capecitabine. However, given the known challenges of poor bioavailability of curcumin and the erratic and low plasma and tissue levels seen in this study, it is likely that curcumin was not present at consistent and therapeutic levels at the time these patients received RT. As numerous metabolites of curcumin have been identified (49), it is possible that the plasma and tissue levels measured in our study were not reflective of the levels of active compound metabolites. These studies would be challenging to perform given the number and varied distribution of each compound. Regardless of the erratic measurements of curcumin levels, there was no overall improved effect seen in this small patient cohort.
Although observational and cancer prevention studies have shown benefits from the simple ingestion of curcumin (22), it is possible that the mechanism of action for curcumin to effect colorectal tumor response or improve conventional treatment efficacy requires more than local contact during digestion in the gastrointestinal tract. If curcumin is further evaluated as a clinical radiosensitizer, studies should examine formulations with proven biodistribution profiles.
Conclusions
In conclusion, we conducted a phase two randomized double-blind study to evaluate the efficacy of curcumin with preoperative CRT for LARC patients. We saw no improvement in pCR rates of rectal tumors at the time of surgery. Additionally, we saw no improvement in tumor downstaging, TTLRF, or survival endpoints. On analysis of both plasma and tissue levels of curcumin, we observed low and variable levels of curcumin. This variability is a source of significant concern for the clinical utility of native curcumin as a therapeutic anti-cancer agent; future studies should evaluate curcumin formulations with proven bioavailability.
Acknowledgments
Funding: This work was supported in part by the National Institutes of Health National Cancer Institute, Cancer Center Support (Core) (grant CA 016672) to the University of Texas MD Anderson Cancer Center and a grant from the Gateway for Cancer Research (to SK).
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-259/rc
Trial Protocol: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-259/tp
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-259/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-259/coif). JRG reports unrelated funding from RSNA, honoraria from the Osler Review and Maryland Boards courses, and leadership roles in ROECSG and ILROG. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) and approved by the MD Anderson Cancer Center institutional review board (protocol 2006-0644). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702-15. [Crossref] [PubMed]
- Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29-42. [Crossref] [PubMed]
- Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol 2020;38:abstr 4008.
- Park IJ, You YN, Agarwal A, et al. Neoadjuvant treatment response as an early response indicator for patients with rectal cancer. J Clin Oncol 2012;30:1770-6. [Crossref] [PubMed]
- Rödel C, Martus P, Papadoupolos T, et al. Prognostic significance of tumor regression after preoperative chemoradiotherapy for rectal cancer. J Clin Oncol 2005;23:8688-96. [Crossref] [PubMed]
- Valentini V, Coco C, Picciocchi A, et al. Does downstaging predict improved outcome after preoperative chemoradiation for extraperitoneal locally advanced rectal cancer? A long-term analysis of 165 patients. Int J Radiat Oncol Biol Phys 2002;53:664-74. [Crossref] [PubMed]
- Crane CH, Skibber JM, Feig BW, et al. Response to preoperative chemoradiation increases the use of sphincter-preserving surgery in patients with locally advanced low rectal carcinoma. Cancer 2003;97:517-24. [Crossref] [PubMed]
- Janjan NA, Khoo VS, Abbruzzese J, et al. Tumor downstaging and sphincter preservation with preoperative chemoradiation in locally advanced rectal cancer: the M. D. Anderson Cancer Center experience. Int J Radiat Oncol Biol Phys 1999;44:1027-38. [Crossref] [PubMed]
- Bonnen M, Crane C, Vauthey JN, et al. Long-term results using local excision after preoperative chemoradiation among selected T3 rectal cancer patients. Int J Radiat Oncol Biol Phys 2004;60:1098-105. [Crossref] [PubMed]
- Rahma OE, Yothers G, Hong TS, et al. NRG-GI002: a phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC)—Pembrolizumab experimental arm (EA) primary results. J Clin Oncol 2021;39:abstr 8.
- Zheng J, Feng X, Hu W, et al. Systematic review and meta-analysis of preoperative chemoradiotherapy with or without oxaliplatin in locally advanced rectal cancer. Medicine (Baltimore) 2017;96:e6487. [Crossref] [PubMed]
- Mohiuddin M, Winter K, Mitchell E, et al. Randomized phase II study of neoadjuvant combined-modality chemoradiation for distal rectal cancer: Radiation Therapy Oncology Group Trial 0012. J Clin Oncol 2006;24:650-5. [Crossref] [PubMed]
- Crane CH, Eng C, Feig BW, et al. Phase II trial of neoadjuvant bevacizumab, capecitabine, and radiotherapy for locally advanced rectal cancer. Int J Radiat Oncol Biol Phys 2010;76:824-30. [Crossref] [PubMed]
- Czito BG, Willett CG, Bendell JC, et al. Increased toxicity with gefitinib, capecitabine, and radiation therapy in pancreatic and rectal cancer: phase I trial results. J Clin Oncol 2006;24:656-62. [Crossref] [PubMed]
- McMullen KP, Blackstock AW. Chemoradiation with novel agents for rectal cancer. Clin Colorectal Cancer 2002;2:24-30. [Crossref] [PubMed]
- Zhu AX, Willett CG. Chemotherapeutic and biologic agents as radiosensitizers in rectal cancer. Semin Radiat Oncol 2003;13:454-68. [Crossref] [PubMed]
- Arteaga CL. EGF receptor as a therapeutic target: patient selection and mechanisms of resistance to receptor-targeted drugs. J Clin Oncol 2003;21:289s-91s. [Crossref] [PubMed]
- Sharma RA, Gescher AJ, Steward WP. Curcumin: the story so far. Eur J Cancer 2005;41:1955-68. [Crossref] [PubMed]
- Aggarwal B, Prasad S, Sung B, et al. Prevention and Treatment of Colorectal Cancer by Natural Agents From Mother Nature. Curr Colorectal Cancer Rep 2013;9:37-56. [Crossref] [PubMed]
- Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: preclinical and clinical studies. Anticancer Res 2003;23:363-98. [PubMed]
- Carroll RE, Benya RV, Turgeon DK, et al. Phase IIa clinical trial of curcumin for the prevention of colorectal neoplasia. Cancer Prev Res (Phila) 2011;4:354-64. [Crossref] [PubMed]
- Furness MS, Robinson TP, Ehlers T, et al. Antiangiogenic agents: studies on fumagillin and curcumin analogs. Curr Pharm Des 2005;11:357-73. [Crossref] [PubMed]
- Moragoda L, Jaszewski R, Majumdar AP. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res 2001;21:873-8. [PubMed]
- Li L, Aggarwal BB, Shishodia S, et al. Nuclear factor-kappaB and IkappaB kinase are constitutively active in human pancreatic cells, and their down-regulation by curcumin (diferuloylmethane) is associated with the suppression of proliferation and the induction of apoptosis. Cancer 2004;101:2351-62. [Crossref] [PubMed]
- Wei SC, Lin YS, Tsao PN, et al. Comparison of the anti-proliferation and apoptosis-induction activities of sulindac, celecoxib, curcumin, and nifedipine in mismatch repair-deficient cell lines. J Formos Med Assoc 2004;103:599-606. [PubMed]
- Mahran RI, Hagras MM, Sun D, et al. Bringing Curcumin to the Clinic in Cancer Prevention: a Review of Strategies to Enhance Bioavailability and Efficacy. AAPS J 2017;19:54-81. [Crossref] [PubMed]
- Lao CD, Ruffin MT 4th, Normolle D, et al. Dose escalation of a curcuminoid formulation. BMC Complement Altern Med 2006;6:10. [Crossref] [PubMed]
- Garcea G, Berry DP, Jones DJ, et al. Consumption of the putative chemopreventive agent curcumin by cancer patients: assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol Biomarkers Prev 2005;14:120-5. [Crossref] [PubMed]
- Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 1999;94:496-509. [Crossref]
- Fu XL, Fang Z, Shu LH, et al. Meta-analysis of oxaliplatin-based versus fluorouracil-based neoadjuvant chemoradiotherapy and adjuvant chemotherapy for locally advanced rectal cancer. Oncotarget 2017;8:34340-51. [Crossref] [PubMed]
- George TJ, Yothers G, Hong TS, et al. Utilizing total neoadjuvant therapy (TNT) in rectal cancer: NRG-GI002, a phase II clinical trial platform. J Clin Oncol 2017;35:abstr TPS814.
- George TJ, Yothers G, Hong TS, et al. NRG-GI002: a phase II clinical trial platform using total neoadjuvant therapy (TNT) in locally advanced rectal cancer (LARC)—First experimental arm (EA) initial results. J Clin Oncol 2019;37:abstr 3505.
- Goel A, Boland CR, Chauhan DP. Specific inhibition of cyclooxygenase-2 (COX-2) expression by dietary curcumin in HT-29 human colon cancer cells. Cancer Lett 2001;172:111-8. [Crossref] [PubMed]
- Chen H, Zhang ZS, Zhang YL, et al. Curcumin inhibits cell proliferation by interfering with the cell cycle and inducing apoptosis in colon carcinoma cells. Anticancer Res 1999;19:3675-80. [PubMed]
- Korutla L, Kumar R. Inhibitory effect of curcumin on epidermal growth factor receptor kinase activity in A431 cells. Biochim Biophys Acta 1994;1224:597-600. [Crossref] [PubMed]
- Chen A, Xu J. Activation of PPAR{gamma} by curcumin inhibits Moser cell growth and mediates suppression of gene expression of cyclin D1 and EGFR. Am J Physiol Gastrointest Liver Physiol 2005;288:G447-56. [Crossref] [PubMed]
- Bhaumik S, Jyothi MD, Khar A. Differential modulation of nitric oxide production by curcumin in host macrophages and NK cells. FEBS Lett 2000;483:78-82. [Crossref] [PubMed]
- Gao X, Kuo J, Jiang H, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol 2004;68:51-61. [Crossref] [PubMed]
- Sandur SK, Deorukhkar A, Pandey MK, et al. Curcumin modulates the radiosensitivity of colorectal cancer cells by suppressing constitutive and inducible NF-kappaB activity. Int J Radiat Oncol Biol Phys 2009;75:534-42. [Crossref] [PubMed]
- Ruiz de Porras V, Layos L, Martínez-Balibrea E. Curcumin: A therapeutic strategy for colorectal cancer? Semin Cancer Biol 2021;73:321-30. [Crossref] [PubMed]
- Cheng AL, Hsu CH, Lin JK, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res 2001;21:2895-900. [PubMed]
- Sharma RA, Euden SA, Platton SL, et al. Phase I clinical trial of oral curcumin: biomarkers of systemic activity and compliance. Clin Cancer Res 2004;10:6847-54. [Crossref] [PubMed]
- Sharma RA, McLelland HR, Hill KA, et al. Pharmacodynamic and pharmacokinetic study of oral Curcuma extract in patients with colorectal cancer. Clin Cancer Res 2001;7:1894-900. [PubMed]
- Mansouri K, Rasoulpoor S, Daneshkhah A, et al. Clinical effects of curcumin in enhancing cancer therapy: A systematic review. BMC Cancer 2020;20:791. [Crossref] [PubMed]
- Howells LM, Iwuji COO, Irving GRB, et al. Curcumin Combined with FOLFOX Chemotherapy Is Safe and Tolerable in Patients with Metastatic Colorectal Cancer in a Randomized Phase IIa Trial. J Nutr 2019;149:1133-9. [Crossref] [PubMed]
- Garcea G, Jones DJ, Singh R, et al. Detection of curcumin and its metabolites in hepatic tissue and portal blood of patients following oral administration. Br J Cancer 2004;90:1011-5. [Crossref] [PubMed]
- Anand P, Kunnumakkara AB, Newman RA, et al. Bioavailability of curcumin: problems and promises. Mol Pharm 2007;4:807-18. [Crossref] [PubMed]
- Allegra A, Innao V, Russo S, et al. Anticancer Activity of Curcumin and Its Analogues: Preclinical and Clinical Studies. Cancer Invest 2017;35:1-22. [Crossref] [PubMed]
- Helson L. Curcumin (diferuloylmethane) delivery methods: a review. Biofactors 2013;39:21-6. [Crossref] [PubMed]


