
A randomized controlled trial of positive end-expiratory pressure on pulmonary oxygenation and biventricular function in esophageal cancer patients receiving one-lung ventilation under a lower FiO2
Introduction
One lung ventilation (OLV) offers an operable and mobile minimally surgical field during thoracic surgery. However, lung atelectasis during OLV leads to a mismatched ventilation/perfusion ratio (V/Q) and threatens hypoxemia (1-4). Although high fraction of inspiration O2 (FiO2) (1.0) improved oxygenation (5), the following higher levels of inflammation remarkably raised acute respiratory distress syndrome (ARDS) risk, leading to increased postoperative mortality (6,7). Several reports have shown the independent risk factors for ARDS are high FiO2 and airway pressure, as well as prolonged OLV (6-8), and maximizing the use of minimum FiO2 under adequate oxygenation is the current recommended strategy for protective ventilation (3).
Our previous animal study has shown low FiO2 (0.6) could produce less inflammation, oxidative stress, and lung injury than FiO2 (1.0) after OLV [positive end-expiratory pressure (PEEP) =0 cmH2O, VT =10 mL/kg] (8). However, 30% (3/10) of animals developed hypoxemia and required elevated FiO2. We further studied the effect of low FiO2 (0.6) in humans under a protective strategy (PEEP =5 cmH2O, VT =6 mL/kg) and all patients had lower oxidative stress and complications after OLV and only 6.7% (2/30) developed hypoxemia (9). Recently, Ferrando et al. showed an “individualized” PEEP level measurement of approximately 10 cmH2O during OLV improved pulmonary oxygenation (10). However, whether the increased PEEP could improve pulmonary oxygenation and decrease hypoxemia under low FiO2 (0.6) remains unknown. This research aimed to explore whether increased PEEP could improve pulmonary oxygenation and decrease hypoxemia under low FiO2 (0.6) during OLV. The secondary objective was to study whether increased PEEP could induce lower inflammatory and lung injury under low FiO2 (0.6).
Given the PEEP decrement trial results of Ferrando et al., PEEP (8 cmH2O) and PEEP (10 cmH2O) were used as higher levels of PEEP in this research. The baseline level of pulmonary oxygenation was defined by the patients with protective strategy (FiO2 =0.6, PEEP =5 cmH2O). Previous studies have shown that IL-6 and IL-10 were associated with lung complications after OLV (11,12), and their serum levels were also assessed. This study aimed to explore the safety and lung protective effect of this ventilation mode in patients with OLV, so as to seek an optimal and suitable lung protective ventilation mode for patients, and to open up a new design idea for lung protective ventilation strategies during OLV, which has important scientific theoretical value and broad clinical application prospects. We present the following article in accordance with the CONSORT reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-522/rc).
Methods
Patients
This study enrolled patients with esophageal cancer (EC) who were scheduled to undergo elective open radical surgery in The Affiliated Cancer Hospital of Nanjing Medical University. EC was diagnosed under clinical data, laboratory index, gastroscopy and pathology. Patients were excluded if they met the following criteria: (I) pulse oxygen saturation (SpO2) <90% during this trial; (II) 20% reduction in blood pressure; (III) severe intraoperative arrhythmia and hemodynamic instability; (IV) operation time more than 6 hours or less than 1 hour; (V) patients with immune, endocrine, neurological, or blood vessel disease; (VI) patients with liver and kidney dysfunction, glaucoma, or mental illness. Patients were randomly divided into four parallel groups on a ratio of 1:1:1:1 using a computer-generated list: Group A (FiO2 =0.6, PEEP =0 cm, n=30), Group B (FiO2 =0.6, PEEP =5 cm, n=30), Group C (FiO2 =0.6, PEEP =8 cm, n=30), and Group D (FiO2 =0.6, PEEP =10 cm, n=30). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Nanjing Medical University (No. 2017_550). All patients were enrolled after obtaining written informed consent.
Anesthesia and intervention
All patients received left-sided double-lumen intratracheal intubation under total intravenous anesthesia, with intramuscular injection of phenobarbital 0.1 g and atropine 0.5 mg 30 minutes before admission. After entering the operating room, a central venous catheter was placed in the right internal jugular vein and midazolam 0.05 mg·kg-1, fentanyl 3–4 µg·kg-1, propofol 1 mg·kg-1, and cis-atracurium 0.2 mg·kg-1 were induced with sequential intravenous injections. The left-sided double-lumen intratracheal tube was placed and confirmed by fiberoptic bronchoscope. Ventilation parameters in this trial were tidal volume (VT) =6–8 mL/ideal body weight, respiratory rate (RR) =12–14 beats/min, inspiratory to expiratory ratio =1:2, ETCO2 =35–45 mmHg. Theses parameters remained constant, and the non-ventilated lung was directly connected to indoor air during OLV. FiO2 was 0.6 in the four groups. Remifentanil, propofol, and cis-atracurium were continuously pumped intravenously to maintain anesthesia, and the depth was monitored to maintain BIS between 40 and 60 intraoperatively.
VT was 6 mL/IBW, RR was 12–14 beats/min, and respiratory parameters were adjusted to maintain SpO2 >90% and end-expiratory carbon dioxide partial pressure (PCO2) at 35–45 mmHg. At the beginning of chest closure, 2 µg/kg fentanyl was intravenously injected, and two-lung ventilation (TLV) was restored by manual lung swelling (airway pressure was limited to less than 30 cmH2O, lung swelling duration was 30–40 s). At the end of the operation, all patients were admitted to the ICU for synchronized intermittent mandatory ventilation. When the patients were awake, the double-lumen bronchial catheter was extubated. Postoperative analgesia was controlled intravenous analgesia.
Primary endpoints
An arterial catheter was introduced into the radial artery, and a central vein line (two lumens 20 cm long) was placed through the right internal jugular vein into the right atrium and its location confirmed by chest X-ray. Before OLV (T1), OLV 10 minutes (T2), OLV 15 minutes (T3), OLV 30 minutes (T4), OLV 60 minutes (T5), and OLV 120 minutes (T6) for blood gas analyses, artery and venous blood samples were collected. At the same time, peak airway pressure (Ppeak), mean arterial pressure (MAP), heart rate (HR), and airway pressure (Paw) were documented.
The calculation formulas of the shunt fraction were as follows:
Qs/Qt = (CcO2 − CaO2)/(CcO2 − CvO2) (11,12);
CaO2 = (1.36 × hemoglobin × SaO2) + (0.0031 × PaO2);
CvO2 = (1.36 × hemoglobin × SvO2) + (0.0031 × PvO2);
CcO2 = ((FiO2 × (PB-PH2O) − PaCO2/Respiratory quotient) × 0.0031) + 1.36 × hemoglobin;
PB, 760 mmHg; PH2O, 47 mmHg; respiratory quotient, 0.8.
Dynamic compliance was calculated as VT/(Ppeak − PEEP).
Venous blood samples were collected at T1, T5, TLV 30 minutes (T7), and 24 h postoperatively (T8). The serum samples of all patients were centrifugated at 3,000 rpm for 20 min, then stored at −80 ℃ for biochemical evaluation. Il-6 and IL-10 concentrations were measured using human IL-6 and IL-10 ELISA kits, respectively, as described in the instructions.
Secondary endpoints
The hospital stays, clinical pulmonary infection score (CPIS) and complications of patients as the secondary endpoints were assessed in this trial.
Statistical analysis
Data analysis was performed by SPSS 20.0 software (IBM Corporation, Armonk, NY, USA). Continuous data with normal distribution were performed using ANOVA and Kolmogorov-Smirnov analyses with mean ± standard deviation (mean ± SD). Continuous data with skewed distribution were analyzed by Kruskal-Wallis and Fisher's precision probability tests, and presented as median and quartile [M (Q1, Q3)]. Categorical data were expressed by the numbers and proportions [n (%)], and the chi-square test was utilized to compare differences between groups. Two-sided P<0.05 was considered statistically significant.
Results
A total of 120 EC patients were enrolled between June 1, 2015 and June 30, 2018. All patients were followed up until discharge. The participant flow showed in Figure 1. In Group A, hypoxemia occurred in two patients, requiring FiO2 enhancement during OLV. The two patients were excluded. In Group D, hypotension occurred in two patients (blood pressure dropped from 152/80 to 98/64 mmHg, and from 130/80 to 85/50 mmHg) requiring reduction of PEEP. The two patients were excluded. Then the data of 116 patients (Group A =28, Group B =30 Group C =30, and Group D =28) were assessed for final analysis. Clinical characteristics of 118 patients in the four groups are shown in Table 1. While the bleeding volumes of patients in Group D were significantly higher than others, no significant differences were observed in age, gender, BMI, drinking, smoking, HB, infusion volume, urination volume, ASA grade, TNM grade, lung function, OLV, and operation time.
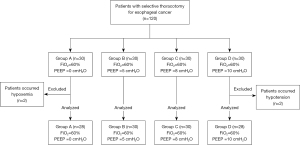
Table 1
| Variables | Group A (n=28) | Group B (n=30) | Group C (n=30) | Group D (n=28) | P |
|---|---|---|---|---|---|
| Age (years), mean ± SD | 63.10±5.985 | 63.90±5.720 | 63.33±6.493 | 64.37±4.694 | 0.829 |
| Male, n (%) | 11 (39.2) | 12 (40.0) | 17 (56.7) | 13 (43.3) | 0.502 |
| BMI (kg/m2), mean ± SD | 23.83±6.223 | 23.69±3.513 | 23.67±3.409 | 23.33±2.315 | 0.927 |
| Smoking index, M (Q1, Q3) | 600 (500, 1,100) | 600 (375, 600) | 600 (375, 850) | 460 (400, 600) | 0.362 |
| Smoking cessation, n (%) | 14 (50.0) | 16 (53.3) | 12 (40.0) | 18 (64.2) | 0.342 |
| Drinking, n (%) | 14 (50.0) | 15 (50.0) | 13 (43.3) | 19 (63.3) | 0.429 |
| OLV duration (min), mean ± SD | 172.5±44.3 | 170.8±58.0 | 162.8±42.5 | 178.7±47.8 | 0.212 |
| Surgery duration (min), mean ± SD | 205.6±52.8 | 194.2±51.6 | 193±45.2 | 213.3±43.1 | 0.230 |
| FEV1 >80%, n (%) | 28 (100.0) | 29 (96.7) | 29 (96.57) | 26 (92.9) | 0.602 |
| Infusion volume (mL), mean ± SD | 1,982±356 | 2,224±709 | 1,946±208 | 1,922±417 | 0.465 |
| Bleeding volume (mL), mean ± SD | 183.3±58 | 162.1±40.0 | 169.6±73.7 | 236.2±153.4 | 0.011 |
| Urine output (mL), mean ± SD | 230.7±83.7 | 248.6±132.6 | 232.1±178.6 | 226.6±88 | 0.818 |
| Hb (g/L), mean ± SD | 12.2±1.5 | 12.0±1.4 | 11.6±1.6 | 12.5±1.4 | 0.245 |
| ASA, n | 0.950 | ||||
| II | 27 | 29 | 28 | 26 | |
| III | 1 | 1 | 2 | 2 | |
| TNM stage, n | 0.957 | ||||
| T1N0M0/T2N0M0/T1N1M0 | 2/14/10 | 1/15/11 | 0/16/12 | 1/14/12 | |
| T2N1M0/T2N2M0/T3N0M0 | 2/0/0 | 3/0/0 | 2/0/0 | 1/0/0 |
BMI, body mass index; OLV, one-lung ventilation; FEV1, forced expiratory volume in first second; Hb, hemoglobin; ASA, American Standards Association; TNM, tumor, node, metastasis.
Within the first 30 minutes after OLV initiation, PaO2 decreased (Figure 2A), while Qs/Qt increased (Figure 2B), with PaO2 and Qs/Qt reaching their nadir. Group D had significantly higher PaO2 and lower Qs/Qt than Group B from OLV 15 minutes to OLV 60 minutes. Similarly, the dynamic compliance in Group D were significantly higher than Group B between 15 and 60 minutes during OLV (Figure 3A). During the whole OLV, there were no differences in PaO2 and Qs/Qt between Groups B and C. PaO2 were significantly higher and Qs/Qt in group B were lower than those in Group A at OLV 30 minutes. No significant difference was found in SaO2, PvO2, ETCO2, and PaCO2 among the four groups at each point (Table 2).
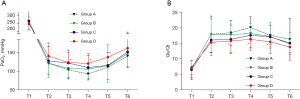
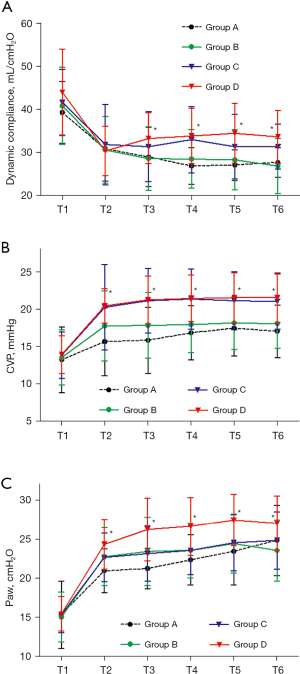
Table 2
| Variables | Group | T1 | T2 | T3 | T4 | T5 | T6 |
|---|---|---|---|---|---|---|---|
| SaO2* (mmHg), mean ± SD | A | 99.9±0.3 | 98.1±2.0 | 97.5±2.0 | 96.3±2.1 | 97.7±1.7 | 98.7±1.2 |
| B | 99.9±0.2 | 97.5±2.2 | 97.6±1.9 | 96.9±2.1 | 96.9±2.5 | 98.6±1.4 | |
| C | 99.9±0.2 | 98.2±1.8 | 97.8±2.1 | 97.0±2.1 | 97.2±2.2 | 98.8±1.4 | |
| D | 99.8±0.3 | 98.1±1.8 | 97.6±1.7 | 96.9±2.1 | 97.8±1.8 | 99.0±1.0 | |
| PvO2 (mmHg), mean ± SD | A | 43.0±2.9 | 37.4±3.5 | 36.3±3.8 | 35.3±3.0 | 35.4±4.3 | 35.0±2.7 |
| B | 43.5±3.7 | 37.0±2.5 | 35.8±3.2 | 34.9±3.9 | 35.8±5.0 | 35.2±2.0 | |
| C | 43.7±2.5 | 36.3±4.1 | 34.6±2.9 | 34.1±3.2 | 34.6±2.9 | 36.1±3.2 | |
| D | 43.3±2.1 | 34.8±3.8 | 35.0±4.2 | 33.9±2.6 | 36.0±3.9 | 38.3±3.7 | |
| PaCO2 (mmHg), mean ± SD | A | 42.5±4.3 | 41.3±6.1 | 39.5±3.7 | 41.3±5.8 | 40.6±6.3 | 39.8±7.1 |
| B | 43.4±4.8 | 43.3±5.4 | 41.4±5.9 | 41.7±6.8 | 43.0±4.4 | 42.4±6.6 | |
| C | 43.6±6.1 | 42.3±11.4 | 39.7±3.6 | 39.3±4.2 | 42.5±4.3 | 39.7±3.8 | |
| D | 44.7±5.6 | 40.3±6.1 | 41.2±6.0 | 40.5±6.2 | 42.5±4.0 | 40.4±7.6 | |
| ETCO2 (mmHg), mean ± SD | A | 35.6±5.3 | 36.6±4.2 | 36.0±4.1 | 36.0±3.8 | 35.2±3.3 | 35.3±3.3 |
| B | 35.0±3.5 | 38.4±3.8 | 37.7±4.1 | 37.5±4.5 | 37.0±4.9 | 37.0±4.5 | |
| C | 35.4±3.9 | 38.3±5.8 | 37.9±5.8 | 37.0±6.9 | 37.0±4.7 | 36.9±4.7 | |
| D | 34.1±4.0 | 36.3±2.9 | 36.4±3.1 | 35.4±3.4 | 35.6±3.7 | 36.4±4.1 | |
| HR (beats/min), mean ± SD | A | 74.2±12.5 | 75.0±12.1 | 77.3±9.6 | 76.8±10.8 | 73.6±12.8 | 72.5±18.8 |
| B | 76.7±11.8 | 77.8±11.3 | 78.2±11.6 | 78.3±12.9 | 74.3±11.0 | 69.2±10.6 | |
| C | 74.7±9.9 | 76.3±9.1 | 78.3±9.3 | 77.2±11.7 | 73.0±10.8 | 67.6±9.7 | |
| D | 74.3±13.0 | 75.5±13.6 | 77.1±15.5 | 73.9±11.9 | 70.1±11.4 | 68.3±10.1 | |
| MAP (mmHg), mean ± SD# | A | 97.8±11.8 | 98.3±13.1 | 13.2±2.4 | 98.3±11.0 | 101.6±94.3 | 98.8±12.4 |
| B | 99.8±13.9 | 102.2±12.7 | 12.3±2.3 | 101.5±14.3 | 97.7±12.5 | 96.1±12.4 | |
| C | 97.3±14.9 | 104.5±10.2 | 98.9±11.9 | 103.5±12.6 | 98.9±13.2 | 93.7±10.8 | |
| D | 95.8±14.2 | 105.4±14.9 | 100.6±13.7 | 99.4±14.0 | 101.0±13.0 | 97.8±11.6 |
T1, preoperative; T2, OLV 10 min; T3, OLV 15 min; T4, OLV 30 min; T5, OLV 60 min; T6, OLV 120 min; *, the SaO2 in patients with hypoxemia was significantly lower than patients in other groups; #, the MAP in patients with hypotension in Group D was significantly lower than patients in other groups. SaO2, oxygen saturation; SD, standard deviation; PvO2, partial pressure of oxygen in venous blood; PaCO2, partial pressure of carbon dioxide in artery; ETCO2, partial pressure of end-tidal carbon dioxide; HR, heart rate; MAP, mean arterial pressure; OLV, one-lung ventilation.
In the first 10 minutes, CVP and Paw increased after OLV initiation then remained stable during OLV (Figure 3B,3C). Compared with Group A and B during OLV, the levels of CVP in Groups C and D were higher, while the Paw level in Group D was significantly higher than in the other three groups.
During OLV, the levels of IL-6 and IL-10 slowly increased and there were no differences between the four groups at 60 minutes (Figure 4). After restarting TLV, the levels of IL-6 and IL-10 rapidly increased, and 30 minutes after restart and 24 h postoperatively, the levels of IL-6 and IL-10 in Group A and B were higher and lower, respectively, compared with those in Groups C and D (Figure 4). After surgery, there were more patients with CPIS over 6 in Group A (P<0.001). While there were no significantly differences in the hospital stay and complications among these four groups (P>0.05), complications in Groups A and D were more serious (Table 3).

Table 3
| Variables | Group A (n=28) | Group B (n=30) | Group C (n=30) | Group D (n=28) | P |
|---|---|---|---|---|---|
| Hospital stays (d), M (Q1, Q3) | 14 (13, 17) | 13 (13, 14) | 14 (13, 14) | 14 (13, 18) | 0.135 |
| CPIS*, n | <0.001 | ||||
| ≥6 | 9 | 0 | 1 | 2 | |
| 0–6 | 17 | 30 | 29 | 26 | |
| Complications, n | 3 | 7 | 4 | 6# | 0.228 |
| Empyema | 1 | ||||
| Incision infection | 2 | 4 | 2 | 1 | |
| Hydrothorax | 1 | 2 | |||
| Pneumonia | 1 | 1 | 2 | 1 | |
| Deliration | 1 | ||||
| Hypoxemia | 1 | 1¶ |
*, Group B, C, and D were combined when Fish test was performed; #, another two complications: urinary tract bleeding and chylothorax; ¶, the patient was transferred to a superior hospital due to refractory hypoxemia; M (Q1, Q3), median and quartile; n, number; CPIS, clinical pulmonary infection score.
Discussion
Although a study previously published showed a higher PEEP could reduce pulmonary shunt and increase PaO2 during OLV (10), it remains unclear whether higher PEEP can increase oxygenation at low FiO2 during OLV. Our study showed a higher PEEP (10 cmH2O) could increase the levels of PaO2 and decrease those of Qs/Qt during the first 60 minutes of OLV. In Group D, two patients were excluded due to hypotension, and the levels of CVP and Paw also increased in the higher PEEP group during OLV. More importantly, the levels of IL-6 and IL-10 increased in higher PEEP patients, and although there were no significant differences in complications, those in patients with 10 cmH2O PEEP were more serious.
Hypoxic pulmonary vasoconstriction (HPV) refers to the reflex contraction of vascular smooth muscle due to low regional partial pressure of oxygen (PO2) in the pulmonary circulation (13). It has two distinct phases (14-19), with phase 1 beginning in a matter of seconds and reaching its lowest point at 15 minutes. When moderate hypoxia (PO2 30 to 50 mmHg) lasts for over 30 to 60 minutes, HPV begins to enter phase 2 and PVR is increased further, reaching its peak at 2 hours. Therefore, PaO2 reaches its lowest level 20 to 30 minutes after the onset of OLV, then gradually increases during the following 1 to 2 hours. In our study, a higher PEEP (10 cmH2O) increased levels of PaO2 and decreased levels of Qs/Qt between 15 minutes and 60 minutes during OLV. As shown in Figure 3A, the higher degree of dynamic compliance partially overcame the mismatched V/Q ratio, leading to the increase in PaO2. Therefore, patients can benefit from a higher PEEP (10 cmH2O) to pass the hypoxic period within the first 30 minutes of OLV.
A higher PEEP led to haemodynamics and respiratory mechanics changes. First, two patients in Group D who were excluded developed hypotension and their blood pressures returned after reducing PEEP to 5 cmH2O. Second, the remaining patients had higher CVP and Paw during OLV. Kim et al. showed that the more the biventricular diastolic function decreased, the more PEEP increased (20), suggesting this may be the main reason for haemodynamics changes. Third, the bleeding volume in Group D was higher than other groups, and haemodynamics changes may be the reason for higher bleeding during surgery.
As previously shown (21), we did not find significant differences in the levels of inflammation during OLV. However, after OLV, inflammation levels were higher in Group D than Group B. Previous studies showed hyperoxia during OLV increased the levels of inflammation and oxidative stress, leading to a higher risk of complications after surgery (21,22). Our previous research studied the benefits of lower FiO2 in lowering inflammation, oxidative stress, and lung injury (8,9). Although higher PEEP increased oxygenation, the levels of inflammation also increased. Given the higher levels of inflammation and more serious complications in Group D, we believe the small sample size led to the similar rates of complications in the four groups.
There are several limitations in this trial that require caution in interpreting our findings. Firstly, further subgroup analyses based on age or gender could not be performed owing to sample size limitations. Secondly, there is no significant deterioration of pulmonary function in the patients. Whether the PEEP titration strategy is valuable for critically ill patients still needs further explore. Future studies with well-design, large sample size should confirm our findings.
Conclusions
The findings show a higher PEEP increased oxygenation under 60% O2 during OLV. However, the haemodynamics and respiratory mechanics changed, and the levels of inflammation increased. Given the effect of higher FiO2 under protective strategy, a higher PEEP under 60% O2 during OLV is not recommended.
Acknowledgments
Funding: This work was in part supported by grants from the talents program of Jiangsu Cancer Hospital (No.YC201805). The funding body did not play a role in the design of the study or collection, analysis, or interpretation of data, or writing the manuscript.
Footnote
Reporting Checklist: The authors have completed the CONSORT reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-522/rc
Trial Protocol: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-522/tp
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-522/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-522/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Nanjing Medical University (No. 2017_550). All participants were enrolled after written informed consent was obtained.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Templeton TW, Miller SA, Lee LK, et al. Hypoxemia in young children undergoing one-lung ventilation: A retrospective cohort study. Anesthesiology 2021;135:842-53. [Crossref] [PubMed]
- Yoon S, Kim BR, Min SH, et al. Repeated intermittent hypoxic stimuli to operative lung reduce hypoxemia during subsequent one-lung ventilation for thoracoscopic surgery: A randomized controlled trial. PLoS One 2021;16:e0249880. [Crossref] [PubMed]
- Brassard CL, Lohser J, Donati F, et al. Step-by-step clinical management of one-lung ventilation: continuing professional development. Can J Anaesth 2014;61:1103-21. [Crossref] [PubMed]
- Karzai W, Schwarzkopf K. Hypoxemia during one-lung ventilation: prediction, prevention, and treatment. Anesthesiology 2009;110:1402-11. [Crossref] [PubMed]
- Lytle FT, Brown DR. Appropriate ventilatory settings for thoracic surgery: intraoperative and postoperative. Semin Cardiothorac Vasc Anesth 2008;12:97-108. [Crossref] [PubMed]
- García-de-la-Asunción J, García-del-Olmo E, Perez-Griera J, et al. Oxidative lung injury correlates with one-lung ventilation time during pulmonary lobectomy: a study of exhaled breath condensate and blood. Eur J Cardiothorac Surg 2015;48:e37-44. [Crossref] [PubMed]
- Miskovic A, Lumb AB. Postoperative pulmonary complications. Br J Anaesth 2017;118:317-34. [Crossref] [PubMed]
- Xu Z, Gu L, Bian Q, et al. Oxygenation, inflammatory response and lung injury during one lung ventilation in rabbits using inspired oxygen fraction of 0.6 vs. 1.0. J Biomed Res 2016;31:56-64. [PubMed]
- Li P, Gu L, Bian Q, et al. Effects of prostaglandin E1 nebulization of ventilated lung under 60%O2 one lung ventilation on patients' oxygenation and oxidative stress: a randomised controlled trial. Respir Res 2020;21:113. [Crossref] [PubMed]
- Ferrando C, Mugarra A, Gutierrez A, et al. Setting individualized positive end-expiratory pressure level with a positive end-expiratory pressure decrement trial after a recruitment maneuver improves oxygenation and lung mechanics during one-lung ventilation. Anesth Analg 2014;118:657-65. [Crossref] [PubMed]
- Kim HJ, Seo JH, Park KU, et al. Effect of combining a recruitment maneuver with protective ventilation on inflammatory responses in video-assisted thoracoscopic lobectomy: a randomized controlled trial. Surg Endosc 2019;33:1403-11. [Crossref] [PubMed]
- van der Woude MC, Bormans L, van der Horst RP, et al. Pulmonary levels of biomarkers for inflammation and lung injury in protective versus conventional one-lung ventilation for oesophagectomy: A randomised clinical trial. Eur J Anaesthesiol 2020;37:1040-9. [Crossref] [PubMed]
- Lumb AB, Slinger P. Hypoxic pulmonary vasoconstriction: physiology and anesthetic implications. Anesthesiology 2015;122:932-46. [Crossref] [PubMed]
- Zeng C, Lagier D, Lee JW, et al. Perioperative pulmonary atelectasis: Part I. biology and mechanisms. Anesthesiology 2022;136:181-205. [Crossref] [PubMed]
- Dunham-Snary KJ, Wu D, Sykes EA, et al. Hypoxic Pulmonary Vasoconstriction: From Molecular Mechanisms to Medicine. Chest 2017;151:181-92. [Crossref] [PubMed]
- Fabo C, Oszlanyi A, Lantos J, et al. Non-intubated Thoracoscopic Surgery-Tips and Tricks From Anesthesiological Aspects: A Mini Review. Front Surg 2022;8:818456. [Crossref] [PubMed]
- Boehme S, Hartmann EK, Tripp T, et al. PO2 oscillations induce lung injury and inflammation. Crit Care 2019;23:102. [Crossref] [PubMed]
- Lohser J, Slinger P. Lung injury after one-lung ventilation: A review of the pathophysiologic mechanisms affecting the ventilated and the collapsed lung. Anesth Analg 2015;121:302-18. [Crossref] [PubMed]
- Burrowes KS, Clark AR, Wilsher ML, et al. Hypoxic pulmonary vasoconstriction as a contributor to response in acute pulmonary embolism. Ann Biomed Eng 2014;42:1631-43. [Crossref] [PubMed]
- Kim N, Lee SH, Choi KW, et al. Effects of positive end-expiratory pressure on pulmonary Ooxygenation and biventricular function during one-lung ventilation: A randomized crossover study. J Clin Med 2019;8:740. [Crossref]
- Olivant Fisher A, Husain K, Wolfson MR, et al. Hyperoxia during one lung ventilation: inflammatory and oxidative responses. Pediatr Pulmonol 2012;47:979-86. [Crossref] [PubMed]
- Theroux MC, Fisher AO, Horner LM, et al. Protective ventilation to reduce inflammatory injury from one lung ventilation in a piglet model. Paediatr Anaesth 2010;20:356-64. [Crossref] [PubMed]
(English Language Editor: B. Draper)


