
Obstructive sleep apnea and the incidence and mortality of gastrointestinal cancers: a systematic review and meta-analysis of 5,120,837 participants
Introduction
Cancer is a leading cause of mortality in the world (1). Among them, gastrointestinal (GI) cancers, including esophageal, stomach, liver, pancreas, and colorectal cancers, account for 26% of incident cancers and 35% of cancer-related death worldwide in 2020 (1). Due to the large disease burden of GI cancers, there has been rising interest in identifying risk factors, which may play a critical role in risk stratification and early diagnosis (2). Furthermore, recent studies have suggested that up to half of all GI cancers could be caused by modifiable risk factors amenable to intervention, which further emphasizes the importance of identifying these risk factors (3).
Obstructive sleep apnea (OSA) is a highly prevalent disorder, with close to one billion adults suffering from OSA globally (4). While OSA has been well-established as an independent risk factor for both cardiovascular and neuropsychiatric disease (5-10), emerging evidence has shown higher overall cancer incidence in individuals with OSA, suggesting that it could be an important risk factor for GI cancers as well (11-16). Laboratory-based studies have shown the underlying mechanism to be related to repetitive hypoxia and reoxygenation, particularly intermittent hypoxia which is a potent enhancer of the hallmarks of cancer, such as angiogenesis, immune evasion, and metastasis (10,11,17-24). However, epidemiological results have been inconclusive, particularly for GI cancers, with studies reporting increased risk (25-27), no association (28,29), or even reduced risk (26,30). A meta-analysis which sought to clarify the relationship reported no association between OSA and colorectal cancer (31). However, they did not comment on the heterogeneity of the three studies nor did they take into account other gastrointestinal cancers. Moreover, there have been two new cohort studies detailing the association between gastrointestinal cancers and OSA since the publication of the meta-analysis, including a large cohort of 1.3 million veterans (27,29).
Therefore, it is now timely to clarify this association, which has potentially significant clinical implications, given the conflicting epidemiological studies and the surfacing of new cohort studies. We hence conducted a meta-analysis to pool the association of OSA with each of the GI cancers. This systematic review was conducted in accordance with a protocol registered a priori on PROSPERO (CRD42021220836). We present the following article in accordance with the PRISMA reporting checklist (32) (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-153/rc).
Methods
Search strategy
We systematically examined four electronic databases (Cochrane Library, Embase, PubMed, Scopus) from inception to 15 November 2020 with the following search terms: (sleep apnea OR nocturnal hypoxia OR nocturnal hypoxemia) AND cancer AND (incidence OR incident OR mortality). Given that “sleep-disordered breathing” is a heterogenous blanket term encompassing central sleep apnea, OSA, primary snoring and sleep-related hypoventilation syndromes (33,34), we did not include it in our search strategy. We did not limit our initial search strategy to gastrointestinal cancers as some papers have reported on the incidence and mortality of gastrointestinal cancers within an overall cohort study of many cancer types. Instead, we assessed all potentially relevant full-text articles for gastrointestinal cancers. We hand-searched the reference lists in included articles and relevant reviews, thereby including one additional relevant record (28).
Study selection
Two authors independently screened studies using the Rayyan application (35). Articles were screened by title and abstract in the first phase of study selection, and by article full-texts where available in the second phase. We included observational studies and randomized controlled trials of adults aged 18 years and above, which reported on the association of OSA with gastrointestinal cancer incidence compared to healthy controls with neither sleep apnea nor nocturnal hypoxemia, or with less severe forms of these conditions. Gastrointestinal cancers include, but were not limited to, esophageal, stomach, colorectal, liver, pancreas, and biliary cancers. Obstructive sleep apnea was defined by the apnea-hypopnea index (AHI), respiratory disturbance index (RDI), or clinical diagnosis e.g., International Classification of Diseases (ICD) diagnostic codes. Nocturnal hypoxemia was measured by pulse oximetry or any other objective assessments of oxygen saturation e.g., sleep duration with arterial oxygen saturation <90% (T90%), oxygen desaturation index (ODI). As per protocol, we included conference abstracts and other grey literature which fulfilled the above criteria, and excluded reviews, letters, case reports, and publications not in English.
Data extraction
Two authors performed data extraction of the following data from each included study into a standardized template: name of first author, year of publication, study design, country, sample size, mean/median age, percentage male, body mass index (BMI), intervention/exposure, outcomes, covariates, statistical approaches, and main findings.
Statistical analysis
We obtained sufficient data through the systematic review to meta-analyze the longitudinal association between baseline OSA (defined based on ICD codes) and the incidence of colorectal, liver, and pancreatic cancers (also defined using ICD codes). We utilized the generic inverse variance approach favoring estimates with maximal adjustments for covariates where available. We utilized a random-effects model to account for anticipated heterogeneity in the observational estimates (36), and evaluated between-study heterogeneity with the I2 statistic (37). We considered an I² of less than 30% to represent low heterogeneity between studies, 30% to 60% to represent moderate heterogeneity, and more than 60% to represent substantial heterogeneity. We conducted pre-specified subgroup analyses of follow-up duration, covariate adjustment, and study quality. We investigated for publication bias through visual assessment of funnel plots for asymmetry, trim-and-fill, or Egger’s bias, as planned in our protocol (38-40). We performed all analyses in RevMan (version 5.4) following statistical methods outlined in the Cochrane Handbook, and considered a two-sided P value of less than 0.05 to denote statistical significance.
Quality of evidence
Given that the included studies were all observational in nature, we used the Newcastle-Ottawa Scale (NOS) to assess the risk of bias of each study (Table S1) (41,42). Two authors independently graded studies to have a high (<5 stars), moderate (5–7 stars), or low risk of bias (≥8 stars) following the NOS scoring in past reviews (43,44). At the outcome level, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (45) to assess the quality of pooled evidence, based on risk of bias, statistical heterogeneity, statistical imprecision, indirectness, and publication bias, as shown in Table S2.
Results
The process of study selection is presented in Figure S1. The systematic search retrieved 1,705 articles, and hand-searching identified one additional study (28). After removing 197 duplicates, we excluded a further 1,509 articles through title and abstract screening and 27 articles through full text screening. We included seven articles in the review (25-30,46).
Baseline characteristics
The seven studies formed a combined cohort of 5,120,837 patients, with follow-up durations of 3.8 to 11 years. Across the seven studies, four were retrospective, two were prospective, and one was a case-control study. Four studies took place in North America, two in Asia, and one in Europe. The baseline characteristics of the seven studies are summarized in Table 1.
Table 1
| First author, year | Title | DOI | Study design | Sample size | Region/Country | Mean age | % Male | Covariates | Median follow-up duration, years | NOS score |
|---|---|---|---|---|---|---|---|---|---|---|
| Chen 2020 (25) | Increased incidence of colorectal cancer with obstructive sleep apnea: a nationwide population-based cohort study | 10.1016/ j.sleep.2019.02.016 |
Matched retrospective cohort | 20,900 | Taiwan | NR | 80 | Age, sex, and chronic diseases such as chronic obstructive pulmonary disease, diabetes mellitus, coronary artery disease, hypertension, alcohol related conditions, hypercholesterolemia, peptic ulcer, liver cirrhosis, chronic hepatitis, inflammatory bowel disease, cystic fibrosis, acromegaly, prostate cancer | 8 | 8 |
| Gozal 2016 (26) | Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample | 10.5665/sleep.6004 | Retrospective matched cohort | 3,408,906 | United States | NR | 50.2 | Age, sex, morbid obesity, hypertension, type 2 diabetes mellitus, ischemic heart disease, coronary heart failure, stroke, cardiac arrhythmias, and depression | 3.75 (mean) | 8 |
| Justeau 2020 (46) | Association Between Nocturnal Hypoxemia and Cancer Incidence in Patients Investigated for OSA: Data From a Large Multicenter French Cohort | 10.1016/j.chest.2020.06.055 | Prospective cohort | 8,748 | France | 61 | 64.5 | Age, sex, body mass index, smoking status, alcohol intake, diabetes, hypertension, medical history of cardiac disease and COPD, marital status, type of sleep study, and study site | 5.8 | 9 |
| Jara 2020 (27) | The Association of Sleep Apnea and Cancer in Veterans | 10.1177/0194599819900487 | Retrospective matched cohort | 1,377,285 | United States | 55.2 | 94 | Age, sex, year of cohort entry, smoking status, alcohol use, obesity, and comorbidity | 7.4 | 7 |
| Sillah 2018 (30) | Sleep apnea and subsequent cancer incidence | 10.1007/s10552-018-1073-5 | Retrospective cohort | 34,402 | United States | 51.6 | 57.4 | Age, sex | 5.3 | 6 |
| Fang 2015 (28) | Risk of Cancer in Patients with Insomnia, Parasomnia, and Obstructive Sleep Apnea: A Nationwide Nested Case-Control Study | 10.7150/jca.12490 | Nested case-control | 205,266 | Taiwan | NR | 54.4 | Age, sex, income, region, urbanization, and Charlson Comorbidity Index | 11 | 8 |
| Huang 2021 (29) | Associations of Self-reported Obstructive Sleep Apnea with Total and Site-specific Cancer Risk in Older Women: A Prospective Study | 10.1093/sleep/zsaa198 | Prospective cohort | 65,330 | United States | 73 | 0 | Age, race/ethnicity, family history of cancer, body mass index, height, pack-years of smoking, alcohol drinking, physical activity, sleep duration, duration of hypertension drug use by type, history of type 2 diabetes, aspirin use, and recent physical examination | 8 | 5 |
NR, not reported; NOS, Newcastle-Ottawa scale; OSA, obstructive sleep apnea; COPD, chronic obstructive pulmonary disease.
The diagnosis of obstructive sleep apnea was made using the ICD diagnostic codes, except for Justeau 2020 (46) which diagnosed OSA with T90%. All studies reported obstructive sleep apnea, except for Sillah 2018 (30) which reported sleep apnea. Additionally, Sillah 2018 (30) did not adjust for important comorbidities such as obesity, and hence was excluded from the meta-analysis. Six studies, comprising 5,086,435 patients, which focused on obstructive sleep apnea and adjusted for both demographics and comorbidities were included in the meta-analysis.
Colorectal cancer incidence
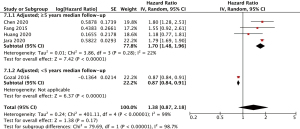
Five studies were included (25-29), all of which had low or moderate risk of bias. Justeau 2020 (46) was not included in the analysis as the study stratified the incidence rates according to the severity of OSA rather than the presence of OSA. The five adjusted studies had adjusted for age, sex, and comorbidities (25-29), included but were not limited to chronic obstructive pulmonary disease (25,27,28), hypertension (25,26,29), diabetes mellitus (25-29), obesity/body-mass index (26,27,29), liver disease (25,27,28), peptic ulcer disease (27,28), stroke (26-28), and cardiac diseases (25-29). All studies had median follow-up duration of five or more years, except for Gozal 2016 (26). The pooled incidence outcomes are shown in Figure 1. In the overall analysis, there was no association observed between OSA and incident colorectal cancer (HR 1.38, 95% CI: 0.87–2.18, I2=99). For the meta-analysis on OSA and colorectal cancer incidence, which had high heterogeneity (I2=99%), pre-specified sensitivity analyses showed that when including (I) a median duration of follow-up ≥5 years (I2=93%), patients with OSA had significantly higher pooled hazards of incident cancer (HR 1.70, 95% CI: 1.48–1.96, I2=22%) compared to patients without OSA. Notably, the heterogeneity decreased to 22% (low heterogeneity). When pre-specified sensitivity analyses were performed for (II) moderate or low risk of bias (I2=99%), the association remained insignificant.
Liver cancer incidence
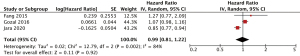
Three studies were included (26-28). All three studies adjusted for important confounders, including age, sex, comorbidities (26-28), of which two studies had additionally adjusted for obesity/body-mass index (26,27). The pooled incidence outcomes are shown in Figure 2. There was no association observed between OSA and incident liver cancer (HR 0.99, 95% CI: 0.81–1.22, I2=84). Due to the lack of studies, pre-specified sensitivity analyses were not performed.
Pancreatic cancer incidence
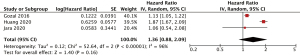
Three studies were included (26,27,29). All three studies adjusted for important confounders, such as age, sex, comorbidities including obesity and body-mass index (26,27,29). The pooled incidence outcomes are shown in Figure 3. There was no association observed between OSA and incident pancreatic cancer (HR 1.36, 95% CI: 0.88–2.09, I2=96). Due to the lack of studies, pre-specified sensitivity analyses were not performed.
Cancer mortality
Gozal 2016 (26) investigated the longitudinal association between OSA and colorectal cancer mortality (HR: 0.92; 95% CI: 0.69 to 1.21), and OSA and liver cancer (HR: 0.85; 95% CI: 0.56 to 1.32).
Discussion
In this pair-wise meta-analysis, we found that among four studies with 7–11 years of median follow-up, patients with OSA experienced a 70% increased incidence of colorectal cancer compared to patients without OSA. There was lack of association between OSA and liver and pancreatic cancers. This is in the context of the recent publication of two new large cohort studies since the last meta-analysis, against the backdrop of conflicting epidemiological studies investigating the relationship between OSA and cancers. Despite previous systematic reviews and meta-analyses illustrating an increased overall cancer incidence in OSA patients (47,48), our analysis supports suggestions that OSA confers differential risk to site-specific cancers, even within GI cancers. Cellular and murine models have demonstrated several possible biological relationships between OSA and cancers (18-21). Among the many biological processes, one possible mechanism that may explain the differential risk is the role of hypoxia-inducible factors (HIF). OSA, through intermittent hypoxia, is thought to upregulate HIF, which subsequently increases angiogenesis and metabolism and thus leading to tumor progression. However, studies have also shown paradoxical roles of HIF in different cancers: while it increases tumorigenesis and growth of renal cell carcinoma (49), it acts as tumor suppressors in non-small cell lung cancer, hepatocellular carcinoma, and glioma (50-52). Even within the same site-specific cancer, different cell lines have been shown to display different hypoxic behaviors (53). The confusing relationship between hypoxia and pancreatic cancer has also been well documented, with HIF having potentially different oncogenic roles on cancer cells and tumor microenvironment (54,55). This suggests that the relationship between OSA, hypoxia and the tumor microenvironment may be more complicated than previously thought and could possibly explain the lack of association seen in our meta-analyses of liver and pancreatic cancer. This is as opposed to other cancers such as cutaneous melanoma which has consistently demonstrated an increased risk in patients with OSA (11). Thus, future studies should investigate the risk of specific cancers in patients with OSA rather than overall cancer incidence, to better understand the interaction between hypoxia and different cancers.
Importantly, we found that in a subgroup analysis, patients with OSA were 70% more likely to develop colorectal cancer, after adjustment for important comorbidities and exclusion of studies with less than five years of median follow-up duration. Significantly, the heterogeneity was markedly lower in this subgroup analysis. It is thus likely that the high heterogeneity seen in the overall analysis of colorectal cancer could be attributed to the combination of these two factors: (I) whether the studies adjusted for comorbidities and (II) difference in follow-up duration. These two factors are critical when investigating the relationship between OSA and cancers Adjustment of comorbidities, particularly obesity, removes the effect of an important confounder (11), since patients with obesity are at an increased risk of developing OSA, and at the same time, epidemiological studies have also described the association between obesity and various types of cancer (56,57), possibly through the mechanism of pro-inflammatory cytokines (58). Furthermore, a longer duration of follow-up is also critical in detecting new cancers, given that cancers typically develop following a time lag after risk factor exposure (27). This is particularly important in the context of colorectal cancer, which when following the adenoma—carcinoma sequence, often takes 5–10 years to fully develop (59). Hence, such an association could only be detected on longer term epidemiological studies. Thus, this subgroup analysis among four studies with 7–11 years of median follow-up, excluding a study with three years of follow-up, may provide a clearer picture of the true relationship between OSA and colorectal cancer. The discrepancy between the finding of our subgroup analysis and previous meta-analyses is likely due to our inclusion of two new studies, which both adjusted for comorbidities and had long-term follow-up of at least 5 years.
Our finding of a positive association of OSA and colorectal cancer in this subgroup analysis has some important implications. Firstly, we recommend that future studies investigating risk of OSA and site-specific cancers should have at least five years of follow-up and multi-adjust for key confounders such as obesity. Secondly, the possible association of OSA and colorectal cancer calls for further investigation on whether early detection and treatment of OSA reduces the incidence of colorectal cancer, as most cases of OSA remain largely undiagnosed and untreated (4). Future research should explore the possibility of additional population based OSA screening through the STOP-BANG questionnaire in identifying high-risk OSA individuals as part of colorectal screening guidelines in reducing the global incidence and burden of colorectal cancer.
Limitations
Our study comprises a large number of participants with appropriate adjustment for key confounders and sound methodological decisions such as consideration of type-specific cancers, follow-up duration, and covariate adjustment in this analysis of cancer risk. All seven included studies also achieved a score of at least five on the Newcastle-Ottawa scale, indicating low to moderate risk of bias. The findings should nonetheless be considered in view of the following limitations. First, there exists significant heterogeneity in our overall findings. While the heterogeneity became significantly reduced (99% to 22%) in the sensitivity analysis of ≥5 years of follow-up and covariate adjustment for colorectal cancer, due to insufficient studies we could not comment if the heterogeneity will be similarly reduced if such sensitivity analyses was performed for liver and pancreatic cancer. Second, the lack of a statistically significant relationship between OSA and pancreatic cancer in our meta-analysis does not necessarily imply the true absence of an association. Among the three included studies, Gozal et al. and Huang et al. had found a statistically significant association (26,29). Therefore, the lack of a significant pooled association may imply a lack of precision from insufficient studies. Further studies, ideally with ≥5 years of follow-up and adequate adjustment for comorbidities, are required before we can draw definitive epidemiological conclusions can be drawn on the association of OSA with liver and pancreatic cancer. Third, most epidemiological studies to date evaluated cancer as a single outcome variable (11), and there exists a need to identify if the association holds true for the aggressiveness and progression of individual cancers. Only Gozal 2016 has examined the effect of OSA on cancer mortality, and there are insufficient studies to be meta-analyzed for cancer mortality hence we could not comment if OSA will be associated with any increased mortality from gastrointestinal cancers. Fourth, while confounders were adjusted for where possible, certain potential confounders were not amenable to analysis due to lack of reporting in the included studies. These potential confounders include alcohol consumption and non-steroidal anti-inflammatory drugs, which are associated with an increased and decreased risk of malignancies, respectively (60,61). Fifth, this meta-analysis is suggestive, but cannot demonstrate a causal relationship between OSA and colorectal cancer, as there may be residual unadjusted confounding among the cohort studies.
Conclusions
In our pooled cohort comprising more than five million patients, we demonstrated an increased risk of colorectal cancer in patients with OSA across studies with long-term median follow-up of at least five years. Associations between OSA and other types of GI cancers were insignificant. Further studies are required before definite epidemiological conclusions can be made on the association of OSA with other types of GI cancer.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-153/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-153/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Arnold M, Abnet CC, Neale RE, et al. Global Burden of 5 Major Types of Gastrointestinal Cancer. Gastroenterology 2020;159:335-349.e15. [Crossref] [PubMed]
- Islami F, Goding Sauer A, Miller KD, et al. Proportion and number of cancer cases and deaths attributable to potentially modifiable risk factors in the United States. CA Cancer J Clin 2018;68:31-54. [Crossref] [PubMed]
- Benjafield AV, Ayas NT, Eastwood PR, et al. Estimation of the global prevalence and burden of obstructive sleep apnoea: a literature-based analysis. Lancet Respir Med 2019;7:687-98. [Crossref] [PubMed]
- Baranchuk A. Sleep apnea, cardiac arrhythmias, and conduction disorders. J Electrocardiol 2012;45:508-12. [Crossref] [PubMed]
- Chokesuwattanaskul A, Lertjitbanjong P, Thongprayoon C, et al. Impact of obstructive sleep apnea on silent cerebral small vessel disease: a systematic review and meta-analysis. Sleep Med 2020;68:80-8. [Crossref] [PubMed]
- Emamian F, Khazaie H, Tahmasian M, et al. The Association Between Obstructive Sleep Apnea and Alzheimer’s Disease: A Meta-Analysis Perspective. Front Aging Neurosci 2016;8:78. [Crossref] [PubMed]
- Garbarino S, Bardwell WA, Guglielmi O, et al. Association of Anxiety and Depression in Obstructive Sleep Apnea Patients: A Systematic Review and Meta-Analysis. Behav Sleep Med 2020;18:35-57. [Crossref] [PubMed]
- Gonzaga C, Bertolami A, Bertolami M, et al. Obstructive sleep apnea, hypertension and cardiovascular diseases. J Hum Hypertens 2015;29:705-12. [Crossref] [PubMed]
- Hakim F, Gozal D, Kheirandish-Gozal L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front Neurol 2012;3:7. [Crossref] [PubMed]
- Gozal D, Almendros I, Phipps AI, et al. Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders? Int J Mol Sci 2020;21:8779. [Crossref] [PubMed]
- Tan BKJ, Teo YH, Tan NKW, et al. Association of obstructive sleep apnea and nocturnal hypoxemia with all-cancer incidence and mortality: a systematic review and meta-analysis. J Clin Sleep Med 2022;18:1427-40. [Crossref] [PubMed]
- Yap DWT, Tan NKW, Tan BKJ, et al. The Association of Obstructive Sleep Apnea With Breast Cancer Incidence and Mortality: A Systematic Review and Meta-analysis. J Breast Cancer 2022;25:149-63. [Crossref] [PubMed]
- Cheong AJY, Tan BKJ, Teo YH, et al. Obstructive Sleep Apnea and Lung Cancer: A Systematic Review and Meta-Analysis. Ann Am Thorac Soc 2022;19:469-75. [Crossref] [PubMed]
- Tan NKW, Yap DWT, Tan BKJ, et al. The association of obstructive sleep apnea with melanoma incidence and mortality: a meta-analysis of 5,276,451 patients. Sleep Med 2021;88:213-20. [Crossref] [PubMed]
- Tan BKJ, Tan NKW, Teo YH, et al. Association of obstructive sleep apnea with thyroid cancer incidence: a systematic review and meta-analysis. Eur Arch Otorhinolaryngol 2022;279:5407-14. [Crossref] [PubMed]
- Samanta D, Semenza GL. Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim Biophys Acta Rev Cancer 2018;1870:15-22. [Crossref] [PubMed]
- Gozal D, Almendros I, Hakim F. Sleep apnea awakens cancer: A unifying immunological hypothesis. Oncoimmunology 2014;3:e28326. [Crossref] [PubMed]
- Almendros I, Farré R, Planas AM, et al. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: differential effect of obstructive apneas and intermittent hypoxia. Sleep 2011;34:1127-33. [Crossref] [PubMed]
- Briançon-Marjollet A, Pépin JL, Weiss JW, et al. Intermittent hypoxia upregulates serum VEGF. Sleep Med 2014;15:1425-6. [Crossref] [PubMed]
- Lacedonia D, Carpagnano GE, Crisetti E, et al. Mitochondrial DNA alteration in obstructive sleep apnea. Respir Res 2015;16:47. [Crossref] [PubMed]
- Akbarpour M, Khalyfa A, Qiao Z, et al. Altered CD8+ T-Cell Lymphocyte Function and TC1 Cell Stemness Contribute to Enhanced Malignant Tumor Properties in Murine Models of Sleep Apnea. Sleep 2017; [Crossref] [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Saxena K, Jolly MK. Acute vs. Chronic vs. Cyclic Hypoxia: Their Differential Dynamics, Molecular Mechanisms, and Effects on Tumor Progression. Biomolecules 2019;9:339. [Crossref] [PubMed]
- Chen CY, Hu JM, Shen CJ, et al. Increased incidence of colorectal cancer with obstructive sleep apnea: a nationwide population-based cohort study. Sleep Med 2020;66:15-20. [Crossref] [PubMed]
- Gozal D, Ham SA, Mokhlesi B. Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample. Sleep 2016;39:1493-500. [Crossref] [PubMed]
- Jara SM, Phipps AI, Maynard C, et al. The Association of Sleep Apnea and Cancer in Veterans. Otolaryngol Head Neck Surg 2020;162:581-8. [Crossref] [PubMed]
- Fang HF, Miao NF, Chen CD, et al. Risk of Cancer in Patients with Insomnia, Parasomnia, and Obstructive Sleep Apnea: A Nationwide Nested Case-Control Study. J Cancer 2015;6:1140-7. [Crossref] [PubMed]
- Huang T, Lin BM, Stampfer MJ, et al. Associations of self-reported obstructive sleep apnea with total and site-specific cancer risk in older women: a prospective study. Sleep 2021;44:zsaa198. [Crossref] [PubMed]
- Sillah A, Watson NF, Schwartz SM, et al. Sleep apnea and subsequent cancer incidence. Cancer Causes Control 2018;29:987-94. [Crossref] [PubMed]
- Al momani L, Alomari M, Nehme F, et al. Tu1011 Myths about obstructive sleep apnea debunked: a systematic review and meta-analysis. Gastroenterology 2020;158:S1005-6.
- Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372: [PubMed]
- Carden KA, Chervin RD. Consistency and Clarity in Sleep Medicine Terminology. J Clin Sleep Med 2016;12:157-8. [Crossref] [PubMed]
- Sateia MJ. International classification of sleep disorders-third edition: highlights and modifications. Chest 2014;146:1387-94. [Crossref] [PubMed]
- Ouzzani M, Hammady H, Fedorowicz Z, et al. Rayyan—a web and mobile app for systematic reviews. Systematic Reviews 2016;5:210. [Crossref] [PubMed]
- DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials 1986;7:177-88. [Crossref] [PubMed]
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med 2002;21:1539-58. [Crossref] [PubMed]
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50:1088-101. [Crossref] [PubMed]
- Egger M, Davey Smith G, Schneider M, et al. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997;315:629-34. [Crossref] [PubMed]
- Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics 2000;56:455-63. [Crossref] [PubMed]
- Wells GA, Shea B, O'Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses 2012. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Cochrane Collaboration. Section 13.5.2.3. Tools for assessing methodological quality or risk of bias in non-randomized studies. In: Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions, Version 510. London 2011.
- Kojima G, Avgerinou C, Iliffe S, et al. Adherence to Mediterranean Diet Reduces Incident Frailty Risk: Systematic Review and Meta-Analysis. J Am Geriatr Soc 2018;66:783-8. [Crossref] [PubMed]
- Saraiva MD, Suzuki GS, Lin SM, et al. Persistent pain is a risk factor for frailty: a systematic review and meta-analysis from prospective longitudinal studies. Age Ageing 2018;47:785-93. [Crossref] [PubMed]
- Guyatt GH, Oxman AD, Vist GE, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336:924-6. [Crossref] [PubMed]
- Justeau G, Gervès-Pinquié C, Le Vaillant M, et al. Association Between Nocturnal Hypoxemia and Cancer Incidence in Patients Investigated for OSA: Data From a Large Multicenter French Cohort. Chest 2020;158:2610-20. [Crossref] [PubMed]
- Palamaner Subash Shantha G, Kumar AA, Cheskin LJ, et al. Association between sleep-disordered breathing, obstructive sleep apnea, and cancer incidence: a systematic review and meta-analysis. Sleep Med 2015;16:1289-94. [Crossref] [PubMed]
- Zhang XB, Peng LH, Lyu Z, et al. Obstructive sleep apnoea and the incidence and mortality of cancer: a meta-analysis. Eur J Cancer Care (Engl) 2017;26:e12427. [Crossref] [PubMed]
- Kaelin WG. Von Hippel-Lindau disease. Annu Rev Pathol 2007;2:145-73. [Crossref] [PubMed]
- Acker T, Diez-Juan A, Aragones J, et al. Genetic evidence for a tumor suppressor role of HIF-2alpha. Cancer Cell 2005;8:131-41. [Crossref] [PubMed]
- Mazumdar J, Hickey MM, Pant DK, et al. HIF-2alpha deletion promotes Kras-driven lung tumor development. Proc Natl Acad Sci U S A 2010;107:14182-7. [Crossref] [PubMed]
- Sun HX, Xu Y, Yang XR, et al. Hypoxia inducible factor 2 alpha inhibits hepatocellular carcinoma growth through the transcription factor dimerization partner 3/ E2F transcription factor 1-dependent apoptotic pathway. Hepatology 2013;57:1088-97. [Crossref] [PubMed]
- Zhou W, Dosey TL, Biechele T, et al. Assessment of hypoxia inducible factor levels in cancer cell lines upon hypoxic induction using a novel reporter construct. PLoS One 2011;6:e27460. [Crossref] [PubMed]
- Fuentes NR, Phan J, Huang Y, et al. Resolving the HIF paradox in pancreatic cancer. Cancer Lett 2020;489:50-5. [Crossref] [PubMed]
- Tan Z, Xu J, Zhang B, et al. Hypoxia: a barricade to conquer the pancreatic cancer. Cell Mol Life Sci 2020;77:3077-83. [Crossref] [PubMed]
- Vainio H, Kaaks R, Bianchini F. Weight control and physical activity in cancer prevention: international evaluation of the evidence. Eur J Cancer Prev 2002;11:S94-100. [PubMed]
- Wiseman M. The second World Cancer Research Fund/American Institute for Cancer Research expert report. Food, nutrition, physical activity, and the prevention of cancer: a global perspective. Proc Nutr Soc 2008;67:253-6. [Crossref] [PubMed]
- Vucenik I, Stains JP. Obesity and cancer risk: evidence, mechanisms, and recommendations. Ann N Y Acad Sci 2012;1271:37-43. [Crossref] [PubMed]
- Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging 2016;11:967-76. [Crossref] [PubMed]
- Bindu S, Mazumder S, Bandyopadhyay U. Non-steroidal anti-inflammatory drugs (NSAIDs) and organ damage: A current perspective. Biochem Pharmacol 2020;180:114147. [Crossref] [PubMed]
- Rumgay H, Murphy N, Ferrari P, et al. Alcohol and Cancer: Epidemiology and Biological Mechanisms. Nutrients 2021;13:3173. [Crossref] [PubMed]


