
Progression of the tumor in a patient with a gastrointestinal stromal tumor with the PDGFRA exon 12 mutation who received multiple surgeries and multiple lines of tyrosine kinase inhibitor therapies: a case report
Introduction
The use of tyrosine kinase inhibitors (TKIs) in the treatment of gastrointestinal stromal tumors (GISTs) is the classic example in the field of solid tumor targeted therapy (1). Since imatinib was first used to treat GIST in the early 2000s, understandings of the mutation types of the GIST pathogenic genes, KIT and platelet-derived growth factor receptor alpha (PDGFRA) have deepened, and new TKIs, such as sunitinib and regorafenib, have been successively applied to the treatment of GIST (2-4). However, not all GIST patients with gene mutations are sensitive to the TKIs recommended in the current guidelines (5). For patients with rare types of GIST, such as the PDGFRA exon 12 mutation, there is insufficient clinical evidence to determine whether TKIs significantly improve their prognosis (6). In particular, the efficacy of ripretinib in patients with PDGFRA exon 12 mutation has not yet been reported. We report the case of a PDGFRA exon 12-mutated small intestinal GIST patient who received multiple surgeries and multiple lines of TKI therapy. Additionally, the tumor of this patient also had pathological characteristics, such as regional lymph node metastasis, visible vascular tumor thrombus, and Succinate dehydrogenase (SDHB) was negative. We hope that our report will provide insights into the diagnosis and treatment of such patients with GISTs in the future. We present the following article in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-791/rc).
Case presentation
We report the case of a 42-year-old female, who underwent an appendectomy 23 years ago, and a cesarean section 18 years ago, and had no special personal history, marriage history, or family history. In March 2020, the patient was admitted to a local hospital, complaining of suffering from intermittent abdominal cramps for >1 month, which had worsened over the last 2 days. Abdominal computed tomography (CT) showed small intestinal type intussusception on the left side of the mid-abdominal midline small intestine (see Figure 1A). Thus, the patient underwent an emergency exploratory laparotomy under general anesthesia.
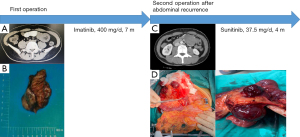
During the operation, a tumor was found in the proximal and middle segments of the small intestine, which was approximately 4 cm × 3 cm × 2 cm in size and tough in texture (see Figure 1B). No other abnormal signs, such as ascites or intraperitoneal metastasis, were observed. Next, a resection of the tumor in the small intestine and the surrounding small intestine was performed.
The patient’s postoperative pathology results were as follows: an epithelioid GIST [60 mitoses/50 high power field (HPF) in the active area], invading the deep myometrium, with intravascular tumor thrombus and no nerve infiltration. The immunohistochemical staining results were as follows: pan-cytokeratin (CKpan)(−), Vimentin(+), cluster of differentiation (CD)117(+), CD34(−), delay of germination 1 (DOG-1)(−), smooth muscle actin (SMA)(−), S100(−), Desmin(−), chromogranin A (CgA)(−), synaptophysin (Syn)(−), CD56(+), somatostatin receptor 2 (SSTR2)(−), P53(scattered +), and a Ki-67 positive rate of about 30%. No tumor was observed at the resection margin, but tumor metastasis was observed in the regional lymph nodes (1/2).
The patient recovered smoothly after the operation and was admitted to our hospital for tumor gene detection. The results identified the PDGFRA 12 exon c.1741C>T (p.P581S) mutation. According to the Chinese Society of Clinical Oncology (CSCO) guidelines (7), the patient’s GIST belonged to the high-risk grade and there was a high risk of recurrence, metastasis and tumor-related mortality. Thus, in April 2020, we started to treat the patient with oral imatinib (400 mg/day). The patient had no adverse drug reactions during the drug treatment, and in June 2020, the abdominal CT examination results of the patient showed no abnormality.
In October 2020, the patient developed symptoms of dull pain in the left lower abdomen. In November 2020, a CT scan of the abdomen showed a small amount of fluid in the pelvic cavity, a mass of soft tissue shadow in the left upper abdominal cavity of the umbilicus (about 43 mm × 43 mm × 39 mm in size), which was adjacent to the aforementioned anastomosis observed in the small intestine surgery (see Figure 1C). The possibility of recurrence of GIST was considered, and metastasis of the GIST was not excluded. A positron emission tomography/CT examination was performed, and the results showed that after the GIST surgery, there was no definite sign of recurrence in the anastomotic area, and there was a hypermetabolic soft tissue mass adjacent to the left abdomen. Thus, the possibility of lymph node metastasis was considered. The patient was admitted to our hospital and diagnosed as follows: (I) postoperative recurrence of GIST; (II) postoperative appendicitis; and (III) postoperative cesarean section.
The results of the physical examination were as follows: fullness on palpation of the left upper abdomen; no other positive signs. In November 2020, the patient underwent an exploratory laparotomy under general anesthesia. During the operation, a tumor about 8 cm × 7 cm × 6 cm in size, located on the small intestinal mesentery below the original jejunal-jejunal anastomosis, was found (see Figure 1D). The small intestinal mesentery tumor and part of the small intestine were resected. The patient recovered smoothly after the operation.
Based on the postoperative pathology results, a diagnosis of GIST metastasis was considered. The mitotic figures were >50 mitoses/50 HPF, and no tumor was found in the paraintestinal, middle and mesenteric root lymph nodes (0/34). The immunohistochemistry results were as follows: AE1/AE3(−), Vimentin(+), Syn(+/−), CgA(−), CD56(+), neuron specific enolase (NSE)(+/−), P63(−), CK5/6(−), Wilms’ tumour 1 (WT1)(−), Calretinin(−), CD99(+), Ki-67 (70% positive cells), epithelial membrane antigen (EMA)(−), Bcl-2(+/−), Desmin(−), CD34(−), S100(−), Fli-1 proto-oncogene, ETS transcription factor (FLI-1)(+), CD117(+/−), DOG-1(−), and SDHB(−). The tumor gene detection results once again revealed the PDGFRA 12 exon c.1741C>T (p.P581s) mutation.
Given that the tumor was resistant to imatinib, the patient was given oral sunitinib (37.5 mg/day) after the surgery. The patient remained in a good condition, and no adverse drug reactions occurred. An abdominal CT scan in December 2020 showed no obvious abnormalities. An abdominal magnetic resonance imaging re-examination in April 2021 showed an abnormal signal in the right anterior lobe of the liver, which was new compared to the December 2020 results, and the possibility of hemangioma was considered high (see Figure 2A). Additionally, an enlarged lymph node was observed on the left side of the abdominal aorta (see Figure 2B), as was a small amount of pelvic effusion (see Figure 2C).
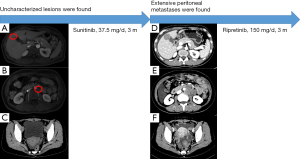
The patient’s abdominal CT in July 2021 revealed multiple soft tissue nodules and masses in the abdomen and pelvis, which were new compared to the April 2021 results, and metastasis was considered. We also noted that the low-density lesion in the right anterior lobe of liver was larger than before, and metastasis was considered (see Figure 2D). Additionally, multiple swollen lymph nodes were observed around the abdominal aorta, and metastasis was considered (see Figure 2E), and abdominal pelvic effusion (see Figure 2F) was also observed. Given the type of gene mutation of the patient’s tumor, the likelihood of the patient benefiting from the 3rd-line TKI regorafenib was small, so the patient was treated with oral ripretinib (150 mg/day). In September 2021, the patient continues to take the medicine and is in a good condition but has a slight abdominal distension. She will be reviewed as scheduled.
All the procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Discussion
It is well known that the causative factors of GIST at the genetic level are usually KIT or PDGFRA mutations (8). The KIT exon 11 mutation is the most common among all the primary mutated GIST cases, and accounts for about 60–70% of such cases, followed by the KIT exon 9 mutation, which accounts for about 10% of such cases, and the KIT exon 13 and 17 mutations both of which account for about 1% of such cases (9). The most common PDGFRA mutation is the exon 18 mutation, which accounts for about 6% of such cases, and the PDGFRA exon 12 and 14 mutations both of which account for 1% of such cases (9). The genotype of the remaining cases is the wild type (9). In relation to the secondary mutation cases, the KIT exon 13 mutation accounts for approximately 56% of such cases, followed by the KIT exon 17 mutation, which accounts for approximately 41% of such cases, and the PDGFRA exon 18 mutation, which accounts for approximately 3% of such cases (10).
The current clinical evidence for GIST treatment suggests that imatinib is mainly sensitive to GIST in patients with the KIT exon 11 mutations and is recommended as a 1st-line drug (11). Conversely, sunitinib is mainly sensitive to GIST in patients with the KIT exon 13 and PDGFRA exon 14 mutations and is recommended as a 2nd-line drug (12), while regorafenib is mainly sensitive to most GIST in patients with the KIT exon 17 and PDGFRA exon 18 mutations, and is recommended as a 3rd-line drug (13).
In our case, there was a high possibility of tumor recurrence and metastasis, and the patient had a poor prognosis in terms of mitoses, vascular tumor thrombus, and regional lymph node metastasis. The 2 postoperative tumor recurrences and rapid disease progression also confirmed the patient’s high-risk tumor classification. This patient had a tumor with the PDGFRA exon 12 mutation, which is an extremely rare mutation. We could not control the recurrence and progression of the patient’s tumor after successively applying 2 TKIs (i.e., imatinib and sunitinib, see Figure 3).
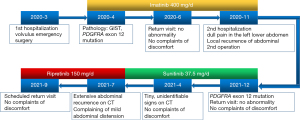
In a completed phase I clinical trial study examining the selection of 3rd-line drugs for patients (14), ripretinib was superior to its counterparts, sunitinib and regorafenib, in improving the objective response rate and progression-free survival in the 2nd and 3rd-line treatments of patients with GISTs. In an ongoing phase III clinical trial (15) comparing ripretinib to sunitinib, which is currently recommended as the 2nd-line treatment for GIST, which demonstrated the high interest of experts in the unique mechanism by which ripretinib inhibits not only the tyrosine protein kinase activation ring but also its binding to the switch pocket (16). Given the rapid growth of relapsed lesions in patients, if regorafenib is selected for treatment and if the patient’s condition continues to progress, there is likely to be no other chance to change the treatment drug. Thus, after the patient developed extensive intra-abdominal recurrence and metastasis, we prescribed ripretinib, which is different from previous drug treatments for patients with the PDGFRA exon 12-mutant GIST.
There were some deficiencies in reviewing the diagnosis and treatment process of our case. For example, after the 2nd postoperative recurrence, a pathological biopsy and genetic testing of the new lesions were not performed. Additionally, the interval between the follow-up and review of the patient was too long, especially in April 2021 when the CT scan revealed a new microscopic mass that was difficult to characterize, but the patient was not reexamined until some 3 months later, and extensive abdominal metastases was already present at the re-examination in July 2021.
In conclusion, we reported a case of a patient with PDGFRA exon 12-mutated small intestinal GIST who underwent multiple surgeries and multiple lines of TKIs. The conventional TKIs of imatinib and sunitinib were not effective in treating this patient’s tumor, which suggests that ripretinib treatment should be considered as soon as possible in treating PDGFRA exon 12-mutated GISTs in the future. Additionally, if a patient’s tumor has a high risk of adverse biological behaviors, such as high mitotic figures, vascular tumor thrombus, SDHB(−), and regional lymph node metastasis, the postoperative follow-up and review should be close and strict, and the time interval should be 2 months or even 1 month. One of the metastases in the patient’s 2nd postoperative recurrence was also considered to have developed from the para-aortic lymph nodes (see Figure 2E), which suggests that consideration should be given to expanding the dissection of the regional lymph nodes during reoperation to the para-aortic lymph nodes.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-791/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-791/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the procedures performed in this study were conducted in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Helsinki Declaration (as revised in 2013). Written informed consent was obtained from the patient for the publication of this case report and the accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sborov D, Chen JL. Targeted therapy in sarcomas other than GIST tumors. J Surg Oncol 2015;111:632-40. [Crossref] [PubMed]
- Zhao Q, Zhang C, Qi C, et al. Preclinical model-based evaluation of Imatinib resistance induced by KIT mutations and its overcoming strategies in gastrointestinal stromal tumor (GIST). Am J Transl Res 2021;13:13608-24. [PubMed]
- Den Hollander D, Van der Graaf WTA, Desar IME, et al. Predictive factors for toxicity and survival of second-line sunitinib in advanced gastrointestinal stromal tumours (GIST). Acta Oncol 2019;58:1648-54. [Crossref] [PubMed]
- Tamoschus D, Draexler K, Chang J, et al. Cost-Effectiveness Analysis of Regorafenib for Gastrointestinal Stromal Tumour (GIST) in Germany. Clin Drug Investig 2017;37:525-33. [Crossref] [PubMed]
- Aickara DJ, McBride J, Morrison B, et al. Multidrug resistant gastrointestinal stromal tumor with multiple metastases to the skin and subcutaneous soft tissue: A case report and review of literature. J Cutan Pathol 2020;47:398-401. [Crossref] [PubMed]
- Brohl AS, Demicco EG, Mourtzikos K, et al. Response to sunitinib of a gastrointestinal stromal tumor with a rare exon 12 PDGFRA mutation. Clin Sarcoma Res 2015;5:21. [Crossref] [PubMed]
- Li J, Ye Y, Wang J, et al. Chinese consensus guidelines for diagnosis and management of gastrointestinal stromal tumor. Chin J Cancer Res 2017;29:281-93. [Crossref] [PubMed]
- Nannini M, Urbini M, Astolfi A, et al. The progressive fragmentation of the KIT/PDGFRA wild-type (WT) gastrointestinal stromal tumors (GIST). J Transl Med 2017;15:113. [Crossref] [PubMed]
- Shen YY, Li XQ, Yang LX, et al. Clinicopathological features and prognosis of gastrointestinal stromal tumors with KIT/PDGFRA gene "homozygous mutation": a multicenter retrospective cohort study. Zhonghua Wei Chang Wai Ke Za Zhi 2021;24:804-13. [PubMed]
- Grunewald S, Klug LR, Mühlenberg T, et al. Resistance to Avapritinib in PDGFRA-Driven GIST Is Caused by Secondary Mutations in the PDGFRA Kinase Domain. Cancer Discov 2021;11:108-25. [Crossref] [PubMed]
- Incorvaia L, Fanale D, Vincenzi B, et al. Type and Gene Location of KIT Mutations Predict Progression-Free Survival to First-Line Imatinib in Gastrointestinal Stromal Tumors: A Look into the Exon. Cancers (Basel) 2021;13:993. [Crossref] [PubMed]
- Tan S, Chen P, Ji J, et al. Genomic Subtypes of GISTs for Stratifying Patient Response to Sunitinib following Imatinib Resistance: A Pooled Analysis and Systematic Review. Dis Markers 2018;2018:1368617. [Crossref] [PubMed]
- Grellety T, Kind M, Coindre JM, et al. Clinical activity of regorafenib in PDGFRA-mutated gastrointestinal stromal tumor. Future Sci OA 2015;1:FSO33. [Crossref] [PubMed]
- Janku F, Abdul Razak AR, Chi P, et al. Switch Control Inhibition of KIT and PDGFRA in Patients With Advanced Gastrointestinal Stromal Tumor: A Phase I Study of Ripretinib. J Clin Oncol 2020;38:3294-303. [Crossref] [PubMed]
- Smith BD, Kaufman MD, Lu WP, et al. Ripretinib (DCC-2618) Is a Switch Control Kinase Inhibitor of a Broad Spectrum of Oncogenic and Drug-Resistant KIT and PDGFRA Variants. Cancer Cell 2019;35:738-751.e9. [Crossref] [PubMed]
- Meng Y, Li LL, Wang H, et al. Ripretinib in the treatment of advanced gastrointestinal stromal tumor with metastases in liver, lung and bone: a case report. Zhonghua Wei Chang Wai Ke Za Zhi 2021;24:823-4. [PubMed]
(English Language Editor: L. Huleatt)


