
SGLT-2 as a potential target in pancreatic cancer: the preliminary clue from The Cancer Genome Atlas data
Introduction
Pancreatic cancer (PC) is the most malignant solid tumor of the digestive system. It has been estimated that by 2020, about 420,000 new cases of PC and 410,000 deaths will occur globally each year (1). By 2030, PC will become the second most fatal malignancy (2). The prognosis of PC is very poor, with a 5-year survival rate of only about 6% (3). Many patients are diagnosed at a very late stage and have already progressed beyond the opportunity for radical surgery due to atypical symptoms at the early stage. Relapse and metastasis also attribute to the poor prognosis of PC (4). Currently, radical surgery remains the only possible cure for PC. For resectable PC, gemcitabine may be used postoperatively as adjuvant chemotherapy. For PC patients with metastasis, fluorouracil, formyl tetrahydro folic acid, irinotecan, and oxaliplatin (FOLFIEINOX), gemcitabine and paclitaxel can be adopted for those with better conditions while gemcitabine is the main therapy for those with poor conditions (5). Despite the progress of precision surgery and neoadjuvant therapy in recent years, the five-year survival rate of PC has not improved significantly, partially due to drug resistance. A novel method for early diagnosis and effective treatment is urgently required.
Various metabolic abnormalities are involved in tumorigenesis and progression. The utilization of nutrients in benign cells is closely related to their abundance (6). Through multiple nutrition-sensing pathways, cells detect nutrient levels and respond through AMP-activated protein kinase (AMPK) and mTOR complex 1 (mTORC1) (7,8). Through metabolic reprogramming, cancer cells break the above rules to maintain intracellular nutrient levels and achieve a competitive growth advantage over normal cells (9). As an aggressive malignant tumor, pancreatic cancer thrives in an environment with insufficient energy supply via metabolic reprogramming, in which continuous glucose uptake is an important part (10). Up-regulation of glucose transporter 1 (GLUT1) in PC has been revealed (11), and abnormal glucose metabolism has also been demonstrated as associated with resistance to gemcitabine in PC (12).
Two families of glucose transporters have been identified in mammals: the glucose transporter families (GLUTs) and the sodium glucose transporter families (SGLTs) (13,14). The human GLUTs are expressed in all tissues and transport glucose in the form of cisconcentration gradient (14), and the SGLTs, encoded by the solute carrier family 5 (SLC5) gene family, are expressed in cells with active metabolism (14). Different from GLUTs, SGLTs enable active transport of glucose against concentration gradient. The most studied members of the SGLT family are SGLT1 and SGLT-2: SGLT1 is responsible for glucose uptake in small intestine and proximal renal tubular cells (15); SGLT-2, encoded by the SLC5A2 gene, is mainly distributed in the renal cortex and plays a key role in renal glucose reabsorption (16). The SGLTs and GLUTs cooperate with each other in physiological conditions, while their distribution and weightings in tumor cells remain unclear. A previous study has reported elevated expression of GLUT1 in PC (11). Later, up-regulation of SGLT-2 at both messenger RNA (mRNA) and protein levels in human PC and prostate cancer was also reported (17). Further, SGLT-2 inhibitors have been found to reduce the glucose absorption of PC in a xenograft model in nude mice and to inhibit tumor growth either as a single agent or in combination with gemcitabine (17). The above finding suggested that SGLT-2 is involved in the carcinogenesis and progression of PC. Thereafter, the abnormal expression of SGLT-2 and the anti-tumor potential of SGLT-2 inhibitors have been reported in other malignancies including, colon, breast, lung and liver cancer (18-21). More recently, SGLT-1 has been reported significantly overexpressed in pancreatic ductal adenocarcinoma and was an independent predictor for a better prognosis (22). It is unrealistic to target GLUT1 for cancer treatment as GLUT1 is expressed on the blood-brain barrier for glucose supply of the brain (23). In contrast, due to the tissue specificity of SGLT-2 distribution, SGLT-2 inhibitors have potential therapeutic benefit with fewer side effects for malignancies with up-regulated SGLT-2 expression. Moreover, as widely used hypoglycemic drugs, the safety of SGLT-2 inhibitors has been fully verified. If the anticancer potential of such drugs is validated, their clinical transformation will be very rapid.
In the present study, we analyzed the expression of SLC5A2 in PC using data from The Cancer Genome Atlas (TCGA). Different from previous studies of SGLT-2 in PC, we investigated the relationship between its expression and clinical features, pathological stages and prognosis of PC, respectively. In addition, correlated genes were identified and enriched to explore the underlying mechanism of its pro-oncogenic role in PC. High SLC5A2 expression was found correlated with impaired cell replication surveillance, enhanced cellular metabolism, and drug metabolism. Finally, we unexpectedly revealed that SLC5A2 expression was closely related with the pancreatic progenitor subtype of PC. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-900/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
TCGA database analysis
The RNA sequencing data of PC was provided by TCGA database. The online database Gene Expression Profiling Interactive Analysis (GEPIA; http://gepia.cancer-pku.cn/index.html) is an interactive web that includes 9,736 tumors and 8,587 normal samples from TCGA and the Genotype-Tissue Expression (GTEx) projects (24), which was used to analyze the RNA sequencing expression. We validated the differential expression of the SLC5A2 gene in PC and healthy donor samples and generated the Kaplan-Meier curves for overall survival (OS) and disease-free survival (DFS) between groups with high and low SLC5A2 expression.
Functional annotation
We conduct the functional and pathway enrichment analyses by using the Database for Annotation Visualization and Integrated Discovery (DAVID) (https://david.ncifcrf.gov/) (25). We performed Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analyses to detect the potential biological functions and pathways of the overlapping genes.
Correlated gene expression analysis
We analyzed the genetic correlations of SLC5A2 gene in PC by using the cBioPortal (http://www.cbioportal.org/) and LinkedOmics (http://www.linkedomics.orglogin.php) databases, which are both open-access, open-source resource comprising multidimensional cancer genomics datasets (26,27).
Statistical analysis
Data were compared using the t-test through the software SPSS24.0 (IBM Corp., Armonk, NY, USA). A P value <0.05 was considered statistically significant. All values were presented as the mean ± SD.
Results
SLC5A2 expression and pathoclinical features
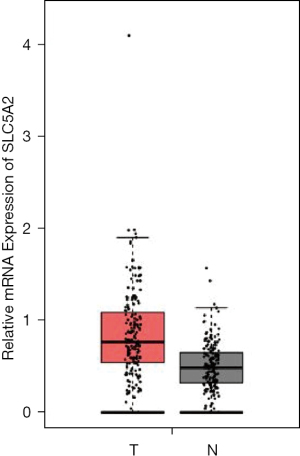
The expression of SGLT-2 was higher in PC (n=179) than in normal pancreas samples (n=171) (Figure 1). In view of epidemiology, the incidence of PC is 50% higher in males than in females. It is also a malignance of older adults, with most cases diagnosed in patients between 60 and 80 years old. In the USA, African-Americans have a higher incidence of PC than other racial groups. In light of this, we subcategorized PC patients according to gender (male and female), age (<60, 60–80, and >80 y), and race (White, African-American, and Asian), and analyzed the expression of SGLT-2. As shown in Figure 2A-2C, no significant differences in SGLT-2 expression were observed between different genders, or among the different age and race subgroups.
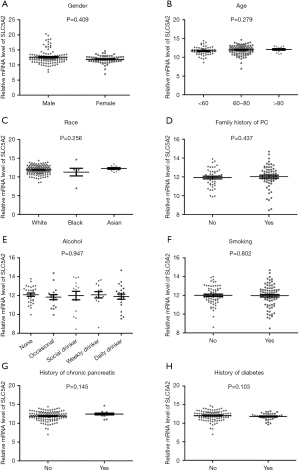
Since the history of chronic pancreatic and diabetes have been identified as risk factors for PC, we also grouped these patients according to history of these two diseases. The patients were also subcategorized with other risk factors such as the consumption of alcohol (none, occasional, social drinker, weekly drinker, and daily drinker) and smoking (non-smoker or smoker). Family history of PC was analyzed as another substantial risk factor because 10% of patients have a positive family history. As shown in Figure 2D-2H, no significant difference was found among the above subgroups.
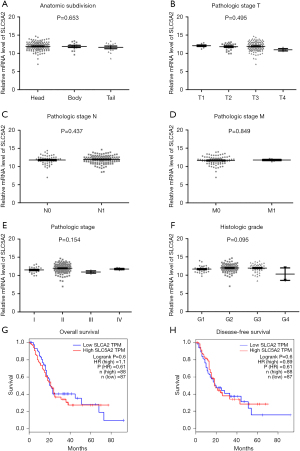
We further analyzed SGLT-2 expression in PC with different anatomical location (head, body, and tail) and no significant differences were observed (Figure 3A). Although SGLT-2 was previously presumed to be more important for bigger tumors (17), neither pathological tumor-node-metastasis (TNM) stages (I, II, III, and IV) (Figure 3B-3E) nor histology grade (G1, G2, G3, and G4) of PC was correlated with SGLT-2 expression (Figure 3F). The prognosis of patients with high (upper 50%) and low expression (lower 50%) of SGLT-2 was analyzed. There were no significant differences between these two subgroups with regard to OS or DFS (Figure 3G,3H).
SGLT-2 correlated genes
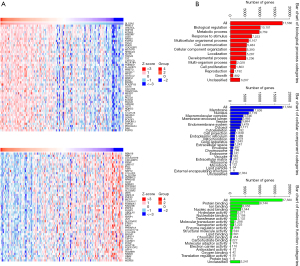
We next identified the genes positively or negatively correlated with high expression of SGLT-2 (Figure 4A). Both GO and KEGG enrichment analyses were performed. As shown in Figure 4B, most correlated genes are located at the membrane and nucleus, responsible for protein and ion binding, and participate in biological regulation, metabolic process, and response to stimuli.
Consistently, several signaling pathways related with cellular metabolism were positively correlated with SGLT-2 expression (Table 1). In addition, the activity of chemical carcinogenesis pathway became activated as SGLT-2 expression increased. In contrast, cell cycle regulation, nucleotide excision repair, and mRNA surveillance became suppressed as SGLT-2 expression increased. Remarkably, drug metabolisms mediated by cytochrome P450 and other enzymes were enhanced when SGLT-2 was upregulated. FOLFIEINOX is one alternative for the patients who are not eligible for surgery but with good performance status. As presented in Figure 5, the metabolism of fluorouracil and irinotecan is enhanced in PC with high SGLT-2 expression. These results suggested that PC with higher SGLT-2 expression is characterized with instable cell replication, exuberant cellular metabolism, and high capacity of chemo drug metabolism. Notably, the pathway of maturity onset diabetes of the young (MODY) was also positively correlated with SGLT-2 expression.
Table 1
| ID | Name | Number of genes | FDR |
|---|---|---|---|
| Positively correlated | |||
| hsa00140 | Steroid hormone biosynthesis - Homo sapiens (human) | 55 | 0.00E+00 |
| hsa00830 | Retinol metabolism - Homo sapiens (human) | 61 | 0.00E+00 |
| hsa00980 | Metabolism of xenobiotics by cytochrome P450 - Homo sapiens (human) | 70 | 0.00E+00 |
| hsa00982 | Drug metabolism - cytochrome P450 - Homo sapiens (human) | 66 | 0.00E+00 |
| hsa05204 | Chemical carcinogenesis - Homo sapiens (human) | 75 | 0.00E+00 |
| hsa04976 | Bile secretion - Homo sapiens (human) | 68 | 1.99E-04 |
| hsa00100 | Steroid biosynthesis - Homo sapiens (human) | 17 | 2.23E-04 |
| hsa04950 | Maturity onset diabetes of the young - Homo sapiens (human) | 26 | 2.55E-04 |
| hsa00590 | Arachidonic acid metabolism - Homo sapiens (human) | 58 | 2.98E-04 |
| hsa00561 | Glycerolipid metabolism - Homo sapiens (human) | 53 | 3.68E-04 |
| hsa04978 | Mineral absorption - Homo sapiens (human) | 51 | 9.95E-04 |
| hsa00480 | Glutathione metabolism - Homo sapiens (human) | 52 | 1.08E-03 |
| hsa00860 | Porphyrin and chlorophyll metabolism - Homo sapiens (human) | 40 | 1.18E-03 |
| hsa00591 | Linoleic acid metabolism - Homo sapiens (human) | 27 | 1.44E-03 |
| hsa04972 | Pancreatic secretion - Homo sapiens (human) | 94 | 4.65E-03 |
| hsa00564 | Glycerophospholipid metabolism - Homo sapiens (human) | 85 | 4.75E-03 |
| hsa00983 | Drug metabolism - other enzymes - Homo sapiens (human) | 44 | 4.77E-03 |
| hsa00071 | Fatty acid degradation - Homo sapiens (human) | 42 | 4.81E-03 |
| hsa00430 | Taurine and hypotaurine metabolism - Homo sapiens (human) | 11 | 4.81E-03 |
| hsa04146 | Peroxisome - Homo sapiens (human) | 82 | 5.89E-03 |
| hsa04975 | Fat digestion and absorption - Homo sapiens (human) | 37 | 7.51E-03 |
| hsa04974 | Protein digestion and absorption - Homo sapiens (human) | 88 | 7.81E-03 |
| hsa04964 | Proximal tubule bicarbonate reclamation - Homo sapiens (human) | 23 | 7.87E-03 |
| hsa00053 | Ascorbate and aldarate metabolism - Homo sapiens (human) | 25 | 1.62E-02 |
| hsa00120 | Primary bile acid biosynthesis - Homo sapiens (human) | 17 | 1.91E-02 |
| Negatively correlated | |||
| hsa04110 | Cell cycle - Homo sapiens (human) | 118 | 2.29E-02 |
| hsa03013 | RNA transport - Homo sapiens (human) | 158 | 2.59E-02 |
| hsa03060 | Protein export - Homo sapiens (human) | 22 | 2.69E-02 |
| hsa03420 | Nucleotide excision repair - Homo sapiens (human) | 45 | 3.15E-02 |
| hsa04120 | Ubiquitin mediated proteolysis - Homo sapiens (human) | 135 | 4.26E-02 |
| hsa03015 | mRNA surveillance pathway - Homo sapiens (human) | 87 | 4.38E-02 |
| hsa00532 | Glycosaminoglycan biosynthesis - chondroitin sulfate/dermatan sulfate - Homo sapiens (human) | 20 | 4.66E-02 |
DAVID, the Database for Annotation Visualization and Integrated Discovery; SGLT-2, sodium-glucose co-transporters-2; ID, identity; FDR, false discovery rate; RNA, Ribonucleic Acid; mRNA, messenger RNA.
SGLT-2 expression and genomic subtypes of PC
Bailey et al. defined PC as comprising 4 subtypes: squamous, pancreatic progenitor, immunogenic, and aberrantly differentiated endocrine exocrine (ADEX) (28). The molecular evolution, pathogenesis, and prognosis differ greatly among these subtypes. Squamous tumors completely lose endodermal identity, preferentially expressed genes for the C2-squamous-like class of tumors of TCGA pan-cancer studies, and have a poor prognosis. Pancreatic progenitor tumors preferentially express the genes involved in early pancreatic development. The ADEX tumors are characterized by KRAS activation and upregulation of genes involved exocrine and endocrine differentiation. In immunogenic tumors, networks of acquired immune suppression are upregulated.
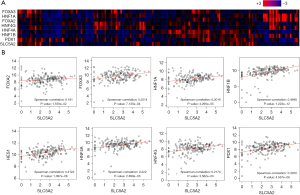
The KEGG enrichment analysis suggested that a high SGLT-2 level coexists with activation of pathways including steroid hormone biosynthesis, drug metabolism, and MODY, which fit the pattern of the pancreatic progenitor subtype. The co-expression of SGLT-2 and the gene symbols, which are critical for pancreatic endoderm cell-fate determination and closely related with MODYs was further investigated. As seen in Figure 5A,5B, SGLT-2 expression was positively correlated with these transcription factors. Among these transcription factors, the highest degrees of relevance were between SGLT-2 and hepatocyte nuclear factor 1B (HNF1B) (Spearman-correlation: 0.4998), followed by hairy and enhancer of split homolog-1 (HES1) (Spearman-correlation: 0.4122). Pancreatic and duodenal homeobox 1 (PDX1), a biomarker for embryonic progenitor cells which both exocrine and endocrine cells derive from, was also found positively correlated expressed with SGLT-2 (Spearman-correlation: 0.3359). It should also be noted that HNF1A (Spearman-correlation: 0.3016) and HNF4A (Spearman-correlation: 0.222) have been demonstrated to participate in the regulation of SGLT-2, and the binding site of HNF1A has been identified in promoter sites.
Due to its participation in the pancreatic progenitor subtype, the possible involvement of SGLT-2 in the ADEX subtype was next explored. As a subclass of the pancreatic progenitor subtype, ADEX exhibits later stages of pancreatic development and differentiation. Instead of preferentially expressing genes for either endocrine or exocrine lineages, its characterized with upregulation of genes for both of them. As shown in Figure 6, the significant but low degree positive correlation between SGLT-2 expression and some of the gene symbols for the ADEX subtype was observed.
Discussion
Due to the high morbidity and mortality, PC is a public health issue. Novel methods, both for early diagnosis and treatment for PC are highly sought after. As PC progresses, it changes the surrounding tumor microenvironment (TME) towards a tumor-promoting one. With excessive fibrosis and extracellular matrix (ECM), the TME of PC is characterized with high interstitial pressure, hypovascularity and low nutrient and oxygen diffusion (29). These factors contribute to the therapeutic resistance and poor prognosis of PC (30). Numerous studies have focused on targeting components of the TMEs for PC therapy, including regulation of the pancreatic stellate cells, degradation of extracellular matrices and vessel embolization. Besides the above traditional approaches, cell-penetrating peptides and other TME-responsive biomaterials have also been investigated to enhance penetration into solid tumor tissue. Activation of anti-tumor immunity is an alternative potential approach (29).
To survive and thrive in TME with hypoxia and low nutrient levels, PC cells reprogramme their metabolism by shifting to non-canonical metabolic pathways and adapting metabolic scavenging pathways such as autophagy and macropinocytosis (31). Investigating this process could provide insights into its pathogenesis and ultimately new therapeutic interventions. The SGLTs are glucose transporters which transport glucose transmembrane utilizing sodium gradient. Among the isoforms, SGLT-2 is mainly expressed in the kidneys and takes major responsibility in glucose reabsorption (16). It has also been identified in human cancers. Scafoglio et al. reported upregulation of SGLT-2 in PC and prostate cancer (17). Their results suggested that SGLT-specific radioactive glucose analog, α-methyl-4-deoxy-4-([18]F) fluoro-D-glucopyranoside (Me4FDG) could be used to detect and stage PC and that SGLT-2 inhibitors could be applied in PC treatment. After this milestone study, the expression of SGLT-2 and the potential of SGLT-2 inhibitors have been revealed in many other malignancies including colon, breast, lung and hepatocellular carcinoma (18-21). More recently, SGLT-1 has been reported significantly overexpressed in pancreatic ductal adenocarcinoma and was an independent predictor for a better prognosis. In the present study, we explored the correlation between SGLT-2 expression and pathological features and prognosis; the possible underlying mechanism was also investigated. No relationship between SGLT-2 expression and PC risk factors, tumor location, or histology grade was identified. Scafoglio et al. also found that SGLT-2 inhibitors as an adjuvant therapy was more effective in larger tumors with limited glucose acquisition for the central area (17). In sight of this, we investigated the relationship between SGLT-2 expression and tumor size. Unexpectedly, there was no correlation detected between SGLT-2 expression and the pathologic T stage. A possible explanation was that SGLT-2 might not be equally distributed but preferably expressed in cells in the central area or those in charge of glucose acquisition. According to our analysis, SGLT-2 could not be used as predictor for lymph node involvement, metastasis, or prognosis (either for DFS or OS), indicating that it facilitated instead of dominated the tumorigenesis or progressing of PC.
Pathway enrichment revealed that high SGLT-2 expression pathways in tumors was related to chemical carcinogenesis. Meanwhile, nucleotide excision repair, mRNA surveillance, and ubiquitin-mediated proteolysis were inefficient, resulting impaired cell replication surveillance at the DNA, RNA, and protein levels. These insufficiencies might not have been the consequent results of abnormal SGLT-2 expression, but more likely coexisted in a subgroup of PC cells with instability.
A more plausible finding was that high SGLT-2 expression was correlated with enhanced cellular metabolism, especially glucose metabolism. It is well recognized that PC cells thrive in a more hostile living environment than most other tumors through metabolic reprogramming, including increased glucose uptake. Here we found that SLC5A2 expression positively correlated with the expressions of HNF1A, HNF1B, HNF4A, and PDX1, consistent with previous reports that HNF1A and HNF4A can transcriptionally regulate SLC5A2 expression (32,33). The above genes are key molecules of glucose metabolism and their genetic variation are an important molecular mechanism of T2DM, MODY, or neonatal diabetes mellitus (NDM). Specifically, HNF1A mutation leads to MODY3 (about 50% of the total), and such patients are characterized with decreased renal sugar threshold (32,34). About 10% of the total number of MODY cases are caused by HNF1B mutation. The patients often experience urogenital system malformation, pancreatic atrophy, and impaired exocrine function (34). Mutations in HNF4A and PDX1 [also known as insulin promoter factor 1 (IPF1)] cause MODY1 and MODY4, respectively (34). Further, PDX1 is also involved in permanent NDM [altered expression of the peroxisome proliferator-activated receptor gamma coactivator 1α (PGC-1α) and PDX1 and their methylation status are associated with fetal glucose metabolism in gestational diabetes mellitus] (35). These genes orchestrate in pancreatic development and glucose metabolism (36). Therefore, we hypothesize that their alterations participate in PC metabolic reprogramming partially by regulating SLC5A2 expression.
Besides energy supply, the potential synergistic effects between SGLT-2 inhibitors and chemotherapy comprise another rationale for its application to PC. In clinical practice, the treatment of PC includes surgery and chemotherapy. Specifically, for resectable PC, adjuvant chemotherapies with gemcitabine are arranged after surgery. For metastatic PC, FOLFIEINOX and gemcitabine plus nab-paclitaxel is recommended for patients with good tolerance and gemcitabine alone is advised for those with compromised performance status; common regimens include FOLFIRINOX, gemcitabine, and gemcitabine plus albumin in combination with paclitaxel. According to our data, SGLT-2 expression was correlated with drug-metabolizing enzymes including CYP2C8, CYP3A4, CYP3A5 (Figure 5), which participate the metabolism of drugs such as irinotecan, gemcitabine, and paclitaxel. For example, CYP2C8, CYP3A5, and CYP3A4 are responsible for taxol metabolism, and CYP3A4 is also involved in irinotecan metabolism. Moreover, Scafoglio et al. have previously reported that SGLT-2 inhibitors facilitate gemcitabine in a mouse PC model. The above findings provide rationales for combinations of SGLT-2 inhibitors and these chemotherapy, and further evaluation should be conducted (17).
Compared with GLUTs, which are also targets for cancer therapy, SGLT-2 is a more attractive target. First, unlike the wide distribution of GLUTs in healthy tissues, SGLT-2 is restrictively expressed in kidney tubules, increasing the selectivity and thus reducing the side effects. Second, unlike GLUTs facilitating glucose diffusion, SGLTs concentrate glucose into the cells from a comparatively hypoglycemic microenvironment, so the blocking of this procedure is theoretically more mortal for tumor cells. In addition, as novel oral antidiabetic drugs (OADs) are widely used in clinic, the safety of SGLT-2 inhibitors has been carefully evaluated.
It is well accepted that the incidence of cancer increases in type 2 diabetes mellitus (T2DM). Although the mechanism is not completely understood, hyperglycemia, hyperinsulinemia, chronic inflammation, and enhanced oxidative stress are considered attributors. Among the raft of malignancies, PC is one with a particularly higher relative risk (RR) (37). Meanwhile, compelling evidence indicates that PC induces DM. Possible hypotheses include destruction of the pancreas, β-cell disfunction, and insulin resistance (38). Although we cannot tell whether SGLT-2 inhibitors are helpful to prevent PC in DM patients, SGLT-2 inhibitors might be a preferable antidiabetic option for patients with both DM and PC. Anti-cancer therapy has made great advances in the past decade. Precision medicine enables us to identify patients with certain genetic alterations and select the more effective therapies. In the present study, high SGLT-2 expression was found to be closely related to pancreatic progenitor subtype of PC. A possible explanation is that this subtype is characterized by activation of numerous genes including HNF1A and HNF4A, which have been demonstrated to participate in the regulation of SGLT-2. This finding suggested that preselection of PC patients might further optimize the curative effect.
Conclusions
High SLC5A2 expression in PC was shown to coexist with impaired cell replication surveillance, enhanced cellular metabolism, and drug metabolism. High SLC5A2 expression was also closely associated with the pancreatic progenitor subtype of PC. There is potential for SGLT-2 as a target for PC treatment, and SGLT-2 inhibitors should be further evaluated as a novel therapy in PC.
Acknowledgments
Funding: This study was supported by the National Key R&D Program of China (No. 2018YFC1314800), the National Natural Science Foundation of China (No. 81802829), and the Natural Science Basic Research Plan in Shaanxi Province (No. 2020JQ-501).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-900/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-900/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136:E359-86. [Crossref] [PubMed]
- Rahib L, Smith BD, Aizenberg R, et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res 2014;74:2913-21. [Crossref] [PubMed]
- Oktay E, Levent M, Gelincik H, et al. Perspective of Turkish Medicine Students on Cancer, Cancer Treatments, Palliative Care, and Oncologists (ARES Study): a Study of the Palliative Care Working Committee of the Turkish Oncology Group (TOG). J Cancer Educ 2020;35:69-75. [Crossref] [PubMed]
- Perera RM, Bardeesy N. Pancreatic Cancer Metabolism: Breaking It Down to Build It Back Up. Cancer Discov 2015;5:1247-61. [Crossref] [PubMed]
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Efeyan A, Comb WC, Sabatini DM. Nutrient-sensing mechanisms and pathways. Nature 2015;517:302-10. [Crossref] [PubMed]
- Hardie DG. AMP-activated protein kinase: an energy sensor that regulates all aspects of cell function. Genes Dev 2011;25:1895-908. [Crossref] [PubMed]
- Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol 2011;12:21-35. [Crossref] [PubMed]
- DeBerardinis RJ, Thompson CB. Cellular metabolism and disease: what do metabolic outliers teach us? Cell 2012;148:1132-44. [Crossref] [PubMed]
- Halbrook CJ, Lyssiotis CA. Employing Metabolism to Improve the Diagnosis and Treatment of Pancreatic Cancer. Cancer Cell 2017;31:5-19. [Crossref] [PubMed]
- Ying H, Kimmelman AC, Lyssiotis CA, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell 2012;149:656-70. [Crossref] [PubMed]
- Shukla SK, Purohit V, Mehla K, et al. MUC1 and HIF-1alpha Signaling Crosstalk Induces Anabolic Glucose Metabolism to Impart Gemcitabine Resistance to Pancreatic Cancer. Cancer Cell 2017;32:71-87.e7. [Crossref] [PubMed]
- Wright EM, Turk E. The sodium/glucose cotransport family SLC5. Pflugers Arch 2004;447:510-8. [Crossref] [PubMed]
- Yamazaki Y, Harada S, Tokuyama S. Sodium-glucose transporter as a novel therapeutic target in disease. Eur J Pharmacol 2018;822:25-31. [Crossref] [PubMed]
- Turk E, Zabel B, Mundlos S, et al. Glucose/galactose malabsorption caused by a defect in the Na+/glucose cotransporter. Nature 1991;350:354-6. [Crossref] [PubMed]
- Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 2011;91:733-94. [Crossref] [PubMed]
- Scafoglio C, Hirayama BA, Kepe V, et al. Functional expression of sodium-glucose transporters in cancer. Proc Natl Acad Sci U S A 2015;112:E4111-9. [Crossref] [PubMed]
- Abdel-Rafei MK, Thabet NM, Rashed LA, et al. Canagliflozin, a SGLT-2 inhibitor, relieves ER stress, modulates autophagy and induces apoptosis in irradiated HepG2 cells: Signal transduction between PI3K/AKT/GSK-3β/mTOR and Wnt/β-catenin pathways; in vitro. J Cancer Res Ther 2021;17:1404-18. [Crossref] [PubMed]
- Yamamoto L, Yamashita S, Nomiyama T, et al. Sodium-glucose cotransporter 2 inhibitor canagliflozin attenuates lung cancer cell proliferation in vitro. Diabetol Int 2021;12:389-98. [Crossref] [PubMed]
- Zhou J, Zhu J, Yu SJ, et al. Sodium-glucose co-transporter-2 (SGLT-2) inhibition reduces glucose uptake to induce breast cancer cell growth arrest through AMPK/mTOR pathway. Biomed Pharmacother 2020;132:110821. [Crossref] [PubMed]
- Saito T, Okada S, Yamada E, et al. Effect of dapagliflozin on colon cancer cell Endocr J 2015;62:1133-7. [Rapid Communication]. [Crossref] [PubMed]
- Du J, Gu J, Deng J, et al. The expression and survival significance of sodium glucose transporters in pancreatic cancer. BMC Cancer 2022;22:116. [Crossref] [PubMed]
- Wright EM, Ghezzi C, Loo DDF. Novel and Unexpected Functions of SGLTs. Physiology (Bethesda) 2017;32:435-43. [Crossref] [PubMed]
- Tang Z, Li C, Kang B, et al. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res 2017;45:W98-W102. [Crossref] [PubMed]
- Dennis G Jr, Sherman BT, Hosack DA, et al. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 2003;4:3. [Crossref] [PubMed]
- Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov 2012;2:401-4. [Crossref] [PubMed]
- Vasaikar SV, Straub P, Wang J, et al. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Res 2018;46:D956-63. [Crossref] [PubMed]
- Bailey P, Chang DK, Nones K, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016;531:47-52. [Crossref] [PubMed]
- Huang X, Ding L, Liu X, et al. Regulation of tumor microenvironment for pancreatic cancer therapy. Biomaterials 2021;270:120680. [Crossref] [PubMed]
- Ho WJ, Jaffee EM, Zheng L. The tumour microenvironment in pancreatic cancer - clinical challenges and opportunities. Nat Rev Clin Oncol 2020;17:527-40. [Crossref] [PubMed]
- Encarnación-Rosado J, Kimmelman AC. Harnessing metabolic dependencies in pancreatic cancers. Nat Rev Gastroenterol Hepatol 2021;18:482-92. [Crossref] [PubMed]
- Zhao Y, Gao P, Sun F, et al. Sodium Intake Regulates Glucose Homeostasis through the PPARδ/Adiponectin-Mediated SGLT2 Pathway. Cell Metab 2016;23:699-711. [Crossref] [PubMed]
- Bonner C, Kerr-Conte J, Gmyr V, et al. Inhibition of the glucose transporter SGLT2 with dapagliflozin in pancreatic alpha cells triggers glucagon secretion. Nat Med 2015;21:512-7. [Crossref] [PubMed]
- Owen KR. Monogenic diabetes in adults: what are the new developments? Curr Opin Genet Dev 2018;50:103-10. [Crossref] [PubMed]
- Wang L, Fan H, Zhou L, et al. Altered expression of PGC-1α and PDX1 and their methylation status are associated with fetal glucose metabolism in gestational diabetes mellitus. Biochem Biophys Res Commun 2018;501:300-6. [Crossref] [PubMed]
- Bakhti M, Böttcher A, Lickert H. Modelling the endocrine pancreas in health and disease. Nat Rev Endocrinol 2019;15:155-71. [Crossref] [PubMed]
- Esposito K, Chiodini P, Colao A, et al. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care 2012;35:2402-11. [Crossref] [PubMed]
- Sah RP, Nagpal SJ, Mukhopadhyay D, et al. New insights into pancreatic cancer-induced paraneoplastic diabetes. Nat Rev Gastroenterol Hepatol 2013;10:423-33. [Crossref] [PubMed]


