
Fruquintinib for refractory colorectal cancer in a pre-treated 82-year-old patient achieved a progression-free survival of 25 months: a case report
Introduction
Colorectal cancer (CRC) is one of the most frequently occurring cancers worldwide (1). Chemotherapy is the primary treatment for advanced CRC (2). Advanced age, comorbidities, and poor performance status (PS) may lead to treatment discontinuation (3), which may affect patient prognosis. Elderly patients who are also in poor general condition often have difficulty tolerating standard therapy and require dose reduction or selection of less toxic drugs. Currently, only limited evidence is available to guide the treatment of elderly CRC patients.
Fruquintinib is a highly selective tyrosine kinase inhibitor targeting vascular endothelial growth factor receptors and was approved in China for patients with metastatic CRC who had failed at least 2 lines of treatment (4). In this article, we report the case of an 82-year-old patient with advanced refractory CRC who received fruquintinib as the 2nd-line therapy. To date, it is the oldest metastatic colorectal cancer (mCRC) patient treated with fruquintinib, and this case provides inspiration for refractory elderly CRC patients. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-841/rc).
Case presentation
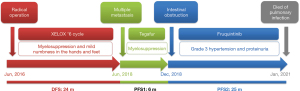
An 82-year-old man with a long history of hypertension was admitted to our hospital due to no defecation for 3 days in December 2018. He was diagnosed with descending colon cancer and underwent a radical operation in June 2016, followed by 6 cycles of XELOX chemotherapy. The postoperative pathological examination revealed stage IIIB cancer (pT4aN1cM0). The disease relapsed in June 2018, and the patient was prescribed Tegafur for 6 months. After front-line chemotherapy, the patient developed bone marrow suppression and mild numbness in the hands and feet.
The patient had a PS score of 3 and nutrition score (NRS2002) of 5. The patients’ carcino-embryonic antigen (CEA) and carbohydrate atigen 19-9 (CA 19-9) levels were 382.27 and 68.62 ng/mL, respectively. CT showed multiple metastatic lesions, including a giant mass (10 cm) in the pelvic cavity, suggesting disease progression (see Figure 1).
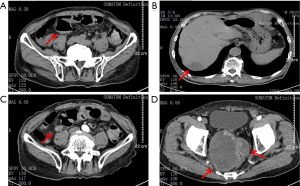
A multidisciplinary team (MDT), including a radiologist, a colorectal surgeon, a radiotherapist, a chemotherapist, a gastroenterologist, a nutritionist, and a geriatrician, was assembled. The results of the MDT meeting were as follows: (I) The intestinal obstruction was due to the external pressure from the giant metastatic lesion at the pelvic wall. Because of the patient’s advanced age and poor general condition, surgery was not considered. Fasting, fluid infusion, and enemas were applied to relieve the intestinal obstruction. (II) The patient had severe malnutrition and required 25–30 kcal/kg every day. Supplements of fish oil, ω-3, and glutamine were suggested. (III) Conservative treatment was suggested for the multiple metastases. Fruquintinib was to be applied as systemic anti-tumor therapy after the patient’s general condition had been stabilized. (IV) The patient had hypertension for 30 years and was taking amlodipine 5 mg qd. The blood pressure needed to be stabilized to prevent cardiovascular adverse events (AEs) during fruquintinib treatment.
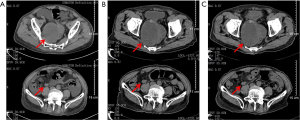
The intestinal obstruction was completely alleviated after symptomatic therapy, and the patient’s nutritional status improved. Fruquintinib treatment (5 mg/day, D1–21, q4w) was started in December 2018. Grade 3 hypertension and proteinuria occurred 10 and 15 days after starting the treatment, respectively, and thus fruquintinib was discontinued for 1 month. Irbesartan hydrochlorothiazide, metoprolol, and nifedipine tablets, and albumin infusion were used as the symptomatic treatment. Hypertension and proteinuria gradually resolved to grade 1, and fruquintinib treatment was then re-started, but the dose was reduced to 3 mg/day. The patient’s CEA levels decreased following fruquintinib administration (see Figure 2). The patient had no intestinal obstruction thereafter, and no disease progression was detected during the 25-month follow-up period (see Figure 3). However, the patient died of pulmonary infection in January 2021, which was considered a non-progression–related death. The whole treatment timeline figure is shown (see Figure 4).
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
Elderly patients have difficulties tolerating standard chemotherapy regimens. Thus, it is essential that new acceptable regimens with reliable efficacies for aged CRC patients are developed. In the present case, given the patient’s general condition and impaired tolerability, single-agent chemotherapy was chosen as the 1st-line treatment when the disease recurred the 1st time. At the time of the 2nd relapse, the patient had an advanced age (82 years) and a poor physical condition (a PS score of 3); thus, treatment was challenging. As per the guidelines for CRC of the Chinese Society of Clinical Oncology (2), an MDT was assembled to control the tumor progression and improve the patient’s quality of life by administering anti-tumor and symptomatic therapies. A previous study has shown fruquintinib is an effective, safe, and tolerable 3rd-line treatment for metastatic CRC (5). Thus, fruquintinib was chosen for this patient.
Grade 3 hypertension and proteinuria occurred during the treatment, and the dose reduction was given careful consideration. In the FRESCO study, the dose of fruquintinib was reduced in two 1 mg/day increments to clinically manage significant AEs (6). As the patient in this case had an underlying disease (i.e., hypertension) and severe malnutrition in addition to an advanced age, safety was the primary concern during the anti-tumor treatment. Thus, the re-start dose of fruquintinib was reduced to 3 mg/day. Notably, dose reduction requires individualized consideration, and in most cases, a primary decrease to 4 mg/day is recommended.
The most common fruquintinib-related AEs include hypertension, hand-foot-skin reaction, and proteinuria (7). Hypertension is a common AE in anti-angiogenic therapy, which is associated with a better prognosis. In metastatic CRC patients treated with bevacizumab, drug-related hypertension may be a predictor of improved survival (8,9). As the pathogenesis of angiogenesis inhibitor-related hypertension includes increased microvascular permeability, decreased endothelial renewal capacity, and the decreased production of vasodilators (10), grade 3 hypertension may suggest a favourable prognosis. The patient in the present study achieved a PFS of 25 months. Thus, while fruquintinib is not yet a standard therapy recommended for CRC, this case provides further evidence of its anti-tumor activity and safety as reported previously (6).
An 82-year-old patient with chemo-refractory CRC who received fruquintinib as the 2nd-line treatment achieved a PFS of 25 months. Typically, CRC patients with advanced age and poor general condition require MDT meetings and individualized treatment plans, and fruquintinib is effective and tolerable in these patients.
Acknowledgments
Funding: This study was supported by Ningbo Medical Key Discipline Oncology (No. 2016019), Zhejiang Provincial Medicine and Health Project (No. 2022KY1135), and Hangzhou Medical College Undergraduate Teaching Reform Innovation Project (No. FYJG202219).
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-841/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-841/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer Statistics, 2021. CA Cancer J Clin 2021;71:7-33. [Crossref] [PubMed]
- Diagnosis And Treatment Guidelines For Colorectal Cancer Working Group CSOCOC. Chinese Society of Clinical Oncology (CSCO) diagnosis and treatment guidelines for colorectal cancer 2018 (English version). Chin J Cancer Res 2019;31:117-34. [Crossref] [PubMed]
- Hoeben KW, van Steenbergen LN, van de Wouw AJ, et al. Treatment and complications in elderly stage III colon cancer patients in the Netherlands. Ann Oncol 2013;24:974-9. [Crossref] [PubMed]
- Chen Z, Jiang L. The clinical application of fruquintinib on colorectal cancer. Expert Rev Clin Pharmacol 2019;12:713-21. [Crossref] [PubMed]
- Zhang Y, Zou JY, Wang Z, et al. Fruquintinib: a novel antivascular endothelial growth factor receptor tyrosine kinase inhibitor for the treatment of metastatic colorectal cancer. Cancer Manag Res 2019;11:7787-803. [Crossref] [PubMed]
- Li J, Qin S, Xu RH, et al. Effect of Fruquintinib vs Placebo on Overall Survival in Patients With Previously Treated Metastatic Colorectal Cancer: The FRESCO Randomized Clinical Trial. JAMA 2018;319:2486-96. [Crossref] [PubMed]
- Li J, Guo W, Bai Y, et al. Safety Profile and Adverse Events of Special Interest for Fruquintinib in Chinese Patients with Previously Treated Metastatic Colorectal Cancer: Analysis of the Phase 3 FRESCO Trial. Adv Ther 2020;37:4585-98. [Crossref] [PubMed]
- De Stefano A, Carlomagno C, Pepe S, et al. Bevacizumab-related arterial hypertension as a predictive marker in metastatic colorectal cancer patients. Cancer Chemother Pharmacol 2011;68:1207-13. [Crossref] [PubMed]
- Tahover E, Uziely B, Salah A, et al. Hypertension as a predictive biomarker in bevacizumab treatment for colorectal cancer patients. Med Oncol 2013;30:327. [Crossref] [PubMed]
- Ranpura V, Pulipati B, Chu D, et al. Increased risk of high-grade hypertension with bevacizumab in cancer patients: a meta-analysis. Am J Hypertens 2010;23:460-8. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)



