
Racial disparities and standard treatment in locally advanced rectal cancer: a National Cancer Database study
Introduction
Colorectal cancer (CRC) is the second leading cause of cancer-related mortality in the United States with an estimate of 45,230 new cases diagnosed in 2021 (1). The incidence and mortality rates of CRC have gradually declined due to improvements in screening and treatment (2). However, CRC mortality rates continue to be higher in Black compared to White patients. The driving mechanisms of this disparity are complex involving demographic and socioeconomic factors, which influence access to prevention, screening, and treatment (2-8). However, while screening disparities contribute to the increased stage at diagnosis in Black patients, differences in mortality rates between Black and White patients continue to exist in CRC even when matched for tumor characteristics (2,3,5).
In the United States, standard treatment modalities for locally advanced rectal cancer consist of surgical excision combined with either neoadjuvant chemoradiation, adjuvant chemoradiation, or short course radiation and chemotherapy, and most recently the addition of total neoadjuvant therapy (TNT) for advanced disease (9-11). Although these treatment regimens have been shown to improve local and distant control, the use of standard treatment is low in practice (12). The effect of various treatments on racial disparities in colon cancer has been previously shown in a Surveillance, Epidemiology, and End Results (SEER) study evaluating survival outcomes between Black and White patients when using a sequential matching process for tumor presentation and treatment (3). In this study, racial survival differences between White and Black patients with stage III disease lowered from 5.1% to 4.3% when matched for tumor characteristics. This difference was further reduced to 2.8% when matched by treatment type, suggesting treatment had an impact on the racial disparity (3). A National Cancer Database (NCDB) study evaluating patients with CRC also showed improved survival differences in Black compared to White patients when matched for treatment (5). Thus, ensuring equitable access to standard treatment is an important modifiable intervention that can ameliorate racial disparities and further supports investigation of the use of standard treatment in rectal cancer.
There are limited studies looking at factors affecting standard treatment in rectal cancer. We hypothesized standard treatment would be received less often in Black compared to White patients, with an associated detriment in survival. To elucidate possible trends and obstacles promoting racial disparities, we evaluated locally advanced rectal adenocarcinoma treatment patterns in the NCDB and sought to determine racial disparities in standard treatment and outcomes based on the receipt of standard treatment. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-542/rc).
Methods
Data source and population
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was determined to be exempt by the University of Southern California institutional review board and individual consent for this retrospective national database analysis was waived. We queried the NCDB, a clinical oncology database sourced from more than 1,500 Commission on Cancer (CoC)-accredited facilities throughout the United States, utilizing user files from 2004 to 2014, for patients ≥18 years old with clinical and pathologic stage II–III rectal adenocarcinoma. We excluded patients with stage 0 and stage I disease as surgical management is the mainstay of treatment, and also excluded patients with stage IV disease as standard treatment varies widely in this population. Patients who received no treatment or were missing follow up data were also excluded. A Consolidated Standards of Reporting Trials (CONSORT) diagram depicting our inclusion and exclusion criteria is shown in Figure 1.
Variables
We defined standard treatment as complete surgical excision—low anterior resection (LAR) or abdominoperineal resection (APR) with either neoadjuvant or adjuvant concurrent chemoradiation (a maximum of 7 days was allowed between initiation of chemotherapy and radiotherapy). We defined all other treatments as nonstandard including (I) sequential chemotherapy and radiation without surgery, (II) definitive concurrent chemoradiation without surgery, (III) surgery alone, (IV) surgery with either radiation or chemotherapy but not both, and (V) trimodality therapy with non-concurrent chemoradiation (receipt of chemotherapy and radiotherapy more than 7 days apart). Any receipt of concurrent chemoradiation and surgery qualified as standard treatment and therefore would also include TNT. We explored additional stratification by short course radiation as this is an acceptable standard treatment, but only identified 426 of 70,677 patients (0.6%) who received 2,000–2,999 cGy in 5 fractions. Therefore, we did not include this in our standard treatment cohort.
Our main analysis evaluated whether standard treatment was associated with race, and whether survival was associated with standard treatment. In our survival analyses, we censored patients for loss to follow-up. We controlled for demographic (age, sex, race, ethnicity, and insurance status), biologic [grade and Charlson-Deyo Comorbidity Index (CCI)], treatment facility (academic vs. community or integrated network and facility location), geographic (income, education, and population density associated with zip code, and distance from treatment facility), and temporal (year of diagnosis) characteristics. These covariates are detailed in Table 1.
Table 1
| Characteristics | Standard (n=38,339), n (%) | Nonstandard (n=32,338), n (%) | P value |
|---|---|---|---|
| Patient characteristics | |||
| Age (years) | <0.001 | ||
| 18–49 | 7,545 (19.7) | 4,754 (14.7) | |
| 50–64 | 16,695 (43.5) | 11,241 (34.8) | |
| 65–79 | 12,065 (31.5) | 10,858 (33.6) | |
| ≥80 | 2,034 (5.3) | 5,485 (17.0) | |
| Sex | <0.001 | ||
| Female | 14,384 (37.5) | 12,684 (39.2) | |
| Male | 23,955 (62.5) | 19,654 (60.8) | |
| Race | <0.001 | ||
| White | 33,471 (87.3) | 27,323 (84.5) | |
| Black | 2,863 (7.5) | 3,160 (9.8) | |
| Asian/Pacific Islander | 1,276 (3.3) | 1,150 (3.6) | |
| Other | 729 (1.9) | 705 (2.2) | |
| Ethnicity | <0.001 | ||
| Non-Hispanic | 36,377 (94.9) | 30,225 (93.5) | |
| Hispanic | 1,962 (5.1) | 2,113 (6.5) | |
| CCI | <0.001 | ||
| 0 | 30,613 (79.8) | 25,215 (78.0) | |
| 1 | 6,242 (16.3) | 5,386 (16.7) | |
| 2 | 1,132 (3.0) | 1,258 (3.9) | |
| 3 | 352 (0.9) | 479 (1.5) | |
| Facility/demographic characteristics | |||
| Facility type | <0.001 | ||
| Academic | 12,968 (35.5) | 11,613 (37.4) | |
| Community | 19,443 (53.2) | 16,439 (52.9) | |
| Integrated network | 4,134 (11.3) | 3,036 (9.8) | |
| Facility location | <0.001 | ||
| East North Central | 7,456 (20.4) | 5,359 (17.2) | |
| East South Central | 2,167 (5.9) | 2,035 (6.5) | |
| Middle Atlantic | 4,856 (13.3) | 5,136 (16.5) | |
| Mountain | 1,740 (4.8) | 1,324 (4.3) | |
| New England | 2,176 (6.0) | 1,780 (5.7) | |
| Pacific | 3,854 (10.5) | 4,248 (13.7) | |
| South Atlantic | 7,736 (21.2) | 6,018 (19.4) | |
| West North Central | 4,017 (11.0) | 2,350 (7.6) | |
| West South Central | 2,543 (7.0) | 2,838 (9.1) | |
| Distance from facility (miles) | <0.001 | ||
| 0–5 | 9,761 (25.7) | 9,548 (29.9) | |
| >5–10 | 7,690 (20.2) | 6,640 (20.8) | |
| >10–25 | 10,123 (26.6) | 8,013 (25.1) | |
| >25 | 10,455 (27.5) | 7,784 (24.3) | |
| Insurance | <0.001 | ||
| Unknown | 518 (1.4) | 1,140 (3.5) | |
| Medicaid | 2,492 (6.5) | 2,058 (6.4) | |
| Medicare | 13,358 (34.8) | 14,724 (45.5) | |
| Not insured | 1,771 (4.6) | 1,524 (4.7) | |
| Other government | 557 (1.5) | 596 (1.8) | |
| Private insurance | 19,643 (51.2) | 12,296 (38.0) | |
| Zip code population density | <0.001 | ||
| Not metropolitan | 8,201 (22.0) | 5,910 (18.9) | |
| Metropolitan | 29,158 (78.0) | 25,429 (81.1) | |
| Median income of zip code | <0.001 | ||
| <$38,000 | 9,507 (25.0) | 7,592 (23.8) | |
| $38,000–$47,999 | 10,547 (27.7) | 8,342 (26.1) | |
| $48,000–$62,999 | 6,409 (16.9) | 6,069 (19.0) | |
| ≥$63,000 | 11,564 (30.4) | 9,956 (31.2) | |
| Proportion of zip code without high school diploma (%) | <0.001 | ||
| <7.0 | 10,034 (26.4) | 8,570 (26.8) | |
| 7.0–12.9 | 12,835 (33.7) | 10,040 (31.4) | |
| 13.0–20.9 | 9,123 (24.0) | 7,089 (22.2) | |
| ≥21.0 | 6,056 (15.9) | 6,283 (19.6) | |
| Year of diagnosis | <0.001 | ||
| 2004 | 2,064 (5.4) | 1,895 (5.9) | |
| 2005 | 2,291 (6.0) | 2,151 (6.7) | |
| 2006 | 2,490 (6.5) | 2,258 (7.0) | |
| 2007 | 2,839 (7.4) | 2,510 (7.8) | |
| 2008 | 3,278 (8.6) | 2,965 (9.2) | |
| 2009 | 3,578 (9.3) | 3,084 (9.5) | |
| 2010 | 3,928 (10.2) | 3,180 (9.8) | |
| 2011 | 3,973 (10.4) | 3,267 (10.1) | |
| 2012 | 4,310 (11.2) | 3,428 (10.6) | |
| 2013 | 4,645 (12.1) | 3,600 (11.1) | |
| 2014 | 4,943 (12.9) | 4,000 (12.4) | |
CCI, Charlson-Deyo Comorbidity Index.
Statistical analyses
In an unadjusted analysis, we compared the distribution of patient characteristics by receipt of standard vs. nonstandard treatment and tested for statistical significance with a Pearson chi-squared test. We plotted survival curves using the Kaplan-Meier estimator, stratifying by standard vs. nonstandard treatment and by race and stage. We used a log-rank test to test for statistical significance.
We adjusted for confounders using multivariable logistic regression to examine whether race was associated with receipt of standard treatment. We used a Cox proportional hazards model to estimate survival differences between standard and nonstandard treatment. To examine whether survival differences were heterogeneous by race and stage of disease, we conducted subgroup analyses by incorporating a full set of interaction terms among receipt of standard treatment, race, and stage of disease. We estimated subgroup-specific hazard ratios (HRs) by recombining coefficients, with 95% confidence intervals (CIs) computed using the delta method.
Our primary analysis included observations with missing values (7%), which we imputed using a multinomial logistic model with chained equations. We conducted 10 imputations and recombined coefficients and standard errors using Rubin’s rules (13). In sensitivity analysis, we conducted a complete case analysis. We used robust standard errors for all multivariable analyses. Two-tailed significance tests were used with α=0.05. Analyses were performed using STATA v. 14 and SAS software v. 9.4 (SAS Institute Inc., USA).
Results
Patient characteristics
A total of 70,677 patients with stage II (35,079) or stage III (35,598) rectal adenocarcinoma met our inclusion criteria. Median follow up was 44 months with an interquartile range of 25–73 months. In the patients who received radiation, the median dose was 4,500 cGy with an interquartile range of 4,500–4,860 cGy. Only 303 (0.4%) patients underwent 2,500 cGy in 5 fractions and an additional 123 (0.2%) received other doses in the range of 2,000–2,999 cGy. Patients who received standard treatment were more likely to be younger, male, White, Asian/Pacific Islander, and non-Hispanic. They were also more likely to have a lower CCI, have private or Medicaid insurance, live in a nonmetropolitan area, reside in a zip code with a higher median income and proportion of high school graduates, and have a more recent diagnosis, as shown in Table 1. Further patient and demographic characteristics by race are shown in Table S1.
Receipt of standard treatment
Treatment type based on race is shown in Table 2, with the largest cohort of patients receiving standard treatment—55.5% of White patients compared to 47.5% of Black patients. Overall, approximately 30% of patients who did not receive standard treatment underwent concurrent chemoradiation without surgery which was not standard up to 2014 and currently still experimental. On multivariate analysis, Black [odds ratio (OR): 0.75; 95% CI: 0.71–0.79; P<0.001] and Hispanic White (OR: 0.86; 95% CI: 0.80–0.92; P<0.001) patients were less likely to receive standard treatment compared to non-Hispanic White patients, as shown in Table 3. Other factors that were independently associated with lower odds of receiving standard treatment include older age, a higher CCI of 2–3, living in a metropolitan area or in a zip code with a lower percentage of high school graduates, having non-private insurance, and having an earlier year of diagnosis. When stratifying by stage of disease, Black patients were less likely to receive standard treatment than non-Hispanic White patients (Table 4). Further information on multivariate analysis evaluating the likelihood of not receiving surgery, chemo, or radiation can be found in the supplemental data (Table S2). Factors related to a reduced likelihood of surgery, chemo, or radiation individually, were similar to those that were related to a reduced risk of standard treatment and include increasing age, Black race, higher comorbidities, increased distance from the treatment facility, and a zip code in a non-metropolitan location. Between not receiving surgery, chemo, or radiation specifically, patients were less likely to receive surgery with any non-private insurances however the likelihood of receiving chemo or radiation varied more depending on the type non-private insurance. When comparing these factors to patient and demographic characteristics by race (Table S1), Black patients were more likely to present at a younger age, at a closer distance to the treatment facility, and live in a non-metropolitan area, and therefore one could presume would be more likely to qualify for standard treatment. In terms of comorbidities, the CCI was similar between White and Black patients. However, White patients were more likely to have private insurance. While receipt of standard treatment is based of multifactorial characteristics, there does not appear to be a clear indication for Black patients receiving decreased rates of standard treatment based on their patient and demographic characteristics.
Table 2
| Treatment | Non-Hispanic White, n (%) | Black, n (%) | Other, n (%) |
|---|---|---|---|
| Standard treatment* | 31,671 (55.5) | 2,863 (47.5) | 3,805 (50.1) |
| Concurrent chemoradiation without surgery | 7,362 (12.9) | 953 (15.8) | 959 (12.6) |
| Trimodality therapy with non-concurrent chemoradiation | 6,301 (11.0) | 791 (13.1) | 937 (12.3) |
| Sequential chemo and/or radiation without surgery | 3,927 (6.9) | 677 (11.2) | 685 (9.0) |
| Surgery alone | 4,068 (7.1) | 362 (6.0) | 566 (7.5) |
| Surgery with radiation or chemo but not both | 3,727 (6.5) | 377 (6.3) | 646 (8.5) |
*, standard treatment was defined as complete surgical excision with either neoadjuvant or adjuvant concurrent chemoradiation. We defined all other treatments listed as nonstandard.
Table 3
| Characteristics | OR (95% CI) | P value |
|---|---|---|
| Patient characteristics | ||
| Age (years) | ||
| 18–49 | Reference | |
| 50–64 | 0.927 (0.887–0.970) | 0.001 |
| 65–79 | 0.727 (0.686–0.772) | <0.001 |
| ≥80 | 0.241 (0.223–0.260) | <0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.017 (0.985–1.050) | 0.298 |
| Race | ||
| Non-Hispanic White | Reference | |
| Hispanic White | 0.860 (0.800–0.924) | <0.001 |
| Black | 0.749 (0.707–0.794) | <0.001 |
| Asian/Pacific Islander | 1.014 (0.930–1.106) | 0.753 |
| Other | 0.874 (0.783–0.975) | 0.016 |
| CCI | ||
| 0 | Reference | |
| 1 | 1.023 (0.980–1.067) | 0.296 |
| 2 | 0.854 (0.783–0.930) | <0.001 |
| 3 | 0.697 (0.603–0.805) | <0.001 |
| Stage | ||
| II | Reference | |
| III | 1.104 (1.079–1.139) | <0.001 |
| Facility/demographic characteristics | ||
| Facility type | ||
| Academic | Reference | |
| Community | 1.086 (1.047–1.126) | <0.001 |
| Integrated network | 1.222 (1.155–1.294) | <0.001 |
| Facility location | ||
| East North Central | Reference | |
| East South Central | 0.741 (0.688–0.797) | <0.001 |
| Middle Atlantic | 0.707 (0.669–0.747) | <0.001 |
| Mountain | 0.883 (0.813–0.960) | 0.003 |
| New England | 0.928 (0.859–1.002) | 0.056 |
| Pacific | 0.653 (0.615–0.693) | <0.001 |
| South Atlantic | 0.921 (0.876–0.969) | 0.002 |
| West North Central | 1.169 (1.095–1.249) | <0.001 |
| West South Central | 0.712 (0.664–0.763) | <0.001 |
| Insurance | ||
| Private insurance | Reference | |
| Unknown | 0.327 (0.293–0.365) | <0.001 |
| Medicaid | 0.818 (0.766–0.873) | <0.001 |
| Medicare | 0.864 (0.822–0.908) | <0.001 |
| Not insured | 0.754 (0.700–0.812) | <0.001 |
| Other government | 0.605 (0.535–0.683) | <0.001 |
| Median income | ||
| <$38,000 | Reference | |
| $38,000–$47,999 | 1.041 (0.988–1.096) | 0.134 |
| $48,000–$62,999 | 1.020 (0.963–1.079) | 0.501 |
| ≥$63,000 | 0.897 (0.840–0.958) | 0.001 |
| Distance from facility (miles) | ||
| 0–5 | Reference | |
| >5–10 | 1.082 (1.034–1.132) | 0.001 |
| >10–25 | 1.109 (1.061–1.158) | <0.001 |
| >25 | 1.149 (1.092–1.209) | <0.001 |
| Zip code population density | ||
| Not metropolitan | Reference | |
| Metropolitan | 0.921 (0.877–0.968) | 0.001 |
| Proportion of zip code without high school diploma (%) | ||
| <7.0 | Reference | |
| 7.0–12.9 | 0.936 (0.894–0.981) | 0.005 |
| 13.0–20.9 | 0.853 (0.807–0.901) | <0.001 |
| ≥21.0 | 0.765 (0.715–0.818) | <0.001 |
| Year of diagnosis | ||
| 2004 | Reference | |
| 2005 | 0.977 (0.894–1.067) | 0.605 |
| 2006 | 1.000 (0.916–1.092) | 0.992 |
| 2007 | 1.028 (0.944–1.120) | 0.522 |
| 2008 | 0.997 (0.918–1.083) | 0.947 |
| 2009 | 1.056 (0.973–1.146) | 0.189 |
| 2010 | 1.131 (1.043–1.226) | 0.003 |
| 2011 | 1.110 (1.024–1.203) | 0.011 |
| 2012 | 1.134 (1.047–1.228) | 0.002 |
| 2013 | 1.183 (1.093–1.280) | <0.001 |
| 2014 | 1.103 (1.021–1.193) | 0.013 |
OR, odds ratio; CI, confidence interval; CCI, Charlson-Deyo Comorbidity Index.
Table 4
| Subgroup | OR (95% CI) | P value |
|---|---|---|
| All stages | ||
| Black vs. non-Hispanic White | 0.75 (0.71–0.79) | <0.0001 |
| Stage II | ||
| Black vs. non-Hispanic White | 0.71 (0.65–0.77) | <0.0001 |
| Stage III | ||
| Black vs. non-Hispanic White | 0.79 (0.73–0.86) | <0.0001 |
OR, odds ratio; CI, confidence interval.
Survival outcomes
In unadjusted analyses, patients receiving standard vs. nonstandard treatment had higher survival (Figure 2; log-rank test P<0.0001). Non-surgical treatments showed the worst outcomes.
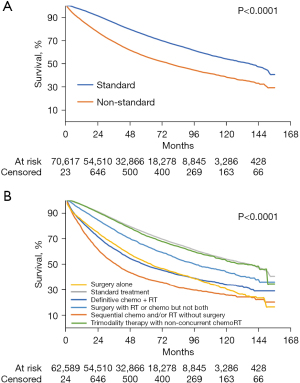
On multivariable Cox regression, after adjusting for confounders, nonstandard treatment was significantly associated with worse survival (HR: 1.69; 95% CI: 1.65–1.73; P<0.001) compared to standard treatment. Other independent predictors of lower survival included being Black, older, male, having treatment at a community or integrated network (vs. academic center), higher CCI, non-private insurance, and residing in a zip code with a lower median income or a lower proportion without a high school diploma (Table 5).
Table 5
| Characteristics | HR (95% CI) | P value |
|---|---|---|
| Treatment type | ||
| Standard | Reference | |
| Nonstandard | 1.689 (1.646–1.734) | <0.001 |
| Patient characteristics | ||
| Age (years) | ||
| 18–49 | Reference | |
| 50–64 | 1.191 (1.139–1.246) | <0.001 |
| 65–79 | 1.716 (1.624–1.813) | <0.001 |
| ≥80 | 3.497 (3.288–3.718) | <0.001 |
| Sex | ||
| Female | Reference | |
| Male | 1.140 (1.110–1.171) | <0.001 |
| Race | ||
| Non-Hispanic White | Reference | |
| Hispanic White | 0.839 (0.784–0.897) | <0.001 |
| Black | 1.145 (1.090–1.202) | <0.001 |
| Asian/Pacific Islander | 0.908 (0.837–0.984) | 0.019 |
| Other | 0.988 (0.898–1.087) | 0.809 |
| CCI | ||
| 0 | Reference | |
| 1 | 1.264 (1.223–1.306) | <0.001 |
| 2 | 1.671 (1.569–1.779) | <0.001 |
| 3 | 2.144 (1.937–2.373) | <0.001 |
| Stage | ||
| II | Reference | |
| III | 1.098 (1.070–1.127) | <0.001 |
| Facility/demographic characteristics | ||
| Facility type | ||
| Academic | Reference | |
| Community | 1.136 (1.102–1.172) | <0.001 |
| Integrated network | 1.071 (1.022–1.123) | 0.004 |
| Facility location | ||
| East North Central | Reference | |
| East South Central | 1.077 (1.014–1.144) | 0.017 |
| Middle Atlantic | 0.971 (0.927–1.017) | 0.207 |
| Mountain | 0.950 (0.884–1.020) | 0.157 |
| New England | 0.998 (0.939–1.061) | 0.950 |
| Pacific | 0.926 (0.880–0.974) | 0.003 |
| South Atlantic | 0.974 (0.994–1.082) | 0.090 |
| West North Central | 0.949 (0.900–1.001) | 0.053 |
| West South Central | 0.920 (0.866–0.977) | 0.007 |
| Insurance | ||
| Private insurance | Reference | |
| Unknown | 0.993 (0.899–1.098) | 0.893 |
| Medicaid | 1.617 (1.526–1.714) | <0.001 |
| Medicare | 1.349 (1.291–1.409) | <0.001 |
| Not insured | 1.554 (1.454–1.662) | <0.001 |
| Other government | 1.399 (1.261–1.552) | <0.001 |
| Median income | ||
| <$38,000 | Reference | |
| $38,000–$47,999 | 0.960 (0.921–1.002) | 0.059 |
| $48,000–$62,999 | 0.914 (0.873–0.958) | <0.001 |
| ≥$63,000 | 0.815 (0.772–0.861) | <0.001 |
| Residence | ||
| Distance from facility (miles) | ||
| 0–5 | Reference | |
| >5–10 | 0.955 (0.920–0.992) | 0.016 |
| >10–25 | 0.922 (0.889–0.956) | <0.001 |
| >25 | 0.893 (0.855–0.932) | <0.001 |
| Zip code population density | ||
| Not metropolitan | Reference | |
| Metropolitan | 0.946 (0.908–0.985) | 0.007 |
| Proportion of zip code without high school diploma (%) | ||
| <7.0 | Reference | |
| 7.0–12.9 | 1.043 (1.002–1.085) | 0.040 |
| 13.0–20.9 | 1.044 (0.996–1.095) | 0.073 |
| ≥21.0 | 1.033 (0.975–1.093) | 0.273 |
| Year of diagnosis | ||
| 2004 | Reference | |
| 2005 | 1.024 (0.963–1.088) | 0.451 |
| 2006 | 0.988 (0.929–1.052) | 0.712 |
| 2007 | 0.979 (0.921–1.041) | 0.498 |
| 2008 | 0.941 (0.886–1.000) | 0.049 |
| 2009 | 0.973 (0.915–1.034) | 0.376 |
| 2010 | 0.981 (0.922–1.043) | 0.533 |
| 2011 | 0.900 (0.845–0.959) | 0.001 |
| 2012 | 0.901 (0.843–0.962) | 0.002 |
| 2013 | 0.881 (0.822–0.944) | <0.001 |
| 2014 | 0.880 (0.817–0.949) | 0.001 |
HR, hazard ratio; CI, confidence interval; CCI, Charlson-Deyo Comorbidity Index.
Racial disparities within standard and nonstandard treatment
Kaplan-Meier survival estimates by race (Figure 3) showed Black patients had worse survival outcomes compared to White patients with both standard and nonstandard treatment. Among patients who received standard treatment, 5- and 10-year survival between White and Black patients were 74.6% and 53.9% vs. 70.3% and 52.3%, respectively. Survival differences at 5 and 10 years increased within nonstandard treatment between White (55.7% and 38.0%, respectively) and Black (50.0% and 34.8%, respectively) patients. Differences were most pronounced in patients receiving nonstandard treatment with stage III disease.
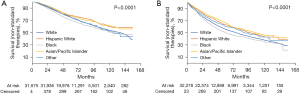
Even after adjusting for demographic, biologic, facility, geographic, and temporal characteristics, Black patients had higher mortality rates compared to White patients in the whole population (HR: 1.15; 95% CI: 1.09–1.20; P<0.0001). This racial disparity in survival persisted when we subgrouped patients into standard (HR: 1.10; 95% CI: 1.03–1.19; P=0.008) and nonstandard treatment (HR: 1.17; 95% CI: 1.10–1.25; P<0.0001) (Table 6). Decreased survival outcomes in Black patients were more pronounced for those who underwent nonstandard treatment, particularly when treating stage III disease (HR: 1.30; 95% CI: 1.19–1.42; P<0.0001). Conversely, Hispanic and Asian/Pacific Islander patients had a decreased risk of death (HR: 0.84; 95% CI: 0.78–0.90; P<0.0001 and HR: 0.91; 95% CI: 0.84–0.98; P=0.019, respectively) compared to non-Hispanic White patients.
Table 6
| Subgroup | Standard, HR (95% CI), P value | Nonstandard, HR (95% CI), P value |
|---|---|---|
| All patients | ||
| Black vs. White | 1.10 (1.03–1.19), 0.008 | 1.17 (1.10–1.25), <0.0001 |
| Other vs. White | 0.98 (0.85–1.13), 0.810 | 0.99 (0.87–1.13), 0.908 |
| Stage II | ||
| Black vs. White | 1.07 (0.96–1.19), 0.198 | 1.07 (0.98–1.17), 0.131 |
| Other vs. White | 0.92 (0.74–1.14), 0.443 | 0.99 (0.83–1.17), 0.890 |
| Stage III | ||
| Black vs. White | 1.13 (1.02–1.25), 0.018 | 1.30 (1.19–1.42), <0.0001 |
| Other vs. White | 1.03 (0.85–1.25), 0.752 | 1.00 (0.83–1.21), 0.964 |
HR, hazard ratio; CI, confidence interval.
All findings were robust to complete case analysis.
Discussion
In this NCDB study including patients with stage II–III rectal adenocarcinoma, receipt of standard treatment was lower in Black and Hispanic White patients compared to non-Hispanic White patients. Moreover, nonstandard treatment was significantly associated with worse survival compared to those undergoing standard treatment. On subgroup analysis by race, Black patients had worse survival compared to White patients as a whole population, and in the standard and nonstandard treatment subgroups. This disparity in survival was most prominent in Black patients with stage III disease who received nonstandard treatment—resulting in a 30% higher probability of death when compared to White patients who received nonstandard treatment. Within the standard treatment subgroups, the increased probability of death between Black and White patients was reduced to 13%.
Standard treatment has been shown to improve oncologic outcomes in rectal cancer, and now includes TNT regimens for advanced cases (10,11). The updated standard treatment recommendations are of particular importance for Black patients as incidence, stage at presentation, and mortality rates are higher when compared to White patients (2). The causes of increased CRC incidence and mortality in Black patients are multifactorial and involve complexities beyond merely treatment regimens, which supports our finding of continued survival discrepancies between Black and White patients undergoing standard treatment. A portion of this concerning disparity can be accounted for by the limited access to preventative, screening, and treatment resources associated with low socioeconomic environments (4,6,14). Further investigation is essential but one potentially modifiable factor is the receipt of standard treatment, which is supported in our study as Black patients had a lower risk of mortality when compared to White patients in the standard treatment group compared to the nonstandard treatment group—particularly for those with stage III disease. Therefore, given the higher propensity for advanced disease in Black patients, the necessity for standard treatment is further heightened as modern TNT regimens have shown improved pathologic complete response and distant metastases rates in the recent RAPIDO and PRODIGE-23 trials for locally advanced rectal cancer (10,11).
Our study showed Black patients had lower rates of standard treatment irrespective of cancer stage and demographic characteristics, and is the first to include strict criteria for standard treatment utilizing concurrent chemoradiation. Although other demographic and socioeconomic factors were also independently associated with lower odds of receiving standard treatment, including age ≥80 years (0.241; 95% CI: 0.223–0.260), a CCI of 3 (0.697; 95% CI: 0.603–0.805), and unknown insurance (0.327; 95% CI: 0.293–0.365), we chose to focus our study on the disparity for Black patients as poor survival is known in this population and the OR was clinically significant at 0.75 (95% CI: 0.71–0.79). The unwarranted disparity in receipt of standard treatment showed increasingly worse outcomes for Black patients, and is supported by prior observational studies (3,5). In a matched NCDB study, the 5-year survival differences between Black and White patients with stage I–IV CRC decreased from a 9.2% absolute difference to 2.3% when matched for insurance and tumor characteristics. This absolute difference was not further reduced when matching by treatment in the whole cohort. However, there was improvement for those with stage II and III disease when stratified by stage, revealing differences in treatment may contribute to the overall racial survival disparity in select patients (5). Another recent NCDB analysis evaluated racial disparities in treatment for stage II and III rectal cancer at minority-serving hospitals. On multivariable analysis, treatment at minority-serving hospitals as well as Black race had lower odds of receiving standard treatment. After adjusting for covariates including standard treatment, patient characteristics, and disease specific variables, Black individuals continued to have a higher risk of mortality compared to White patients (15). This study presented similar findings to ours, however the definition of standard treatment was surgical resection (APR or LAR) as well as neoadjuvant and/or adjuvant systemic chemotherapy and any radiation therapy. It did not clearly define the importance of concurrent chemoradiation (as short course radiation was found to be used in a very small subset of patients). Furthermore, while consistent with our study in showing Black patients continued to have worse survival even when evaluated by subgroups who underwent standard treatment, there was no further evaluation of survival outcomes based on stage of disease.
In our study, while subgroup multivariable analysis shows a higher probability of death for Black compared to White patients undergoing standard treatment (HR: 1.10; 95% CI: 1.03–1.19), the probability was even higher in those receiving nonstandard treatment (HR: 1.17; 95% CI: 1.10–1.25). For Black vs. White patients who received standard treatment, the HR for mortality remained relatively stable for stage II and III disease at 1.07 (95% CI: 0.96–1.19) and 1.13 (95% CI: 1.02–1.25), respectively. In contrast, for Black vs. White patients who received nonstandard treatment, the HR increased in stage II and III disease from 1.07 (95% CI: 0.98–1.17) to 1.30 (95% CI: 1.19–1.42), respectively. Similar to the prior matched NCDB study, treatment type had a stronger contribution in those with stage III disease, pointing to the increased importance of standard treatment in these patients. While improving access among Black patients to standard treatment may only be a portion of the racial survival disparities, it is an important and modifiable factor that can reduce difference in outcomes between White and Black patients.
Racial disparities in receipt of standard treatment has been shown in numerous cancers, including breast, prostate, lung, gynecologic, and gastrointestinal cancers (1,16-20). In a SEER analysis evaluating breast, non-small cell lung, and prostate cancer, Black patients were less likely to receive guideline concordant treatment than White patients, and mortality risks were lowered after adjusting for standard treatment (19). In an NCDB study evaluating standard treatment for locally advanced anal squamous cell carcinoma, racial survival differences that were present between Black and White patients were rectified in the subgroup that received standard treatment (20). In summation, these studies emphasize the importance of eliminating barriers to receipt of standard treatment to decrease racial disparities of standard treatment and survival outcomes.
Limitations of our study include the retrospective nature of the NCDB and potential coding and clerical errors. Data collection is only from CoC-accredited institutions which may not accurately reflect practices in all facilities nationwide. As with other retrospective studies, our results may be biased by additional confounding and unobserved patient and facility characteristics not encompassed within the NCDB. Additionally, while overall survival metrics are available, rectal cancer specific survival is not a collected datapoint and could not be assessed. Another limitation is our study was unable to examine short course radiation due to the small cohort represented within the NCDB. Furthermore, the complex and structurally integrated nature of racial disparities may involve intricate socioeconomic factors and predispositions beyond those stated here. However, while residual confounding factors may still exist, providing equal access to standard treatment should be a top priority and is not excused by unequal racial distributions by socioeconomic status. Our study demonstrates unequal rates of standard treatment in locally advanced rectal cancer between White and Black patients, as well as a widening of survival outcomes with nonstandard treatment. Establishing awareness of modifiable factors contributing to racial disparities is essential to ameliorate the differences in survival outcomes subjected to Black patients and to provide equal access to standard treatment.
Conclusions
Nonstandard treatment in stage II and III rectal cancer is associated with worse survival compared to standard treatment regimens. Black patients are more likely to receive nonstandard treatment and have worse survival outcomes compared to White patients, even when stratified by standard treatment. These survival differences are further amplified when Black patients receive nonstandard treatment, in particular for stage III disease. Ensuring all patients have equal access to standard treatment is critical to ameliorate racial disparities in rectal cancer outcomes.
Acknowledgments
The NCDB is a joint project between the CoC of the American College of Surgeons and the American Cancer Society. The database was established in 1989 and is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that currently captures 72% of all newly diagnosed malignancies in the US annually. The data used in this study is derived from de-identified NCDB files. The CoC has not verified and are not responsible for the analytic or statistical methodology employed or the conclusions derived in this study. The American College of Surgeons has executed a Business Associate Agreement that includes a data use agreement with each of its CoC-accredited hospitals.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-542/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-542/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-542/coif). EL receives salary support from the National Institute of Diabetes and Digestive and Kidney Disease through a K08 award. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was determined to be exempt by the University of Southern California institutional review board and individual consent for this retrospective national database analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- American Cancer Society. Cancer Facts and Figures 2021. Atlanta: American Cancer Society, 2021. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2021.html
- American Cancer Society. Colorectal Cancer Facts and Figures 2020-2022. Atlanta: American Cancer Society, 2020. Available online: https://www.cancer.org/content/dam/cancer-org/research/cancer-facts-and-statistics/colorectal-cancer-facts-and-figures/colorectal-cancer-facts-and-figures-2020-2022.pdf
- Lai Y, Wang C, Civan JM, et al. Effects of Cancer Stage and Treatment Differences on Racial Disparities in Survival From Colon Cancer: A United States Population-Based Study. Gastroenterology 2016;150:1135-46. [Crossref] [PubMed]
- Halpern MT, Holden DJ. Disparities in timeliness of care for U.S. Medicare patients diagnosed with cancer. Curr Oncol 2012;19:e404-13. [Crossref] [PubMed]
- Sineshaw HM, Ng K, Flanders WD, et al. Factors That Contribute to Differences in Survival of Black vs White Patients With Colorectal Cancer. Gastroenterology 2018;154:906-15.e7. [Crossref] [PubMed]
- Carethers JM, Doubeni CA. Causes of Socioeconomic Disparities in Colorectal Cancer and Intervention Framework and Strategies. Gastroenterology 2020;158:354-67. [Crossref] [PubMed]
- Tramontano AC, Chen Y, Watson TR, et al. Racial/ethnic disparities in colorectal cancer treatment utilization and phase-specific costs, 2000-2014. PLoS One 2020;15:e0231599. [Crossref] [PubMed]
- Lee W, Nelson R, Akmal Y, et al. Racial and ethnic disparities in outcomes with radiation therapy for rectal adenocarcinoma. Int J Colorectal Dis 2012;27:737-49. [Crossref] [PubMed]
- National Comprehensive Cancer Network (NCCN). NCCN Clinical Practice Guidelines in Oncology. Rectal Cancer Version 2.2021. 2021 [cited 2021 Dec 8]. Available online: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf
- Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29-42. [Crossref] [PubMed]
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702-15. [Crossref] [PubMed]
- Sineshaw HM, Jemal A, Thomas CR Jr, et al. Changes in treatment patterns for patients with locally advanced rectal cancer in the United States over the past decade: An analysis from the National Cancer Data Base. Cancer 2016;122:1996-2003. [Crossref] [PubMed]
- Azur MJ, Stuart EA, Frangakis C, et al. Multiple imputation by chained equations: what is it and how does it work? Int J Methods Psychiatr Res 2011;20:40-9. [Crossref] [PubMed]
- Demb J, Gupta S. Racial and Ethnic Disparities in Colorectal Cancer Screening Pose Persistent Challenges to Health Equity. Clin Gastroenterol Hepatol 2020;18:1691-3. [Crossref] [PubMed]
- Lu PW, Scully RE, Fields AC, et al. Racial Disparities in Treatment for Rectal Cancer at Minority-Serving Hospitals. J Gastrointest Surg 2021;25:1847-56. [Crossref] [PubMed]
- Mandelblatt JS, Kerner JF, Hadley J, et al. Variations in breast carcinoma treatment in older medicare beneficiaries: is it black or white. Cancer 2002;95:1401-14. [Crossref] [PubMed]
- Mahal BA, Aizer AA, Ziehr DR, et al. Trends in disparate treatment of African American men with localized prostate cancer across National Comprehensive Cancer Network risk groups. Urology 2014;84:386-92. [Crossref] [PubMed]
- Rodriguez VE, LeBrón AMW, Chang J, et al. Racial-Ethnic and Socioeconomic Disparities in Guideline-Adherent Treatment for Endometrial Cancer. Obstet Gynecol 2021;138:21-31. [Crossref] [PubMed]
- Fang P, He W, Gomez D, et al. Racial disparities in guideline-concordant cancer care and mortality in the United States. Adv Radiat Oncol 2018;3:221-9. [Crossref] [PubMed]
- Bian SX, Chen DH, Lin E. Racial disparities in receipt of standard chemoradiation in anal squamous cell carcinoma, an analysis of the National Cancer Database. Cancer Med 2021;10:575-85. [Crossref] [PubMed]



