
Inflammatory bowel disease-associated malignancies and considerations for radiation impacting bowel: a scoping review
Introduction
Inflammatory bowel disease (IBD) is a complex auto-inflammatory condition of the gastrointestinal tract subdivided into Crohn’s disease (CD) and ulcerative colitis (UC). Symptoms include diarrhea, constipation, hematochezia, nausea, vomiting, and weight loss; ultimately resulting in inflamed segments of bowel, ulceration, fistulation, and fibrosis/stricture. IBD is marked by fluctuating episodes of flare and remission, usually reflecting elevations and declines in systemic and local inflammatory markers. It is speculated that this prolonged state of inflammation may result in an increased rate of malignancy seen in patients with IBD (1).
Compared to sporadic cancers in the general population, IBD-associated malignancies present unique challenges to providing quality care. The presence of preexisting diseased and inflamed tissue elicits concerns when selecting therapeutic options. For the Radiation Oncology team, it is critical to understand the risk and presentation of IBD-associated malignancy, the potential for flares in the peri-radiation period, the heightened concern for treatment toxicities, and the expected oncologic outcomes related to IBD-associated malignancy.
Historically, patients with IBD are less likely to receive radiation therapy (RT) due to concerns for increased rates of toxicities and flares (1-6). National Comprehensive Cancer Network (NCCN) guidelines rate active inflammatory disease of the rectum as an absolute contraindication to external beam radiation therapy (EBRT) for prostate cancer (PCa) and inactive UC as a relative contraindication (4). Many have extended this guideline to additional sites and modalities in practice, thus avoiding use of RT in patients with IBD. However, updated analysis has suggested that IBD should not be considered a contraindication for radiation, even in systems neighboring potentially impacted bowel (7-13). Modern improvements in RT conformality, along with documented similar acute and late toxicity rates compared to patients without IBD, suggest that RT could be considered as a treatment option in IBD-associated malignancies (8,9,14-16). Cumulative rates of acute grade ≥3 toxicities across all cancers in patients with IBD are 14–27%, similar to the general population rates (9,14,17-19). The cumulative rates of late grade ≥3 toxicities across all cancers in patients with IBD are 0–29%, also comparable to rates in the general population (9,14,17-19). Case reports of patients with IBD treated with pelvic RT are abundant, highly suggesting that despite guidelines and historical opinion, patients with IBD may, in fact, receive RT as part of their care and determined by their care teams.
This scoping review is organized to describe six categories of malignancy (colorectal carcinoma, anal carcinoma, lymphoma, small bowel adenocarcinoma, prostate cancer, and cancers with no proven association) that have a proposed relationship with IBD (Figure 1) and in which treatment must take into consideration the proximity to nearby bowel impacted by IBD, with a special emphasis on any modifications to the standard treatment planning. It is followed by a review of IBD management considerations during RT and a discussion of the findings. This review assesses the available experimental and non-experimental literature available on the topic, including practice recommendations and available guidelines. We present the following article in accordance with the PRISMA-ScR reporting checklist (20) (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-138/rc).

Methods
A systematic, scoping search of published literature was conducted using applicable PRISMA ScR guidelines (20). The literature search was conducted on PubMed and was searched systematically by screening all publications from January 1990 to June 2021. Citations from the included articles were also manually searched. Relevant National Comprehensive Cancer Network guidelines were reviewed. Information regarding keywords is summarized in Figure 2. Final query was December 2021 in editing. Articles were selected for full text reading if the abstract reported on malignancy in IBD or bowel toxicities. Full English text of the relevant studies was retrieved for further selection.

Inclusion/exclusion criteria
Relevant clinical studies, meta-analyses, case reports, and reviews were included. Reviews were included to better reflect the reasoning and recommendations in the literature that guide decision making in providing RT for patients with IBD. Articles including only familial malignancies, or malignancies with no concern for bowel toxicities during treatment were excluded. The inclusion of case reports and studies with low power reflects the limited availability of data and may introduce a potential for bias in the published literature.
An example of a search conducted as part of this review is as follows: PubMed, date range January 1990 to June 2021, language English, search term “IBD Anal Cancer”, yields 88 titles. After scanning titles for relevance, 21 abstracts were read. Seven articles were excluded based on discussion of IBD-associated anal cancers, not only IBD or sporadic anal cancers. Fourteen full text articles were read in full, and 7 citations were found that specifically addressed IBD-associated anal cancers pathogenesis, prognosis, and treatment, and were ultimately included in this review.
IBD-associated malignancies with proximity to bowel
Colorectal carcinoma (CRC)
CRC is a well-established malignancy associated with IBD, but is not frequently treated with RT. Neoplastic and CRC risk is increased with a diagnosis of colitis involving at least 1/3 of the colon and a duration of disease greater than 7–10 years (21,22). The risk is greater in individuals diagnosed with IBD at a young age (23,24), those with primary sclerosing cholangitis (PSC) (25), and those with fistulizing CD (21,26-28). CRC presents at a mean age 7.7 years younger in patients with IBD than in sporadic cases (29), but with a similar 5-year survival rate (30-32). Historically, patients with IBD present with high grade tumors and 20–24% are mucinous tumors; however, updated studies have shown a 50–60% prevalence of low-grade CRC at diagnosis (32,33).
IBD-associated CRC exhibits unique patterns of development and progression. Whereas sporadic CRC typically develops from a dysplastic precursor adenoma, IBD-associated CRC develops from precursors that can be polypoid, flat, localized, or multifocal (34). The lesions tend to develop in areas of histologic or gross inflammation (35). Reepithelization of ulcerated or damaged tissue with genomically unstable clones can create large or multifocal fields of high neoplastic potential. IBD-associated CRC has a distinct pattern of molecular and genetic changes, acquiring early p53 mutations (36,37) and having hypermethylation of CpG islands preceding dysplasia (38). Based on this aggressive progression, surveillance guidelines have been developed specifically for the IBD population, including yearly colonoscopy in high-risk patients (39). It is important for oncology providers to be aware of these heightened guidelines for their patients with IBD, and to coordinate with their gastroenterologist during follow-up.
Surgical management remains the mainstay of treatment for IBD-associated colon cancer, with adjuvant chemotherapy based on staging, with little established role for RT. In sporadic colon cancer, RT is typically reserved for locally advanced and recurrent disease. Given the high rate of synchronous tumors and poor differentiation, a total proctocolectomy (TPC) with ileal-pouch anal anastomosis (IPAA) is recommended for patients with IBD, in contrast to a segmental approach often utilized in sporadic cases (29). However, TPC with IPAA has significant implications for mortality and quality of life. IBD adversely affects surgical outcomes, with higher rates of complications like wound infections, deep vein thrombosis, postoperative hemorrhage, and poor wound healing (40). Moreover, the surgical removal of the colon does not cure CD, and patients may continue to experience inflammatory symptoms in the small bowel and suffer heightened nutritional and electrolyte disturbances postoperatively.
Sporadic rectal cancers, in contrast to non-rectal colon cancers, are frequently treated with preoperative RT and concurrent chemotherapy; however, IBD-associated adenocarcinomas occur less frequently in the rectum (OR 0.827) (41). In cases where RT is utilized for IBD-associated rectal cancer, dosing is limited by small bowel tolerance. A total of 0%, 7.7%, and 28.6% of patients experienced acute grade ≥3 toxicity for short-course radiation therapy, long-course radiation therapy, and chemoradiotherapy respectively (42). This measure of toxicity was assessed retrospectively in 66 patients in the Dutch pathology registry, and while significant, likely warrants further investigation in a larger population. While hospitalization rates for patients with IBD and rectal cancer during RT compares to those with sporadic rectal cancer, their need for RT breaks and anti-diarrhea medicines is not significantly higher (43), suggesting the concern and close monitoring of IBD patients may lower the threshold to recommend hospitalization. There is some suggestion that malignancies in the rectum are associated with higher rates of IBD flare in the peri-radiation period compared to other sites in the bowel (44). When treating the colon, it may be helpful to treat the patient in the prone position to better spare uninvolved bowel. For rectal cancers, a meta-analysis of eight articles and 127 patients supports that utilizing proton beam therapy compared to photon-beam RT significantly reduced the dose to nearby small bowel (45).
Anal carcinoma
Anal carcinomas are uncommon in the general population and are modestly increased in the IBD population (2). It is more common in women with IBD, with a median age of onset younger than the general population at 42 years (2). In patients with CD, 85% have a history of peri-anal disease and 80% of patients present 10 years or more from IBD diagnosis. Anal carcinomas can be difficult to diagnose in CD, complicated by underlying sepsis, fistulae or fissures, and may present at more advanced stages than the general population (46,47).
The pathogenesis of IBD-associated anal cancers may be multifactorial, including chronic inflammation, human papilloma virus (HPV) infection, decreased defensin production, and drug-induced immunosuppression from IBD therapies as shown in Figure 3 (48). While higher rates of HPV infection are seen in squamous cell carcinoma (SCC) patients with IBD, the relationship is not well understood (49). CD patients are more likely to have abnormal anal cytology regardless of HPV status (49). Defensins, which have been shown to suppress HPV, are lower in CD, leading some to suggest this as a possible explanation for elevated rates of anal SCC (48). The general population has additional risk factors for the development of anal carcinoma, including a history of human immunodeficiency virus (HIV), sexually transmitted infections (STIs), and smoking.
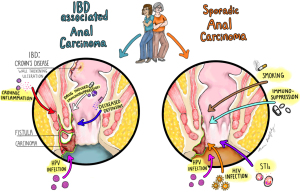
Further complicating the clinical picture, anal cancers in IBD can arise from the native anal tissue, or from fistulas, strictures, and other diseased lesions. Active perianal disease involvement, particularly the presence of fistulas, predominates the documented cases of IBD-associated anal SCC (50). It is theorized that the fistulous tract creates an ideal environment for HPV driven dysplasia (2,46). Adenocarcinomas of fistula tracts arise from migrated columnar rectal tissue, and therefore are more histologically similar to rectal tissue than to glandular tissue (51).
This complex clinical picture is compounded by poor outcomes for IBD-associated anal carcinomas. While sporadic anal carcinomas are usually treated with chemoradiation, 73% of the 33 IBD-anal SCC patients in a systematic review received radical surgery as first line treatment (2). Given the overall low occurrence rate of anal SCC, it is not surprising that even systematic reviews have a small population, however, this creates limitations in the power of the data which could be useful in uncovering subtle differences in outcome. Overall survival at 5 years is just 37% in IBD-associated cases and median disease-free survival is 4 years (2). The current literature has ambiguous suggestions as to why IBD patients undergo surgical management at such a high rate- citing potential increased morbidity of radiotherapy in IBD patients, inability to follow response, potential reduced anal function, and preceding sepsis (46). However, these concerns are not backed by data, and appear to be mostly speculative. Although the data is limited, it is suggested that there were improved outcomes in IBD patients who received multimodal treatment-frequently abdominoperineal resection (APR) with chemoradiation (46). Therefore, despite a history of avoiding RT in these patients, we recommend a multimodal approach to treating IBD-associated anal carcinoma, including the utilization of RT. The advantage of utilizing RT includes treating lymph nodes that may not be dissected with APR and starting with chemoradiation may reduce the need for surgery or allow for more conservative techniques.
Lymphoma
While lymphomas do not exclusively occur in proximity to bowel, the presence of lymphoma in structures adjacent to the bowel elicits concerns for RT planning in patients with IBD. Our choice to include lymphomas in this review also reflects the historic belief that IBD may increase the risk of lymphoma, as in rheumatoid arthritis (RA) and lupus. However, large population-based studies did not reveal a significant relationship between IBD and lymphoma (52-55). Nonetheless, many of the medications used in IBD management have an association with lymphoma development, resulting in a higher incidence of lymphoma in the IBD population.
Medications used for IBD therapy with suspected increased lymphoma risk include corticosteroids (56-60), methotrexate (54), thiopurine analogues (61-63), and some tumor necrosis factor (TNF)-alpha inhibitors [infliximab (60,64), adalimumab (60,65)]. Certolizumab pegol has not been linked with an increased risk of lymphoma (60,66). Notably, there is a particular concern for an increased risk of aggressive and often fatal hepatosplenic T-cell lymphoma (HSTCL) with combination biologic and immunomodulator therapy, however its occurrence has been documented with azathioprine monotherapy as well (52,67,68). It is particularly associated with anti-TNF therapy in combination with immunomodulators (67).
Immunosuppressive therapies and biologics have increased lymphoma risk for the IBD patient and may necessitate therapy changes in the face of a new diagnosis of malignancy. Stopping immunosuppressive therapies during treatment may be advisable to minimize cancer progression but may present a significant opportunity for IBD flare activity. As this could ultimately necessitate breaks and delays in RT and chemotherapy, the decision to halt immunosuppressive therapy must be made with caution, and alternate therapies should be considered in their absence. Discussion among oncology and gastroenterology providers is pivotal to managing patients optimally.
As radiation doses for lymphomas have decreased, it is generally safer with fewer toxicities, and should not require significant alterations in planning for patients with IBD.
Small bowel adenocarcinoma (SBA)
SBA is a rare cancer with greatly increased risk in CD patients, usually presenting in advanced stages (69-74). This same correlation has not been established for patients with UC (75,76). As patients with CD can experience extended periods of inflammation in the small bowel, a possible mechanism of pathogenesis has been proposed as inflammation leading to dysplasia and subsequent adenocarcinoma (48,77,78). Studies have revealed the presence of K-RAS, APC, and MMR genetic mutations in SBA, paralleling CRC (48,79-81). Markers that have been specifically indicated in CD patients with SBA include P53, K-Ras, and signs of microsatellite instability (48,78,82). There is considerable disagreement on the prognosis of CD-associated SBA, largely owing to poor follow up and a lack of matched controls (48,83-86).
For this rare but threatening cancer, standard of treatment includes laparoscopic removal of identified malignancies (48,87). Adjuvant chemotherapy to prevent disease recurrence is usually employed in the setting of lymph node involvement; however, studies have not shown an improvement in disease free or overall survival (48,88). Given the uncommon nature of SBA, and the infrequency of RT use in these cases, no specific guidelines exist for patients with IBD.
Given its poor prognosis despite surgical and medical intervention, it is reasonable to suggest that increased surveillance and more aggressive treatment may be advisable for patients with IBD. Heightened screening guidelines for CRC have been effective in reducing the stage of diagnosis in IBD patients, as covered earlier in this review, provoking the question of screening for SBA in the future as well. In cases of IBD patients with unresectable duodenal SBA, use of intensity-modulated radiotherapy (IMRT) and strict adjacent small bowel limits of 45 Gy are cautiously advisable.
Prostate cancer (PCa)
There is a significantly increased risk of PCa in men with IBD, with a relative risk of approximately 1.81 (89-92). A large cohort study found that the incidence of PCa in men with IBD at 10 years was 4.4% compared to 0.65% in controls (93). A retrospective study found that men with IBD did not differ in age at diagnosis but were diagnosed with a lower Gleason score (3). Pathological analysis of PCa samples from men with IBD showed a greater incidence of B and T cell infiltration into the tumor compared to samples from men without IBD (94). Studies of chemically induced intestinal inflammation in mice mirrored this finding, and further demonstrated increased DNA damage and pro-oncogenic signaling (94). Inflammation may impact have an early impact on the prostate, resulting in a poor predictive value of traditional diagnostic testing in patient with IBD (95). Additionally, some theorize that the link between IBD and PCa may be the introduction of microorganisms to the prostate creating a pro-oncogenic local microbiome (96).
While IBD patients may present with less advanced PCa, historically their cancer management has been more invasive. 40% of patients with IBD and PCa undergo surgical management compared to just 27% of their peers without IBD, and patients with IBD were significantly less likely to receive radiation therapy (3). Use of androgen deprivation therapy (ADT) was similar between groups (3). This represents a hesitation to give radiotherapy to patients with IBD due to a perceived increased risk of complications like IBD flares or bowel toxicities like radiation proctitis and rectal bleeding, reflected in the NCCN PCa treatment guidelines rating active inflammatory disease of the rectum as an absolute contraindication to EBRT and inactive UC as a relative contraindication (4-6).
Although the data are limited, studies of conventional EBRT in patients with IBD and PCa revealed increased rates of both acute and late ≥3 bowel toxicities (6,97). In a retrospective study of 28 patients with IBD and PCa, 21% experienced toxicities severe enough to warrant cessation of radiotherapy (6). Of the patients who experienced those toxicities, 75% had received conventional EBRT. A systematic review of 8 studies revealed severe acute bowel toxicity rates of 20% in patients with IBD who underwent EBRT, and severe late bowel toxicity rates of 15% (97). Strong conclusions on the safety of EBRT in patients with IBD are limited by the lack of published, well-powered studies.
However, the same review showed improved toxicity rates in those who received low dose rate (LDR) brachytherapy, with severe bowel toxicities rates of 7% and 5% for acute and late respectively (97). Similarly, a study of 24 patients with IBD and PCa who underwent LDR brachytherapy found no grade ≥2 rectal toxicities with a median follow up time of 48.5 months (98). A study of 11 patients with IBD and PCa who underwent high dose rate (HDR) brachytherapy revealed no grade ≥2 bowel toxicities with a median follow up time of 6 months (99). The culmination of these studies suggests brachytherapy is well tolerated in IBD patients. That being said, biopsies in the recently radiated field, and the placement of LDR implants in patients with active IBD flare are associated with higher rates of toxicities, suggesting HDR may be safer for patients with IBD, and that biopsies to the rectum after brachytherapy should be limited (100).
Fortunately, use of newer RT approaches which minimize radiation to the nearby bowel and rectum, particularly IMRT, may not have significant increases in either IBD flares or radiation toxicities (7,12,13,101). For patients with IBD with PCa, IMRT produces lower rates of acute grade ≥2 toxicities than 3D-CRT, indicating that precision techniques may be particularly valuable in the approach to the IBD patient (13). In one study of 18 patients with IBD and PCa, all cases of grade 2 proctitis followed treatment with three-dimensional conformal radiation therapy (3DCRT), with no cases following IMRT or LDR brachytherapy (101). Remarkably, this study also showed a decrease in grade 1 diarrhea pre-radiation to post-radiation, suggesting that radiation may have alleviated some symptoms of IBD. Ultimately, there is a growing number of articles which suggest that radiation is a viable treatment option for patients with IBD and PCa (7-10,12-14,101).
Moreover, recent publications reported reduced risk of rectal toxicities in patients with IBD with placement of rectal spacers to displace the anterior rectal wall out of the field of radiation (102-105). In a case report, the use of hydrogel spacer in a patient with IBD and PCa treated with IMRT resulted in reduced rectal dose from 78.7 to 60.4 Gy, without and with the hydrogel spacer, respectively, resulting in Grade 1 GI toxicities of mild diarrhea. (102) One center treating 8 patients with IMRT and HDR brachytherapy boost with an injectable hydrogel spacer, with minimal acute toxicities and no late grade ≥2 gastrointestinal toxicities in the median 36 month follow up period (105). Similarly, the toxicity profile with the use of biodegradable rectal balloon implants inflated with saline is favorable and can be utilized in both the EBRT and brachytherapy setting (103,104). However, in cases which require radiation to the pelvic lymph nodes, radiation to the bowel is more difficult to avoid.
Overall, patients with IBD have an increased risk of PCa, but with lower Gleason scores at diagnosis. Patients with IBD have increased lymphocytic infiltrate into the prostate, suggesting that chronic inflammation plays a significant role in the early changes and eventual neoplasia of the prostate. While contested, there is data that suggests that conventional EBRT results in higher toxicity rates in IBD patients (6,97). However, there is reasonable evidence to suggest that brachytherapy and IMRT are reasonable management options for PCa patients with a history of IBD. At our own institution, HDR brachytherapy is preferred to IMRT in patients with IBD to avoid toxicities associated with treating the pelvis. There is growing support for the use of implantable spacers to reduce rectal toxicities. These recommendations are summarized in Table 1. It is important to have multidisciplinary discussions with surgical and medical oncologists to determine if another mode of treatment would be better tolerated or more in line with the patient’s treatment goals and present the patient with all of the reasonable options, including RT, which may be suitable to treat their PCa.
Table 1
| Malignancy | Management in sporadic cases | Management recommendations in IBD-associated cases |
|---|---|---|
| Colon adenocarcinoma | Segmental resection, concurrent chemotherapy, RT to 45 Gy + boost (106) | TPC with IPAA, concurrent chemotherapy, use of prone positioning and conformational RT |
| Rectal adenocarcinoma | Early stage: surgery + EBRT + chemotherapy | Preferred proton therapy over EBRT (45) |
| Late stage: preoperative RT + 5-FU, definitive surgical resection, adjuvant chemotherapy (106) | ||
| Anal carcinoma | Mitomycin/5-FU + EBRT (50–59 Gy, depending on stage) | Utilize multimodality care when possible, including surgery and radiation with or without chemotherapy (46) |
| Fill bladder and utilize prone position to minimize small bowel dose (107) | ||
| Lymphoma | Depends on subtype: | Limit use of immunosuppressive IBD therapies during treatment |
| Radiation alone for: follicular lymphoma I–II, extranodal MALT IE-IIE, nodal marginal zone lymphoma I–II | ||
| Combined modality (chemotherapy and IFRT) for: classical HL I–II, advanced HL, diffuse large B-cell lymphoma I–II, peripheral T-cell lymphoma I–II (108) | ||
| Small bowel adenocarcinoma | Wide resection and lymph node dissection + chemotherapy | Preferred IMRT for unresectable cases |
| Duodenal: preoperative chemoradiation for unresectable cases (3D conformational or IMRT) | ||
| Jejunal/ileum: RT not indicated (109) | ||
| Prostate cancer | Low risk: similar outcomes for radical prostatectomy, EBRT, or brachytherapy | HDR brachytherapy is most preferred, limit biopsies |
| Intermediate risk: dose escalation above 70 Gy with short-term ADT | IMRT is preferred to 3DCRT and conventional EBRT (13,101) | |
| High risk: EBRT with long-term ADT (4) | Utilize implantable perirectal spacers (hydrogels, balloon) (102-105) |
IBD, inflammatory bowel disease; RT, radiation therapy; TPC, total proctocolectomy; IPAA, ileal-pouch anal anastomosis; EBRT, external beam radiation therapy; 5-FU, 5-fluorouracil; MALT, mucosa associated lymphoid tissue; HL, Hodgkin lymphoma; HDR, high dose rate; ADT, androgen deprivation therapy; IMRT, intensity-modulated radiotherapy; 3DCRT, three-dimensional conformal radiation therapy.
Cancers with no proven association with IBD
Other cancers adjacent to bowel that may warrant radiotherapy include cervical/gynecological cancers, bladder/genitourinary cancers, and upper GI/hepatobiliary cancers; however, these cancers have an unclear relationship with IBD. Cervical cancer risk in patients with IBD has been contested, with conflicting results at different centers, as summarized in the study by Hazenberg et al. (105). The possibility of increased cervical neoplasia in the IBD population was first established in 1994, pointing to azathioprine treatment as a possible cause (110). The theory of immunosuppressant medications being associated with increased cervical neoplasia was extended to include 6-mercaptopurine (MP), methotrexate, and steroids (105). It is theorized that immunosuppressive agents prevent effective clearing of HPV, allowing for greater neoplastic potential. It is not consistently accepted that the IBD disease state is associated with cervical cancer; however, some studies have indicated increased rates of abnormal pap smears and dysplasia in patients with IBD with or without HPV (111-113). There are no clear cervical neoplasia prevention, screening, or treatment guidelines specifically for patients with IBD; therefore, it is reasonable to approach these cases with the same guidelines that apply to the general population. There is no established relationship between IBD and vulvar, vaginal, endometrial, or ovarian cancer (105).
IBD and urinary tract cancers also have a debated correlation. A 2010 pooled analysis found a 2.03 standardized incidence ratio (SIR) of bladder cancer in CD patients, but not in UC patients (114). Additional analysis has found the CD bladder cancer risk to be only marginal at an SIR of 1.19 (115-117). Notably, patients with IBD have an increased risk of renal cell carcinoma (RCC); however, they are known to be diagnosed at a younger age with earlier tumor stage and have favorable outcomes compared to the general population (115,118). It is theorized that more frequent imaging of the abdomen may contribute to higher rates of early recognition and incidental findings of renal malignancy in patients with IBD. Immunosuppressive or biologic therapy for IBD is not associated with worse outcomes in RCC patients; however, thiopurines are associated with higher rates of developing kidney and urinary tract cancers and should be avoided in these patients (118-120). No relationship between IBD and male genital cancers has been established (115,117). Given the marginal relationship with IBD and early detection for urinary tract cancers, it is reasonable to approach treatment using the guidelines applicable to the general population, with special attention to IBD therapy choice during treatment.
Although a part of the gastrointestinal tract, upper GI cancers have a complex relationship with IBD. Gastric cancers do not have a proven association with either CD or UC (121). UC, as associated with PSC, is thus associated with an increased risk of cholangiocarcinoma (122). Notably, colectomy did not reduce the risk of cholangiocarcinoma in patients with IBD-PSC. Additionally, some have proposed a broader relationship between IBD and hepatobiliary cancers (73,123,124); however, others have not replicated the findings of increased rates of hepatobiliary cancer in patients with IBD (125). Ultimately it is difficult to discern whether there is an independent relationship between hepatobiliary cancers and IBD, or only an association of PSC and hepatobiliary cancer (126). And notwithstanding that PSC is associated with gallbladder carcinoma, IBD-PSC does not have a known association with gallbladder carcinoma (127). While management should follow the guidelines used in the general population, it is especially important to monitor nutritional and electrolyte status in these patients, as patients with IBD may experience disturbances in oral intake and GI loses at baseline.
Regardless of an established association, spontaneous cancers in proximity to bowel do arise in patients with IBD. Given the unclear relationship, it is difficult to find firm recommendations and data regarding treatment options for IBD patients with incidental malignancies in proximity to bowel. However, we extend some of the principles established earlier in this review to make the following recommendations when considering RT for IBD patients: assess the severity and location of a patient’s IBD, utilize modalities and positioning which limits unnecessary radiation to the neighboring bowel, consult closely with the patient’s gastroenterologist to determine whether continuation of immunosuppressive and biologic medications is recommended, coordinate with the medical oncologist and surgical oncologist to determine the safety and prognosis of utilizing medical and surgical alternatives, involve a nutritionist to optimize patient recovery and reduce the effects of bowel toxicity related dehydration or electrolyte disturbances, evaluate the need for additional cancer screening as dictated by the patient’s risk factors like IBD duration and history of immunosuppressant use.
IBD during radiation therapy
The presence or history of malignancy warrants special consideration for the management of IBD. It is generally accepted that the approach must be personalized to the patient and consider both the severity of the IBD when inadequately treated, and the risk associated with the diagnosed malignancy. This section will summarize recommendations for the use of IBD therapies during RT, and the concern for IBD flares during this period. Holistic care requires a multidisciplinary approach, incorporating medical oncologists, radiation oncologists, surgeons, gastroenterologists, and nutritionists.
There is a complex interplay of IBD therapies and cancer management- as IBD therapies can impact the development of cancer, and cancer therapies can exacerbate IBD. While there is little high level data to indicate the best therapeutic options for treating IBD during RT, it has been proposed that the use of IBD medication during therapy may predict slightly increased risk for acute GI toxicities in PCa patients (12).
Firstly, we will discuss the use of immunosuppressives, as they may contribute to cancer pathogenesis and poor healing. It is recommended that immunosuppressive agents are held until the malignancy is cured or controlled (14,128,129). Thiopurines, believed to have high oncogenic potential, are stopped or switched to methotrexate (130). A study of colorectal cancer patients undergoing surgical resection demonstrated that patients on immunosuppressive therapies have worse long-term oncologic outcomes, including overall and disease-free survival (131). A French prospective database revealed that patients with IBD are less likely to receive immunomodulatory agents and are more likely to undergo management of IBD via surgeries (130). Based on this body of information, it is reasonable to suggest that patients should consider discontinuing immunosuppressive medications to optimize their oncologic and RT management. Guidelines specify that should immunosuppressant therapy need to be restarted, it is optimal to wait 5 years to limit cancer recurrence; however, the evidence is empirical (129).
Next, we will discuss the use of biologic agents, as they are frequently utilized in the IBD population, and may have a role in both cancer pathogenesis and cancer death. There is little data available on the use of biologics, including TNF-α inhibitors, during radiation therapy. Most of the literature has assessed oncologic outcomes in RA patients utilizing biologics (129). The role of inflammatory cytokines in IBD and in tumor progression remains a highly debated topic with ongoing investigation. TNF-α is thought to promote malignancies through activation of the COX-2 pathway, inducing DNA damage, and upregulating angiogenesis; and TNF-α inhibition is thought to reduce IBD activity and CRC development (29). However, upregulation of TNF-α is being investigated for its role in promoting radiosensitivity and plays a critical role in apoptosis, raising concern that inhibition of TNF-α may hinder adequate cancer suppression (132,133). There is little clinical data on oncologic outcomes for patients with IBD with malignancy treated with TNF-α inhibitors, but some suggest that 5-year survival may be similar regardless of IBD drug choice (134). European Evidence-Based Consensus guidelines include TNF-α inhibitors as agents to withhold at least until cancer therapy is completed (129). IBD patients with a history of malignancy ultimately spend a similar number of years on steroids and anti-TNF agents compared to their IBD peers without malignancy, indicating that these agents are generally restarted after oncologic management is completed (130). Empiric evidence suggests restarting anti-TNF therapy as early as 3 months post-treatment but it is generally recommended to wait at least 2 years (135-137).
Generally, the preferred IBD management in the setting of malignancy includes 5-aminosalicylic acid, local steroid therapy, nutritional therapy, antibiotics, and surgery as needed (129). Other biologics like vedolizumab and ustekinumab, are generally considered safe; however, there are limited data (14). Nutrition should be specifically discussed with patients with IBD undergoing RT. Many patients with IBD chronically suffer from nutritional deficiencies and malabsorption. RT to the pelvis has the potential to exacerbate this, and thus should be addressed early in treatment. Moreover, IBD patients with lower BMI may be more likely to suffer flares in the peri-radiation period (44). Nutritional therapy recommendations for both IBD and pelvic radiation disease (PRD) share common management goals, including increased caloric intake, limiting dairy products, lowering fat content, and increasing protein content (138). There is evidence that the introduction of an elemental/oligomeric enteral diet may improve both IBD and PRD (138-140).
Just as IBD medications can impact the disease course of cancer, the management of cancer may also impact IBD course. Cytotoxic therapies may improve IBD status, while hormonal therapies may exacerbate it (141). For RT, attributed toxicities may overlap with the natural disease course of IBD. Symptoms of pain, fecal urgency, fistulation, and obstruction are signs of both active IBD and RT complications. There is disagreement on whether flare-free interval prior to treatment is predictive of subsequent flare following RT (3,9,12). Barnett et al. found an increased risk of fecal urgency and loose stools in their IBD arm; although, the risk of proctitis, rectal bleeding, stool frequency, or urinary side effects was not increased compared to controls (142). Another study showed an increased risk of adhesions and small bowel obstruction in the IBD-RT arm; however, comparison to the IBD without RT arm suggests that these complications cannot be attributed to RT (43). In PCa, it is unclear whether D’Amico Risk Group or RT is associated with an increased risk for subsequent flare following treatment (4,10,143). RT does not increase the risk of stricture, fistulation, or subsequent IBD activity/flare in the IBD population (9,15,43). Of note, 61% of patients with IBD treated with RT for various primary malignancies were classified as “in remission” from IBD 6 months after RT, with only 2 patients experiencing severe IBD symptoms during follow-up (9,44).
To sum up the approach to IBD management in coordination with RT, immunosuppressive agents like thiopurines should be minimized and biologics like TNF-α inhibitors should be approached with caution. Also, 5-aminosalicylic acid, local steroid therapy, nutritional therapy, antibiotics, and surgery are preferred. Involving a nutritionist is recommended in minimizing both the effects of IBD and acute radiation toxicities. There are little available data on how IBD therapies impact the outcomes of RT, which represents a promising opportunity for future research.
Conclusions
While historically it was believed that IBD-associated malignancies could not be safely managed with RT due to GI toxicities and IBD flares, updated analysis has refuted this belief. Advancements in precision radiation techniques, and utilization of positioning and spacers, allow for lower doses of radiation to the neighboring bowel and rectum than was previously possible. For each disease site, modifications to the standard of treatment for patients with IBD are summarized in Table 1, often making RT a viable treatment modality choice for these patients.
Although there is a substantial body of case reports and small studies on RT in patients with IBD, the power of the data is often lacking. Potential studies with larger power are limited by the infrequency of some cancers, like IBD-associated anal carcinoma. There is a growing body of evidence that patients with IBD fare equally as well as their peers with sporadic malignancies when appropriate RT techniques are utilized (8,9,14-16). The denial of RT to patients with IBD represents a practice based in tradition, rather than in data. Given the critical role RT plays in the management of a number of IBD-associated malignancies, we recommend including RT in the multidisciplinary planning discussions and treatment options presented to the patient.
IBD-associated malignancies are a particularly interesting phenomenon in the greater scheme of understanding the complex relationship between inflammation, cancer pathogenesis, and cancer treatment. While the prolonged state of inflammation in the bowel results in greater incidence of neoplasia (35,69,77,78), even extending into neighboring organs like the prostate (94), many inflammatory markers are associated with cancer cell death and resolution. RT initiates an inflammatory response through the release of alarmins, calreticulin, and type 1 interferon (144-146). While this response is at least partially responsible for clearing the cancer, it is also responsible for the development of some toxicities, like fibrosis (144). This elicits a number of questions relevant to cancer patients with IBD, and on a larger scale, all patients receiving RT. How does the use of anti-cytokine therapies, commonly prescribed to patients with IBD, impact the efficacy of RT? Can systemic anti-inflammatories play a role in reducing radiation toxicities, and can a localized tumor environment high in inflammatory molecules heighten treatment response?
To elucidate the answers to these questions we will have to overcome the limitations of small sample sizes, difficulty differentiating the natural IBD disease course from true bowel toxicities, and confounding variables like polypharmacy and multiple diagnoses, which are common in patients with auto-inflammatory conditions. Much of the data comes from retrospective data analysis derived from large databases, rather than dedicated IBD-malignancy focused studies. While it is clear that more research is required to fully appreciate the unique course of IBD-associated malignancies, it is also evident that our policies on utilizing RT in this special population must be updated.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-ScR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-138/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-138/coif). SKJ reports research grants to Rutgers from Merck & Co. and NPI, consulting fees to Merck & Co., and a position with American Radium Society as GI AUC co-chair. The other authors have no conflicts of interest to declare.
Ethical Statement:
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Axelrad JE, Lichtiger S, Yajnik V. Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 2016;22:4794-801. [Crossref] [PubMed]
- Slesser AA, Bhangu A, Bower M, et al. A systematic review of anal squamous cell carcinoma in inflammatory bowel disease. Surg Oncol 2013;22:230-7. [Crossref] [PubMed]
- Kirk PS, Govani S, Borza T, et al. Implications of Prostate Cancer Treatment in Men With Inflammatory Bowel Disease. Urology 2017;104:131-6. [Crossref] [PubMed]
- Schaeffer E, Srinivas S, Antonarakis E, et al. NCCN Guidelines Prostate Cancer Version 2.2020. NCCN. Available online: https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1459
- Chon BH, Loeffler JS. The effect of nonmalignant systemic disease on tolerance to radiation therapy. Oncologist 2002;7:136-43. [Crossref] [PubMed]
- Willett CG, Ooi CJ, Zietman AL, et al. Acute and late toxicity of patients with inflammatory bowel disease undergoing irradiation for abdominal and pelvic neoplasms. Int J Radiat Oncol Biol Phys 2000;46:995-8. [Crossref] [PubMed]
- Kim J, Feagins LA. Managing Patients with Inflammatory Bowel Disease Who Develop Prostate Cancer. Dig Dis Sci 2020;65:22-30. [Crossref] [PubMed]
- Gaurav B. P042 Inflammatory bowel disease is no longer a contraindication for radiation therapy: A systematic review of outcomes with modern RT techniques. Am J Gastroenterol 2019;114:S11-S12. [Crossref]
- Cantrell J, Vidal G, Bitar H, et al. Should inflammatory bowel disease be a contraindication to radiation therapy: a systematic review of acute and late toxicities. Radiother Pract 2020:1-10.
- Murphy C, Ruth K, Buyyounouski M, et al. Inflammatory Bowel Disease Is Not an Absolute Contraindication to Definitive Radiation Therapy for Prostate Cancer. Int J Radiat Oncol Biol Phys 2013;87:S356. [Crossref]
- Broussard D, Rivière P, Bonnet J, et al. Impact of abdominal or pelvic radiotherapy on disease activity in inflammatory bowel disease: a multicentre cohort study from the GETAID. Aliment Pharmacol Ther 2021;53:400-9. [PubMed]
- Murphy CT, Heller S, Ruth K, et al. Evaluating toxicity from definitive radiation therapy for prostate cancer in men with inflammatory bowel disease: Patient selection and dosimetric parameters with modern treatment techniques. Pract Radiat Oncol 2015;5:e215-22. [Crossref] [PubMed]
- White EC, Murphy JD, Chang DT, et al. Low Toxicity in Inflammatory Bowel Disease Patients Treated With Abdominal and Pelvic Radiation Therapy. Am J Clin Oncol 2015;38:564-9. [Crossref] [PubMed]
- Lin SC, Goldowsky A, Papamichael K, et al. The Treatment of Inflammatory Bowel Disease in Patients With a History of Malignancy. Inflamm Bowel Dis 2019;25:998-1005. [Crossref] [PubMed]
- Rhome R, Axelrad J, Itzkowitz S, et al. Acute and Chronic Complications After Abdominal/Pelvic Radiation in Patients With Inflammatory Bowel Disease. Int J Radiat Oncol Biol Phys 2015;93:E492. [Crossref]
- Chang BW, Kumar AM, Koyfman SA, et al. Radiation therapy in patients with inflammatory bowel disease and colorectal cancer: risks and benefits. Int J Colorectal Dis 2015;30:403-8. [Crossref] [PubMed]
- Radiation therapy in treating patients with stage II prostate cancer. ClinicalTrials.gov identifier: NCT00331773. Updated February 11, 2020. Available online: https://clinicaltrials.gov/ct2/show/NCT00331773
- Chemotherapy plus radiation therapy in treating patients with stage II or stage III anal cancer. ClinicalTrials.gov identifier: NCT00003596. Updated January 4, 2017. Available online: https://www.clinicaltrials.gov/ct2/show/NCT00003596
- Intensity-modulated radiation therapy, fluorouracil, and mitomycin c in treating patients with invasive anal cancer. ClinicalTrials.gov identifier: NCT00423293. Updated February 27, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT00423293
- Tricco AC, Lillie E, Zarin W, et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann Intern Med 2018;169:467-73. [Crossref] [PubMed]
- Biancone L, Zuzzi S, Ranieri M, et al. Fistulizing pattern in Crohn's disease and pancolitis in ulcerative colitis are independent risk factors for cancer: a single-center cohort study. J Crohns Colitis 2012;6:578-87. [Crossref] [PubMed]
- Choi CH, Rutter MD, Askari A, et al. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. Am J Gastroenterol 2015;110:1022-34. [Crossref] [PubMed]
- Ekbom A, Helmick C, Zack M, et al. Increased risk of large-bowel cancer in Crohn's disease with colonic involvement. Lancet 1990;336:357-9. [Crossref] [PubMed]
- Brackmann S, Andersen SN, Aamodt G, et al. Two distinct groups of colorectal cancer in inflammatory bowel disease. Inflamm Bowel Dis 2009;15:9-16. [Crossref] [PubMed]
- Zheng HH, Jiang XL. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis of 16 observational studies. Eur J Gastroenterol Hepatol 2016;28:383-90. [Crossref] [PubMed]
- Ekbom A, Helmick C, Zack M, et al. Ulcerative colitis and colorectal cancer. A population-based study. N Engl J Med 1990;323:1228-33. [Crossref] [PubMed]
- Friedman S, Rubin PH, Bodian C, et al. Screening and surveillance colonoscopy in chronic Crohn's colitis. Gastroenterology 2001;120:820-6. [Crossref] [PubMed]
- Maykel JA, Hagerman G, Mellgren AF, et al. Crohn's colitis: the incidence of dysplasia and adenocarcinoma in surgical patients. Dis Colon Rectum 2006;49:950-7. [Crossref] [PubMed]
- Keller DS, Windsor A, Cohen R, et al. Colorectal cancer in inflammatory bowel disease: review of the evidence. Tech Coloproctol 2019;23:3-13. [Crossref] [PubMed]
- Choi PM, Zelig MP. Similarity of colorectal cancer in Crohn's disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut 1994;35:950-4. [Crossref] [PubMed]
- Sugita A, Greenstein AJ, Ribeiro MB, et al. Survival with colorectal cancer in ulcerative colitis. A study of 102 cases. Ann Surg 1993;218:189-95. [Crossref] [PubMed]
- Delaunoit T, Limburg PJ, Goldberg RM, et al. Colorectal cancer prognosis among patients with inflammatory bowel disease. Clin Gastroenterol Hepatol 2006;4:335-42. [Crossref] [PubMed]
- Fogt F, Vortmeyer AO, Goldman H, et al. Comparison of genetic alterations in colonic adenoma and ulcerative colitis-associated dysplasia and carcinoma. Hum Pathol 1998;29:131-6. [Crossref] [PubMed]
- Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology 2011;140:1807-16. [Crossref] [PubMed]
- Mathy C, Schneider K, Chen YY, et al. Gross versus microscopic pancolitis and the occurrence of neoplasia in ulcerative colitis. Inflamm Bowel Dis 2003;9:351-5. [Crossref] [PubMed]
- Burmer GC, Rabinovitch PS, Haggitt RC, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology 1992;103:1602-10. [Crossref] [PubMed]
- Yin J, Harpaz N, Tong Y, et al. p53 point mutations in dysplastic and cancerous ulcerative colitis lesions. Gastroenterology 1993;104:1633-9. [Crossref] [PubMed]
- Issa JP, Ahuja N, Toyota M, et al. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer Res 2001;61:3573-7. [PubMed]
- Clarke WT, Feuerstein JD. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. World J Gastroenterol 2019;25:4148-57. [Crossref] [PubMed]
- Ramsey M, Krishna SG, Stanich PP, et al. Inflammatory Bowel Disease Adversely Impacts Colorectal Cancer Surgery Short-term Outcomes and Health-Care Resource Utilization. Clin Transl Gastroenterol 2017;8:e127. [Crossref] [PubMed]
- Reynolds IS, O'Toole A, Deasy J, et al. A meta-analysis of the clinicopathological characteristics and survival outcomes of inflammatory bowel disease associated colorectal cancer. Int J Colorectal Dis 2017;32:443-51. [Crossref] [PubMed]
- Bosch SL, van Rooijen SJ, Bökkerink GM, et al. Acute toxicity and surgical complications after preoperative (chemo)radiation therapy for rectal cancer in patients with inflammatory bowel disease. Radiother Oncol 2017;123:147-53. [Crossref] [PubMed]
- Mudgway R, Bryant AK, Heide ES, et al. A Matched Case-Control Analysis of Clinical Outcomes for Patients With Inflammatory Bowel Disease and Rectal Cancer Treated With Pelvic Radiation Therapy. Int J Radiat Oncol Biol Phys 2019;105:994-1004. [Crossref] [PubMed]
- Annede P, Seisen T, Klotz C, et al. Inflammatory bowel diseases activity in patients undergoing pelvic radiation therapy. J Gastrointest Oncol 2017;8:173-9. [Crossref] [PubMed]
- Fok M, Toh S, Easow J, et al. Proton beam therapy in rectal cancer: A systematic review and meta-analysis. Surg Oncol 2021;38:101638. [Crossref] [PubMed]
- Devon KM, Brown CJ, Burnstein M, et al. Cancer of the anus complicating perianal Crohn's disease. Dis Colon Rectum 2009;52:211-6. [Crossref] [PubMed]
- Ky A, Sohn N, Weinstein MA, et al. Carcinoma arising in anorectal fistulas of Crohn's disease. Dis Colon Rectum 1998;41:992-6. [Crossref] [PubMed]
- Wisniewski A, Fléjou JF, Siproudhis L, et al. Anal Neoplasia in Inflammatory Bowel Disease: Classification Proposal, Epidemiology, Carcinogenesis, and Risk Management Perspectives. J Crohns Colitis 2017;11:1011-8. [Crossref] [PubMed]
- Shah SB, Pickham D, Araya H, et al. Prevalence of Anal Dysplasia in Patients With Inflammatory Bowel Disease. Clin Gastroenterol Hepatol 2015;13:1955-61.e1. [Crossref] [PubMed]
- Laukoetter MG, Mennigen R, Hannig CM, et al. Intestinal cancer risk in Crohn's disease: a meta-analysis. J Gastrointest Surg 2011;15:576-83. [Crossref] [PubMed]
- Nishigami T, Kataoka TR, Ikeuchi H, et al. Adenocarcinomas associated with perianal fistulae in Crohn's disease have a rectal, not an anal, immunophenotype. Pathology 2011;43:36-9. [Crossref] [PubMed]
- Siegel CA. Risk of lymphoma in inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2009;5:784-790.
- Lewis JD, Bilker WB, Brensinger C, et al. Inflammatory bowel disease is not associated with an increased risk of lymphoma. Gastroenterology 2001;121:1080-7. [Crossref] [PubMed]
- Farrell RJ, Ang Y, Kileen P, et al. Increased incidence of non-Hodgkin's lymphoma in inflammatory bowel disease patients on immunosuppressive therapy but overall risk is low. Gut 2000;47:514-9. [Crossref] [PubMed]
- Loftus EV Jr, Tremaine WJ, Habermann TM, et al. Risk of lymphoma in inflammatory bowel disease. Am J Gastroenterol 2000;95:2308-12. [Crossref] [PubMed]
- Bernstein L, Ross RK. Prior medication use and health history as risk factors for non-Hodgkin's lymphoma: preliminary results from a case-control study in Los Angeles County. Cancer Res 1992;52:5510s-5s. [PubMed]
- Zhang Y, Holford TR, Leaderer B, et al. Prior medical conditions and medication use and risk of non-Hodgkin lymphoma in Connecticut United States women. Cancer Causes Control 2004;15:419-28. [Crossref] [PubMed]
- Kato I, Koenig KL, Shore RE, et al. Use of anti-inflammatory and non-narcotic analgesic drugs and risk of non-Hodgkin's lymphoma (NHL) (United States). Cancer Causes Control 2002;13:965-74. [Crossref] [PubMed]
- Beiderbeck AB, Holly EA, Sturkenboom MC, et al. No increased risk of non-Hodgkin's lymphoma with steroids, estrogens and psychotropics (Netherlands). Cancer Causes Control 2003;14:639-44. [Crossref] [PubMed]
- Bewtra M, Lewis JD. Safety profile of IBD: lymphoma risks. Med Clin North Am 2010;94:93-113. [Crossref] [PubMed]
- Kandiel A, Fraser AG, Korelitz BI, et al. Increased risk of lymphoma among inflammatory bowel disease patients treated with azathioprine and 6-mercaptopurine. Gut 2005;54:1121-5. [Crossref] [PubMed]
- Beaugerie L, Brousse N, Bouvier AM, et al. Lymphoproliferative disorders in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Lancet 2009;374:1617-25. [Crossref] [PubMed]
- Sokol H, Beaugerie L, Maynadié M, et al. Excess primary intestinal lymphoproliferative disorders in patients with inflammatory bowel disease. Inflamm Bowel Dis 2012;18:2063-71. [Crossref] [PubMed]
- Brown SL, Greene MH, Gershon SK, et al. Tumor necrosis factor antagonist therapy and lymphoma development: twenty-six cases reported to the Food and Drug Administration. Arthritis Rheum 2002;46:3151-8. [Crossref] [PubMed]
- Schiff MH, Burmester GR, Kent JD, et al. Safety analyses of adalimumab (HUMIRA) in global clinical trials and US postmarketing surveillance of patients with rheumatoid arthritis. Ann Rheum Dis 2006;65:889-94. [Crossref] [PubMed]
- Sandborn WJ, Feagan BG, Stoinov S, et al. Certolizumab pegol for the treatment of Crohn's disease. N Engl J Med 2007;357:228-38. [Crossref] [PubMed]
- Shah ED, Coburn ES, Nayyar A, et al. Systematic review: hepatosplenic T-cell lymphoma on biologic therapy for inflammatory bowel disease, including data from the Food and Drug Administration Adverse Event Reporting System. Aliment Pharmacol Ther 2020;51:527-33. [Crossref] [PubMed]
- Lemann M, de La Valussiere G, Bouhnik Y, et al. Intravenous cyclosporine for refractory attacks of Crohn's disease (CD): Long-term follow-up of patients. Gastroenterology 1998;114:A1020. [Crossref]
- Aydinli H, Remzi F, Schwartzberg D, et al. Small bowel Adenocarcinoma in the setting of Crohn’s disease: A systematic review of the literature. Turk J Colorectal Dis 2020;30:220-30. [Crossref]
- Jess T, Gamborg M, Matzen P, et al. Increased risk of intestinal cancer in Crohn's disease: a meta-analysis of population-based cohort studies. Am J Gastroenterol 2005;100:2724-9. [Crossref] [PubMed]
- Canavan C, Abrams KR, Mayberry J. Meta-analysis: colorectal and small bowel cancer risk in patients with Crohn's disease. Aliment Pharmacol Ther 2006;23:1097-104. [Crossref] [PubMed]
- von Roon AC, Reese G, Teare J, et al. The risk of cancer in patients with Crohn's disease. Dis Colon Rectum 2007;50:839-55. [Crossref] [PubMed]
- Bernstein CN, Blanchard JF, Kliewer E, et al. Cancer risk in patients with inflammatory bowel disease: a population-based study. Cancer 2001;91:854-62. [Crossref] [PubMed]
- Lech G, Korcz W, Kowalczyk E, et al. The risk of small bowel adenocarcinoma in patients with Crohn's disease. Prz Gastroenterol 2020;15:309-13. [Crossref] [PubMed]
- Jess T, Loftus EV Jr, Velayos FS, et al. Risk of intestinal cancer in inflammatory bowel disease: a population-based study from olmsted county, Minnesota. Gastroenterology 2006;130:1039-46. [Crossref] [PubMed]
- Bojesen RD, Riis LB, Høgdall E, et al. Inflammatory Bowel Disease and Small Bowel Cancer Risk, Clinical Characteristics, and Histopathology: A Population-Based Study. Clin Gastroenterol Hepatol 2017;15:1900-1907.e2. [Crossref] [PubMed]
- Svrcek M, Piton G, Cosnes J, et al. Small bowel adenocarcinomas complicating Crohn's disease are associated with dysplasia: a pathological and molecular study. Inflamm Bowel Dis 2014;20:1584-92. [Crossref] [PubMed]
- Rashid A, Hamilton SR. Genetic alterations in sporadic and Crohn's-associated adenocarcinomas of the small intestine. Gastroenterology 1997;113:127-35. [Crossref] [PubMed]
- Delaunoit T, Neczyporenko F, Limburg PJ, et al. Pathogenesis and risk factors of small bowel adenocarcinoma: a colorectal cancer sibling? Am J Gastroenterol 2005;100:703-10. [Crossref] [PubMed]
- Kern SE, Redston M, Seymour AB, et al. Molecular genetic profiles of colitis-associated neoplasms. Gastroenterology 1994;107:420-8. [Crossref] [PubMed]
- Redston MS, Papadopoulos N, Caldas C, et al. Common occurrence of APC and K-ras gene mutations in the spectrum of colitis-associated neoplasias. Gastroenterology 1995;108:383-92. [Crossref] [PubMed]
- Chan RC, Katelaris PH, Stewart P, et al. Small bowel adenocarcinoma with high levels of microsatellite instability in Crohn's disease. Hum Pathol 2006;37:631-4. [Crossref] [PubMed]
- Bilimoria KY, Bentrem DJ, Wayne JD, et al. Small bowel cancer in the United States: changes in epidemiology, treatment, and survival over the last 20 years. Ann Surg 2009;249:63-71. [Crossref] [PubMed]
- Kronberger IE, Graziadei IW, Vogel W. Small bowel adenocarcinoma in Crohn's disease: a case report and review of literature. World J Gastroenterol 2006;12:1317-20. [Crossref] [PubMed]
- Weber NK, Fletcher JG, Fidler JL, et al. Clinical characteristics and imaging features of small bowel adenocarcinomas in Crohn's disease. Abdom Imaging 2015;40:1060-7. [Crossref] [PubMed]
- Palascak-Juif V, Bouvier AM, Cosnes J, et al. Small bowel adenocarcinoma in patients with Crohn's disease compared with small bowel adenocarcinoma de novo. Inflamm Bowel Dis 2005;11:828-32. [Crossref] [PubMed]
- Hamdane A. Coexistence of small bowel adenocarcinoma and chronic inflammatory bowel disease (IBD): Case Report and Review of the Literature. SAS J Surg 2021;7:238-9. [Crossref]
- Duerr D, Ellard S, Zhai Y, et al. A Retrospective Review of Chemotherapy for Patients with Small Bowel Adenocarcinoma in British Columbia. J Cancer 2016;7:2290-5. [Crossref] [PubMed]
- Ge Y, Shi Q, Yao W, et al. The association between inflammatory bowel disease and prostate cancer risk: a meta-analysis. Prostate Cancer Prostatic Dis 2020;23:53-8. [Crossref] [PubMed]
- Chen M, Yuan C, Xu T. An increase in prostate cancer diagnosis during inflammatory bowel disease: A systematic review and meta-analysis. Clin Res Hepatol Gastroenterol 2020;44:302-9. [Crossref] [PubMed]
- Carli E, Caviglia GP, Pellicano R, et al. Incidence of Prostate Cancer in Inflammatory Bowel Disease: A Meta-Analysis. Medicina (Kaunas) 2020;56:285. [Crossref] [PubMed]
- Meyers TJ, Weiner AB, Graff RE, et al. Association between inflammatory bowel disease and prostate cancer: A large-scale, prospective, population-based study. Int J Cancer 2020;147:2735-42. [Crossref] [PubMed]
- Burns JA, Weiner AB, Catalona WJ, et al. Inflammatory Bowel Disease and the Risk of Prostate Cancer. Eur Urol 2019;75:846-52. [Crossref] [PubMed]
- Desai AS, Sagar V, Lysy B, et al. Inflammatory bowel disease induces inflammatory and pre-neoplastic changes in the prostate. Prostate Cancer Prostatic Dis 2022;25:463-71. [Crossref] [PubMed]
- Takahashi H, Froemming AT, Bruining DH, et al. Prostate MRI characteristics in patients with inflammatory bowel disease. Eur J Radiol 2021;135:109503. [Crossref] [PubMed]
- Sfanos KS, Joshu CE. IBD as a risk factor for prostate cancer: what is the link? Nat Rev Urol 2019;16:271-2. [Crossref] [PubMed]
- Tromp D, Christie DR. Acute and Late Bowel Toxicity in Radiotherapy Patients with Inflammatory Bowel Disease: A Systematic Review. Clin Oncol (R Coll Radiol) 2015;27:536-41. [Crossref] [PubMed]
- Peters CA, Cesaretti JA, Stone NN, et al. Low-dose rate prostate brachytherapy is well tolerated in patients with a history of inflammatory bowel disease. Int J Radiat Oncol Biol Phys 2006;66:424-9. [Crossref] [PubMed]
- Mohammed W, Hoskin P, Henry A, et al. Short-term Toxicity of High Dose Rate Brachytherapy in Prostate Cancer Patients with Inflammatory Bowel Disease. Clin Oncol (R Coll Radiol) 2018;30:534-8. [Crossref] [PubMed]
- Pai HH, Keyes M, Morris WJ, et al. Toxicity after (125)I prostate brachytherapy in patients with inflammatory bowel disease. Brachytherapy 2013;12:126-33. [Crossref] [PubMed]
- Gestaut MM, Swanson GP. Long term clinical toxicity of radiation therapy in prostate cancer patients with Inflammatory Bowel Disease. Rep Pract Oncol Radiother 2017;22:77-82. [Crossref] [PubMed]
- Singh R, Jackson PS, Blake M, et al. Minimal Rectal Toxicity in the Setting of Comorbid Crohn's Disease Following Prostate Cancer Radiotherapy with a Hydrogel Rectal Spacer. Cureus 2017;9:e1533. [Crossref] [PubMed]
- Vanneste BGL, Van Limbergen EJ, van de Beek K, et al. A biodegradable rectal balloon implant to protect the rectum during prostate cancer radiotherapy for a patient with active Crohn's disease. Tech Innov Patient Support Radiat Oncol 2018;6:1-4. [Crossref] [PubMed]
- Vanneste BGL, Van Limbergen EJ, Marcelissen T, et al. Is prostate cancer radiotherapy using implantable rectum spacers safe and effective in inflammatory bowel disease patients? Clin Transl Radiat Oncol 2021;27:121-5. [Crossref] [PubMed]
- Hazenberg HMJL, de Boer NKH, Mulder CJJ, et al. Neoplasia and Precursor Lesions of the Female Genital Tract in IBD: Epidemiology, Role of Immunosuppressants, and Clinical Implications. Inflamm Bowel Dis 2018;24:510-31. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2021;19:329-59. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. Anal Carcinoma, version 1.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2021. Available online: https://www.nccn.org/guidelines/category_1
- Zelenetz AD, Gordon LI, Abramson JS, et al. B Cell Lymphomas, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2021. Available online: https://www.nccn.org/guidelines/category_1
- Benson AB, Venook AP, Al-Hawary MM, et al. Small Bowel Adenocarcinoma, version 2.2021, NCCN clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2021. Available online: https://www.nccn.org/guidelines/category_1
- Connell WR, Kamm MA, Dickson M, et al. Long-term neoplasia risk after azathioprine treatment in inflammatory bowel disease. Lancet 1994;343:1249-52. [Crossref] [PubMed]
- Rungoe C, Simonsen J, Riis L, et al. Inflammatory bowel disease and cervical neoplasia: a population-based nationwide cohort study. Clin Gastroenterol Hepatol 2015;13:693-700.e1. [Crossref] [PubMed]
- Bhatia J, Bratcher J, Korelitz B, et al. Abnormalities of uterine cervix in women with inflammatory bowel disease. World J Gastroenterol 2006;12:6167-71. [Crossref] [PubMed]
- Marehbian J, Arrighi HM, Hass S, et al. Adverse events associated with common therapy regimens for moderate-to-severe Crohn's disease. Am J Gastroenterol 2009;104:2524-33. [Crossref] [PubMed]
- Pedersen N, Duricova D, Elkjaer M, et al. Risk of extra-intestinal cancer in inflammatory bowel disease: meta-analysis of population-based cohort studies. Am J Gastroenterol 2010;105:1480-7. [Crossref] [PubMed]
- Feng D, Yang Y, Wang Z, et al. Inflammatory bowel disease and risk of urinary cancers: a systematic review and pooled analysis of population-based studies. Transl Androl Urol 2021;10:1332-41. [Crossref] [PubMed]
- Geng Z, Geng Q. Risk of Urinary Bladder Cancer in Patients With Inflammatory Bowel Diseases: A Meta-Analysis. Front Surg 2021;8:636791. [Crossref] [PubMed]
- Zhang C, Liu S, Peng L, et al. Does inflammatory bowel disease increase the risk of lower urinary tract tumors: a meta-analysis. Transl Androl Urol 2021;10:164-73. [Crossref] [PubMed]
- Derikx LA, Nissen LH, Drenth JP, et al. Better survival of renal cell carcinoma in patients with inflammatory bowel disease. Oncotarget 2015;6:38336-47. [Crossref] [PubMed]
- Wauters L, Billiet T, Papamichael K, et al. Incidence of renal cell carcinoma in inflammatory bowel disease patients with and without anti-TNF treatment. Eur J Gastroenterol Hepatol 2017;29:84-90. [Crossref] [PubMed]
- Bourrier A, Carrat F, Colombel JF, et al. Excess risk of urinary tract cancers in patients receiving thiopurines for inflammatory bowel disease: a prospective observational cohort study. Aliment Pharmacol Ther 2016;43:252-61. [Crossref] [PubMed]
- Wan Q, Zhao R, Xia L, et al. Inflammatory bowel disease and risk of gastric, small bowel and colorectal cancer: a meta-analysis of 26 observational studies. J Cancer Res Clin Oncol 2021;147:1077-87. [Crossref] [PubMed]
- Gulamhusein AF, Eaton JE, Tabibian JH, et al. Duration of Inflammatory Bowel Disease Is Associated With Increased Risk of Cholangiocarcinoma in Patients With Primary Sclerosing Cholangitis and IBD. Am J Gastroenterol 2016;111:705-11. [Crossref] [PubMed]
- Mellemkjaer L, Olsen JH, Frisch M, et al. Cancer in patients with ulcerative colitis. Int J Cancer 1995;60:330-3. [Crossref] [PubMed]
- Karlén P, Löfberg R, Broström O, et al. Increased risk of cancer in ulcerative colitis: a population-based cohort study. Am J Gastroenterol 1999;94:1047-52. [Crossref] [PubMed]
- Ekbom A, Helmick C, Zack M, et al. Extracolonic malignancies in inflammatory bowel disease. Cancer 1991;67:2015-9. [Crossref] [PubMed]
- Erichsen R, Olén O, Sachs MC, et al. Hepatobiliary Cancer Risk in Patients with Inflammatory Bowel Disease: A Scandinavian Population-Based Cohort Study. Cancer Epidemiol Biomarkers Prev 2021;30:886-94. [Crossref] [PubMed]
- Lewis JT, Talwalkar JA, Rosen CB, et al. Prevalence and risk factors for gallbladder neoplasia in patients with primary sclerosing cholangitis: evidence for a metaplasia-dysplasia-carcinoma sequence. Am J Surg Pathol 2007;31:907-13. [Crossref] [PubMed]
- Bernheim O, Colombel JF, Ullman TA, et al. The management of immunosuppression in patients with inflammatory bowel disease and cancer. Gut 2013;62:1523-8. [Crossref] [PubMed]
- Annese V, Beaugerie L, Egan L, et al. European Evidence-based Consensus: Inflammatory Bowel Disease and Malignancies. J Crohns Colitis 2015;9:945-65. [Crossref] [PubMed]
- Rajca S, Seksik P, Bourrier A, et al. Impact of the diagnosis and treatment of cancer on the course of inflammatory bowel disease. J Crohns Colitis 2014;8:819-24. [Crossref] [PubMed]
- Khoury W, Lavery IC, Kiran RP. Effects of chronic immunosuppression on long-term oncologic outcomes for colorectal cancer patients undergoing surgery. Ann Surg 2011;253:323-7. [Crossref] [PubMed]
- Mauceri HJ, Beckett MA, Liang H, et al. Translational strategies exploiting TNF-alpha that sensitize tumors to radiation therapy. Cancer Gene Ther 2009;16:373-81. [Crossref] [PubMed]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell 2004;116:205-19. [Crossref] [PubMed]
- Axelrad J, Bernheim O, Colombel JF, et al. Risk of New or Recurrent Cancer in Patients With Inflammatory Bowel Disease and Previous Cancer Exposed to Immunosuppressive and Anti-Tumor Necrosis Factor Agents. Clin Gastroenterol Hepatol 2016;14:58-64. [Crossref] [PubMed]
- Swoger JM, Regueiro M. Stopping, continuing, or restarting immunomodulators and biologics when an infection or malignancy develops. Inflamm Bowel Dis 2014;20:926-35. [Crossref] [PubMed]
- Cosnes J. What Should Be Done in Inflammatory Bowel Disease Patients with Prior Malignancy? Dig Dis 2017;35:50-5. [Crossref] [PubMed]
- Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1-s106. [Crossref] [PubMed]
- DeWitt T, Hegazi R. Nutrition in pelvic radiation disease and inflammatory bowel disease: similarities and differences. Biomed Res Int 2014;2014:716579. [Crossref] [PubMed]
- Craighead PS, Young S. Phase II study assessing the feasibility of using elemental supplements to reduce acute enteritis in patients receiving radical pelvic radiotherapy. Am J Clin Oncol 1998;21:573-8. [Crossref] [PubMed]
- Sanz-Paris A, Martinez-García M, Martinez-Trufero J, et al. Oligomeric Enteral Nutrition in Undernutrition, due to Oncology Treatment-Related Diarrhea. Systematic Review and Proposal of An Algorithm of Action. Nutrients 2019;11:1888. [Crossref] [PubMed]
- Axelrad JE, Fowler SA, Friedman S, et al. Effects of cancer treatment on inflammatory bowel disease remission and reactivation. Clin Gastroenterol Hepatol 2012;10:1021-7.e1. [Crossref] [PubMed]
- Barnett GC, De Meerleer G, Gulliford SL, et al. The impact of clinical factors on the development of late radiation toxicity: results from the Medical Research Council RT01 trial (ISRCTN47772397). Clin Oncol (R Coll Radiol) 2011;23:613-24. [Crossref] [PubMed]
- Feagins LA, Kim J, Chandrakumaran A, et al. Rates of Adverse IBD-Related Outcomes for Patients With IBD and Concomitant Prostate Cancer Treated With Radiation Therapy. Inflamm Bowel Dis 2020;26:728-33. [Crossref] [PubMed]
- De Ruysscher D, Niedermann G, Burnet NG, et al. Radiotherapy toxicity. Nat Rev Dis Primers 2019;5:13. [Crossref] [PubMed]
- Pitt JM, Kroemer G, Zitvogel L. Immunogenic and Non-immunogenic Cell Death in the Tumor Microenvironment. Adv Exp Med Biol 2017;1036:65-79. [Crossref] [PubMed]
- Burnette BC, Liang H, Lee Y, et al. The efficacy of radiotherapy relies upon induction of type i interferon-dependent innate and adaptive immunity. Cancer Res 2011;71:2488-96. [Crossref] [PubMed]


