
Development and validation of a nomogram based on neutrophil-to-lymphocyte ratio and fibrinogen-to-lymphocyte ratio for predicting recurrence of colorectal adenoma
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer in the world (1). More than 1.8 million new cases of CRCs still occur each year (2). Colorectal adenoma (CRA) is a recognized precursor lesion for CRC (3). Therefore, early detection and resection of CRA are essential to reduce the incidence rate of CRC effectively. Endoscopic removal of CRAs is the key to reducing CRC incidence and mortality (4,5). However, although endoscopic treatment has the advantages of simple operations, less trauma and complete resection, there is still a high recurrence rate (6). Therefore, the analysis of risk factors for recurrence after endoscopic removal of CRAs is important clinical guidance for the prevention of recurrence and reduction of canceration rate.
A number of factors associated with CRA recurrence have been identified, including age, sex, lifestyle and adenoma characteristics (7-9). It has been shown that inflammation plays a significant role in the occurrence and development of tumors (10). Peripheral blood count coefficient is a relatively new index of inflammation, including platelet-to-lymphocyte ratio (PLR) and neutrophil-to-lymphocyte ratio (NLR). These indexes can better reflect the systemic inflammatory response, with readily available and low-cost, and have been reported to correlate with the prognosis of CRC (11,12). Fibrinogen has a certain correlation with malignant tumors, which plays a significant role in evaluating the prognosis of tumors (13). Lymphocytes are important antineoplastic factors, which play a significant role in cancer-specific immune response (14). Recently, a novel prognosticator fibrinogen-to-lymphocyte ratio (FLR) has been proposed, which has been proved to evaluate the prognosis in gastric cancer (15), esophageal cancer (16), non-small cell lung cancer (17) and head and neck adenoid-cystic carcinoma (18). However, there is no study on the predictive performance of NLR and FLR in CRA recurrence. The purpose of this research was to explore the predictive performance of NLR and FLR on the CRA recurrence and to develop a new nomogram for a more accurate assessment of recurrence to provide basis for follow-up of patients with CRA. We present the following article in accordance with the TRIPOD reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-410/rc).
Methods
Patients
This research included patients who underwent colonoscopy and CRA resection for the first time in the General Hospital of Tianjin Medical University from October 2017 to October 2019. They were followed up for 2 years to evaluate their recurrence of adenomas by colonoscopy. The inclusion criteria were pathological diagnosis of CRA, at least one follow-up colonoscopy performed at more than 6 months later after CRA resection, with enough data. Patients with one of the following characteristics were excluded: (I) history of CRC or CRA; (II) history of colorectal surgery or colonoscopy with removal of adenomas; (III) history of familial adenomatous polyposis, inflammatory bowel disease or intestinal tuberculosis; (IV) poor bowel preparation resulting in unclear colonoscopic views; (V) incomplete data; (VI) acute infections, blood disorders, autoimmune diseases and other diseases that may cause inflammation. A total of 421 patients were included in the study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Tianjin Medical University General Hospital (No. IRB2022-WZ-014) and individual consent for this retrospective analysis was waived.
Parameters
The patient’s clinical features including age (years), gender, CRC family history, past history of chronic disease (hypertension, diabetes and coronary heart disease) as well as history of smoking and drinking were recorded at the time of first colonoscopy as the baseline time. Blood test indicators included hemoglobin, neutrophil, lymphocyte, monocyte, platelet, albumin and fibrinogen. [NLR = neutrophil level (109/L)/lymphocyte level (109/L); PLR = platelet level (109/L)/lymphocyte level (109/L); lymphocyte-to-monocyte ratio (LMR) = lymphocyte level (109/L)/monocyte level (109/L); FLR = fibrinogen level (g/L)/lymphocyte level (109/L); prognostic nutritional index (PNI) = serum albumin (g/L) + 5 × total lymphocyte count (109/L)]. The date including characteristics of the adenomas (size, number, location, histology and differentiation grade) were recorded. Endoscopy was performed by experienced endoscopists with more than 5 years of experience. Patients were instructed to consume a light diet the day before the examination, and polyethylene glycol was used for bowel preparation. Biopsy specimens were analyzed by two experienced pathologists under a microscope. CRA recurrence are defined as adenomas found during a follow-up colonoscopy at least 6 months after initial resection, whether at the same site or elsewhere (19,20).
Statistical analysis
Statistical analysis of data was performed using IBM SPSS Statistics 23.0 and R version 4.0.4. A t-test for independent samples was carried out on continuous variables with a normal distribution, which were expressed as mean ± standard deviation (SD). Continuous variables with non-normal distribution were expressed by median (interquartile range) and analyzed by Mann-Whitney U tets. Categorical variables were expressed as count and percent and were analyzed by χ2 test. The Youden index was used to determine the cut-off points of the continuous variable. In the training cohort, an analysis of multivariate logistic regression was conducted to determine the independent risk factors for recurrence of CRA. Using R software “rms” package and independent risk factors, a nomogram model for predicting the risk of CRA recurrence was established. A calibration plot with bootstrap sampling (n=1,000) was used to calibrate the nomogram internally. By calculating the area under the curve (AUC) of the receiver operating characteristics (ROC) curve and decision curve analysis (DCA), we assessed the discriminative power and clinical utility of the nomogram model. All tests were two-sided, and a P value of <0.05 was considered to be statistically significant.
Results
Clinical characteristics of patients with CRA
The study ultimately included 421 patients with CRA who were divided into a training cohort (n=301) and a validation cohort (n=120) by random sampling. A significant difference in clinicopathological features between two cohorts was not found (all P>0.05), which meant baseline for the two cohorts was balanced. The percentage of recurrence in the two cohorts were 53.2% (n=160) and 51.7% (n=62). The clinicopathological features of CRA patients in two cohort were shown in Table 1.
Table 1
| Variable | Training cohort (n=301) | Validation cohort (n=120) | P |
|---|---|---|---|
| Age (years) | 61.7±9.7 | 61.5±8.5 | 0.824 |
| Gender | 0.080 | ||
| Male | 186 (61.8%) | 63 (52.5%) | |
| Female | 115 (38.2%) | 57 (47.5%) | |
| Smoking | 0.301 | ||
| Yes | 114 (37.9%) | 39 (32.5%) | |
| No | 187 (62.1%) | 81 (67.5%) | |
| Drinking | 0.435 | ||
| Yes | 79 (26.2%) | 36 (30.0%) | |
| No | 222 (73.8%) | 84 (70.0%) | |
| CRC family history | 0.850 | ||
| Yes | 12 (4.0%) | 5 (4.2%) | |
| No | 289 (96.0%) | 115 (95.8%) | |
| Diabetes | 0.073 | ||
| Yes | 83 (27.6%) | 23 (19.2%) | |
| No | 218 (72.4%) | 97 (80.8%) | |
| Coronary heart disease | 0.799 | ||
| Yes | 43 (14.3%) | 16 (13.3%) | |
| No | 258 (85.7%) | 104 (86.7%) | |
| Hypertension | 0.594 | ||
| Yes | 112 (37.2%) | 48 (40.0%) | |
| No | 189 (62.8%) | 72 (60.0%) | |
| Adenoma location | 0.894 | ||
| Distal colon | 110 (36.5%) | 42 (35.0%) | |
| Proximal colon | 83 (27.6%) | 32 (26.7%) | |
| Both | 108 (35.9%) | 46 (38.3%) | |
| Adenoma size | 0.563 | ||
| ≤10 mm | 224 (74.4%) | 86 (71.7%) | |
| >10 mm | 77 (25.6%) | 34 (28.3%) | |
| No. of adenomas | 0.438 | ||
| 1 | 114 (37.9%) | 38 (31.7%) | |
| 2 | 60 (19.9%) | 24 (20.0%) | |
| ≥3 | 127 (42.2%) | 58 (48.3%) | |
| Villous component | 0.263 | ||
| Yes | 62 (20.6%) | 19 (15.8%) | |
| No | 239 (79.4%) | 101 (84.2%) | |
| Differentiation | 0.386 | ||
| High-grade dysplasia | 29 (9.6%) | 15 (12.5%) | |
| Low-grade dysplasia | 272 (90.4%) | 105 (87.5%) | |
| Hemoglobin (g/L) | 135.4±19.1 | 135.9±15.8 | 0.810 |
| NLR | 1.59 (1.23–2.10) | 1.54 (1.13–2.15) | 0.661 |
| PLR | 109.7 (86.9–136.8) | 110.2 (91.0–135.0) | 0.601 |
| LMR | 4.38 (3.42–5.26) | 4.33 (3.24–5.16) | 0.483 |
| PNI | 52.2±5.9 | 51.4±5.3 | 0.185 |
| FLR | 1.57 (1.25–2.02) | 1.53 (1.31–2.08) | 0.940 |
Data was expressed as n (%) or mean ± standard deviation or median (interquartile range). CRC, colorectal cancer; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; FLR, fibrinogen-to-lymphocyte ratio.
Univariate analysis of factors associated with CRA recurrence in the training cohort
CRA recurrence was found in 160 patients (53.2%) during the 2-year follow-up in the training cohort. The clinical characteristics, blood test indicators and characteristics of the adenomas were all recorded during the first colonoscopy. In patients with recurrent adenomas, the proportions of smokers and diabetic patients were higher (all P<0.001). A significant difference in age, sex, alcohol consumption, coronary heart disease, hypertension and family history of CRC between two groups was not found (all P>0.05). Patients with recurrence had a higher proportion of adenomas located in the whole colon than those without recurrence (46.9% vs. 23.4%, P<0.001). The recurrence group had significantly higher proportions of large adenomas (≥10 mm) and multiple adenomas (≥3) (35.6% vs. 14.2%, P<0.001; 61.3% vs. 20.6%, P<0.001). The proportions of villous adenoma and high-grade dysplasia in recurrence group were significantly higher (25.6% vs. 14.9%, P=0.022; 13.1% vs. 5.7%, P=0.029). NLR, PLR and FLR in recurrence group were significantly higher (all P<0.05), and LMR and PNI in recurrence group were significantly lower (all P<0.05) (Table 2).
Table 2
| Variable | Recurrence group (n=160) | Nonrecurrence group (n=141) | P |
|---|---|---|---|
| Age (years) | 62.4±9.3 | 60.0±10.0 | 0.191 |
| Gender | 0.326 | ||
| Male | 103 (64.4%) | 83 (58.9%) | |
| Female | 57 (35.6%) | 58 (41.1%) | |
| Smoking | <0.001*** | ||
| Yes | 78 (48.8%) | 36 (25.5%) | |
| No | 82 (51.2%) | 105 (74.5%) | |
| Drinking | 0.430 | ||
| Yes | 45 (28.1%) | 34 (24.1%) | |
| No | 115 (71.9%) | 107 (75.9%) | |
| CRC family history | 0.714 | ||
| Yes | 7 (4.4%) | 5 (3.5%) | |
| No | 153 (95.6%) | 136 (96.5%) | |
| Diabetes | <0.001*** | ||
| Yes | 59 (36.9%) | 24 (17.0%) | |
| No | 101 (63.1%) | 117 (83.0%) | |
| Coronary heart disease | 0.479 | ||
| Yes | 25 (15.6%) | 18 (12.8%) | |
| No | 135 (84.4%) | 123 (87.2%) | |
| Hypertension | 0.074 | ||
| Yes | 67 (41.9%) | 45 (31.9%) | |
| No | 93 (58.1%) | 96 (68.1%) | |
| Adenoma location | <0.001*** | ||
| Distal colon | 52 (32.5%) | 58 (41.1%) | |
| Proximal colon | 33 (20.6%) | 50 (35.5%) | |
| Both | 75 (46.9%) | 33 (23.4%) | |
| Adenoma size | <0.001*** | ||
| ≤10 mm | 103 (64.4%) | 121 (85.8%) | |
| >10 mm | 57 (35.6%) | 20 (14.2%) | |
| No. of adenomas | <0.001*** | ||
| 1 | 37 (23.1%) | 77 (54.6%) | |
| 2 | 25 (15.6%) | 35 (24.8%) | |
| ≥3 | 98 (61.3%) | 29 (20.6%) | |
| Villous component | 0.022* | ||
| Yes | 41 (25.6%) | 21 (14.9%) | |
| No | 119 (74.4%) | 120 (85.1%) | |
| Differentiation | 0.029* | ||
| Low-grade dysplasia | 139 (86.9%) | 133 (94.3%) | |
| High-grade dysplasia | 21 (13.1%) | 8 (5.7%) | |
| Hemoglobin (g/L) | 134.2±19.1 | 136.8±19.1 | 0.247 |
| NLR | 1.78 (1.30–2.37) | 1.47 (1.17–1.77) | <0.001*** |
| PLR | 115.3 (92.6–153.9) | 101.8 (80.3–127.3) | <0.001*** |
| LMR | 4.15±1.54 | 4.70±1.41 | 0.002** |
| PNI | 50.9±5.8 | 53.6±5.6 | <0.001*** |
| FLR | 1.70 (1.31–2.36) | 1.55 (1.16–1.85) | 0.001** |
Data was expressed as n (%) or mean ± standard deviation or median (interquartile range). *, P<0.05; **, P<0.01; ***, P<0.001. CRA, colorectal adenoma; CRC, colorectal cancer; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; FLR, fibrinogen-to-lymphocyte ratio.
Multivariate analysis of factors associated with CRA recurrence
According to the univariate analysis, smoking, diabetes, adenoma location, size, number, histology, differentiation grade, peripheral blood markers (NLR, PLR, LMR, PNI and FLR) were significantly correlated with recurrence of adenomas (all with P<0.05). The cut-off points of peripheral blood markers (NLR, PLR, LMR, PNI and FLR) were obtained according to the optimal cut-off values obtained in ROC curve analysis according to Youden’s J index, which were 1.94, 90.5, 4.04, 52.1 and 2.32 (Figure 1). The above risk factors with P<0.05 were included in the multivariate regression analysis. After analysis, the results showed that smoking (OR =2.326; 95% CI: 1.250–4.360; P=0.008), diabetes (OR =3.346; 95% CI: 1.715–6.530; P<0.001), adenoma number (OR =6.436; 95% CI: 3.172–13.056; P<0.001), adenoma size (OR =3.057; 95% CI: 1.291–7.240; P=0.011), NLR (OR =3.388; 95% CI: 1.487–7.723; P=0.004) and FLR (OR =2.726; 95% CI: 1.025–7.252; P=0.045) were served as independent risk factors for CRA recurrence (Table 3).
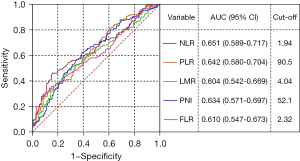
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | ||
| Age (years) | |||||||
| <60 | 1.000 | ||||||
| ≥60 | 1.548 | 0.975–2.456 | 0.064 | ||||
| Gender | |||||||
| Female | 1.000 | ||||||
| Male | 1.263 | 0.792–2.012 | 0.327 | ||||
| Smoking | |||||||
| No | 1.000 | ||||||
| Yes | 2.774 | 1.701–4.525 | <0.001 | 2.326 | 1.250–4.36 | 0.008** | |
| Drinking | |||||||
| No | 1.000 | ||||||
| Yes | 1.231 | 0.734–2.066 | 0.430 | ||||
| CRC family history | |||||||
| No | 1.000 | ||||||
| Yes | 1.244 | 0.386–4.012 | 0.714 | ||||
| Diabetes | |||||||
| No | 1.000 | ||||||
| Yes | 2.848 | 1.653–4.907 | <0.001 | 3.346 | 1.715–6.530 | <0.001*** | |
| Coronary heart disease | |||||||
| No | 1.000 | ||||||
| Yes | 1.265 | 0.658–2.432 | 0.480 | ||||
| Hypertension | |||||||
| No | 1.000 | ||||||
| Yes | 1.537 | 0.957–2.467 | 0.075 | ||||
| Location | |||||||
| Proximal colon | 1.000 | ||||||
| Distal colon | 0.736 | 0.413–1.311 | 0.413 | ||||
| Both | 2.535 | 1.456–4.415 | 0.001 | 1.224 | 0.660–2.497 | 0.578 | |
| Size | |||||||
| ≤10 mm | 1.000 | ||||||
| >10 mm | 3.348 | 1.887–5.939 | <0.001 | 3.057 | 1.291–7.240 | 0.011* | |
| Number | |||||||
| 1 | 1.000 | ||||||
| 2 | 1.486 | 0.779–2.836 | 0.229 | ||||
| ≥3 | 7.033 | 3.975–12.441 | <0.001 | 6.436 | 3.172–13.056 | <0.001*** | |
| Villous component | |||||||
| No | 1.000 | ||||||
| Yes | 1.969 | 1.098–3.530 | 0.023 | 1.197 | 0.543–2.636 | 0.656 | |
| Differentiation | |||||||
| Low-grade dysplasia | 1.000 | ||||||
| High-grade dysplasia | 2.515 | 1.075–5.867 | 0.033 | 0.865 | 0.265–2.820 | 0.809 | |
| Hemoglobin (g/L) | |||||||
| <135 | 1.000 | ||||||
| ≥135 | 0.905 | 0.573–1.429 | 0.669 | ||||
| NLR | |||||||
| <1.94 | 1.000 | ||||||
| ≥1.94 | 4.564 | 2.683–8.074 | <0.001 | 3.388 | 1.487–7.723 | 0.004** | |
| PLR | |||||||
| <90.5 | 1.000 | ||||||
| ≥90.5 | 2.742 | 1.634–4.598 | <0.001 | 1.388 | 0.700–2.751 | 0.348 | |
| LMR | |||||||
| >4.04 | 1.000 | ||||||
| ≤4.04 | 2.439 | 1.513–3.932 | <0.001 | 0.672 | 0.317–1.421 | 0.298 | |
| PNI | |||||||
| >52.1 | 1.000 | ||||||
| ≤52.1 | 2.456 | 1.544–3.907 | <0.001 | 1.339 | 0.709–2.530 | 0.369 | |
| FLR | |||||||
| <2.32 | 1.000 | ||||||
| ≥2.32 | 5.126 | 2.471–10.632 | <0.001 | 2.726 | 1.025–7.252 | 0.045* | |
*, P<0.05; **, P<0.01; ***, P<0.001. CRA, colorectal adenoma; CRC, colorectal cancer; NLR, neutrophil-to-lymphocyte ratio; PLR, platelet-to-lymphocyte ratio; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index; FLR, fibrinogen-to-lymphocyte ratio.
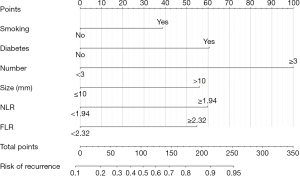
Nomogram development and validation
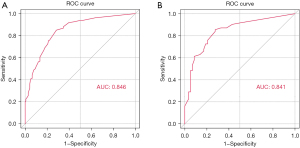
Six independent risk factors associated with risk of recurrence after colonoscopy resection of CRA were identified based on multivariate regression analysis. These six factors were used to create a predictive model for recurrence in CRA patients (Figure 2). The total points of patients were calculated by adding up all individual scores of the six predictors. Different total points corresponded to different risks of recurrence. The nomogram score was 39 for smoking, 61 for diabetes, 100 for adenoma number, 56 for adenoma size, 60 for NLR and 55 for FLR, respectively. The AUCs of the nomogram for predicting the risk of CRA recurrence in two cohorts were 0.846 (95% CI: 0.803–0.890) and 0.841 (95% CI: 0.770–0.913) (Figure 3A,3B).
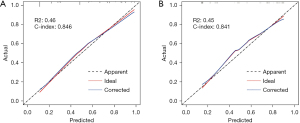
The calibration curves showed that the probability predicted by the model is basically consistent with the actual probability, and had good accuracy (Figure 4A,4B). DCA was a new method to evaluate alternative prognostic strategies with significant advantages over AUC (Figure 5A,5B). The results demonstrated that the line graph model showed a good clinical application value over a wide range of the risks of recurrence of the two cohorts, which indicating that it had a good clinical utility.

Discussion
Studies have shown that more than 95% of CRC develops from adenomatous polyps (21). In recent years, endoscopic resection has become increasingly popular as a method of removing CRAs, thereby reducing CRC incidence and mortality (22). There is a significant clinical issue with the high recurrence rate after resection. Clinical studies showed that 20–50% of CRA patients recurred within 2–5 years after resection (23), or even higher (24), and about 0.3–0.9% of patients could develop CRC (25). Therefore, identifying high-risk factors for recurrence after CRAs removed by colonoscopy can provide a theoretical basis for regular review of colorectal microscopy in patients.
Among 421 patients, CRA recurrence were observed in 222 patients (52.7%). The recurrence rate was high, likely related to the fact that high average age of the population included in the study. Many studies have shown that the pathologic features of adenoma are highly correlated with its recurrence, which are the most commonly used predictors of recurrence (26-28). Our study showed that the size and number of adenomas were significant independent risk factors for recurrence, which was consistent with previous research (29,30). Smoking is a well-known carcinogenic risk factor, which has been proved to be related to the occurrence and progression of CRC (31). Our study found a significant correlation between smoking and CRA recurrence, considering that smoking can promote adenoma development through oxidative DNA damage (32). A foreign study showed a significant increase in the incidence of CRA in people with poor glycemic control (33). The association of hyperglycemia with CRA has been demonstrated in studies (34), which is thought to be related to insulin resistance. Insulin resistance and subsequent hyperinsulinemia lead to increased insulin-like growth factor 1 (IGF1) signaling, both of which are risk factors for CRA (35). Our study also found that the diabetes was an independent risk factor for recurrence (36). Previous researches have found that the family history of CRA or CRC may be a risk factor for adenoma recurrence (19,27,37), which may be associated with autosomal dominant inheritance. However, some studies have found the opposite results (38,39). Our study showed that family history of CRC was not significantly associated with recurrence of CRA, and the sample size needs to be expanded for further studies.
Inflammatory reaction is involved in tumor progression through a series of inflammatory cells, such as neutrophils, lymphocytes, platelets and monocytes (40). Neutrophils are known to regulate the tumor microenvironment and produce cytokines that may promote angiogenesis as well as tumor cell proliferation and migration (41). Lymphocytes play a vital role in antitumor immunity by promoting the apoptosis of tumor cells, thus inhibiting the progression of tumor cells (42). Studies have shown that high NLR is associated with an increase in colorectal adenomatous polyps, which is considered to be related to the continuous inflammatory state in the body (43). Chronic inflammation damages normal colorectal epithelial cells by releasing multiple inflammatory mediators that damage endothelial cells and enhance vascular permeability (44). As a marker of systemic inflammatory response, NLR is of great significance in predicting the prognosis of various cancers (45,46). In patients with progressive CRC, the increase of NLR was associated with a high risk of progression, and the normalization of NLR after chemotherapy improved the progression-free survival of some patients (47). Studies have shown that non-steroidal anti-inflammatory drugs (NSAIDs) can reduce systemic inflammation and the risk of CRC (48). In this study, high NLR was found to be a risk factor for adenoma recurrence, which accorded closely with the above theory.
As well as providing a stable framework for the extracellular matrix of tumors, fibrinogen facilitates tumor angiogenesis, and enhances tumor migration and invasion (49,50). Fibrinogen can facilitate tumor cells evading natural killer cells (51). Many types of cancer have poor prognosis when their fibrinogen levels are high, as shown by recent studies (52-54). Elevated fibrinogen levels are related to an increased risk of CRC (55,56). The increase level of plasma fibrinogen in CRC patients before operation is related to distant metastasis and poor outcomes after radical operation (57). The FLR represents a combination of fibrinogen and lymphocyte that synergistically enhances its individual prognostic value. Moreover, it may reflect the interaction between inflammation and clotting in cancer (16). FLR has been shown to assess tumor prognosis in carcinoma of stomach (15), esophagus cancer (16), non-small cell lung cancer (17) and head and neck adenoid-cystic carcinoma (18). In particular, higher FLR values were associated with poorer outcomes. However, no study has evaluated the relationship between FLR and risk of CRA or CRC. This study suggested that FLR was served as an independent risk factor for adenoma recurrence and had a potential prognostic value.
As mentioned earlier, six independent risk factors contributed to the development of the risk prediction model. The nomogram could shift the complex regression equation to simple and visual graphs, which made the results of the prediction model easier to read. Calibration curve and ROC curve verified that the nomogram had good predictive ability. DCA also confirmed the clinical practicability of this nomogram model.
Our study has a number of limitations. In the first place, the data were collected from only one institution, so the accuracy was limited. Second, the study was retrospective, with incomplete data inclusion, and did not assess the relationship between diet, drugs, physical activity and the risk of adenoma recurrence. We did not evaluate smoking status and alcohol use status, such as daily smoking amount, duration of smoking and variables related to drinking. Third, a 2-year follow-up period may not be enough to observe all recurrent cases. In order to verify these preliminary results, multicenter and prospective trials are needed. Next, we will assess the relationship between different levels of NLR and FLR and the risk of adenoma recurrence, as well as the relationship between different pathological types and adenoma recurrence, so as to provide reference for early intervention and treatment of adenoma recurrence.
Acknowledgments
Funding: This work was supported by funding from Tianjin Science and Technology Bureau Major Disease Prevention and Control Technology Major Special Project (No. 19ZXDBSY00020), the National Natural Science Foundation of China (No. 81900487) and the National Key R&D Program of China (No. 2019YFC0119505).
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-410/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-410/dss
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-410/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-410/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethical Committee of Tianjin Medical University General Hospital (No. IRB2022-WZ-014) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Guren MG. The global challenge of colorectal cancer. Lancet Gastroenterol Hepatol 2019;4:894-5. [Crossref] [PubMed]
- Vogelstein B, Fearon ER, Hamilton SR, et al. Genetic alterations during colorectal-tumor development. N Engl J Med 1988;319:525-32. [Crossref] [PubMed]
- Bonnington SN, Rutter MD. Surveillance of colonic polyps: Are we getting it right? World J Gastroenterol 2016;22:1925-34. [Crossref] [PubMed]
- Rex DK, Schoenfeld PS, Cohen J, et al. Quality indicators for colonoscopy. Gastrointest Endosc 2015;81:31-53. [Crossref] [PubMed]
- Wang J, Zhang XH, Ge J, et al. Endoscopic submucosal dissection vs endoscopic mucosal resection for colorectal tumors: a meta-analysis. World J Gastroenterol 2014;20:8282-7. [Crossref] [PubMed]
- Belderbos TD, Leenders M, Moons LM, et al. Local recurrence after endoscopic mucosal resection of nonpedunculated colorectal lesions: systematic review and meta-analysis. Endoscopy 2014;46:388-402. [Crossref] [PubMed]
- Liu B, Wen P, Gu X, et al. Elevated serum triglyceride predicts recurrence of colorectal polyps in patients with advanced adenomas. Lipids Health Dis 2020;19:211. [Crossref] [PubMed]
- Seo JY, Chun J, Lee C, et al. Novel risk stratification for recurrence after endoscopic resection of advanced colorectal adenoma. Gastrointest Endosc 2015;81:655-64. [Crossref] [PubMed]
- Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature 2008;454:436-44. [Crossref] [PubMed]
- Li Y, Jia H, Yu W, et al. Nomograms for predicting prognostic value of inflammatory biomarkers in colorectal cancer patients after radical resection. Int J Cancer 2016;139:220-31. [Crossref] [PubMed]
- Ming-Sheng F, Mei-Ling D, Xun-Quan C, et al. Preoperative Neutrophil-to-Lymphocyte Ratio, Platelet-to-Lymphocyte Ratio, and CEA as the Potential Prognostic Biomarkers for Colorectal Cancer. Can J Gastroenterol Hepatol 2022;2022:3109165. [Crossref] [PubMed]
- Xu WY, Zhang HH, Yang XB, et al. Prognostic significance of combined preoperative fibrinogen and CA199 in gallbladder cancer patients. World J Gastroenterol 2018;24:1451-63. [Crossref] [PubMed]
- Feng F, Zheng G, Wang Q, et al. Low lymphocyte count and high monocyte count predicts poor prognosis of gastric cancer. BMC Gastroenterol 2018;18:148. [Crossref] [PubMed]
- Huang C, Liu Z, Xiao L, et al. Clinical Significance of Serum CA125, CA19-9, CA72-4, and Fibrinogen-to-Lymphocyte Ratio in Gastric Cancer With Peritoneal Dissemination. Front Oncol 2019;9:1159. [Crossref] [PubMed]
- Fan N, Chen D, Zheng J, et al. A novel preoperative plasma indicator to predict prognoses for patients with esophageal squamous cell carcinoma after radical esophagectomy: fibrinogen-to-lymphocyte ratio. Cancer Manag Res 2019;11:4719-28. [Crossref] [PubMed]
- Liu M, Yang J, Wan L, et al. Elevated Pretreatment Fibrinogen-to-Lymphocyte Percentage Ratio Predict Tumor Staging and Poor Survival in Non-Small Cell Lung Cancer Patients with Chemotherapy or Surgery Combined with Chemotherapy. Cancer Manag Res 2021;13:4921-33. [Crossref] [PubMed]
- Brkic FF, Stoiber S, Friedl M, et al. The Potential Prognostic Value of a Novel Hematologic Marker Fibrinogen-to-Lymphocyte Ratio in Head and Neck Adenoid-Cystic Carcinoma. J Pers Med 2021;11:1228. [Crossref] [PubMed]
- Jacobs ET, Martínez ME, Alberts DS, et al. Association between body size and colorectal adenoma recurrence. Clin Gastroenterol Hepatol 2007;5:982-90. [Crossref] [PubMed]
- Huang Y, Gong W, Su B, et al. Recurrence and surveillance of colorectal adenoma after polypectomy in a southern Chinese population. J Gastroenterol 2010;45:838-45. [Crossref] [PubMed]
- Kalus M. Carcinoma and adenomatous polyps of the colon and rectum in biopsy and organ tissue culture. Cancer 1972;30:972-82. [Crossref] [PubMed]
- Zauber AG, Winawer SJ, O'Brien MJ, et al. Colonoscopic polypectomy and long-term prevention of colorectal-cancer deaths. N Engl J Med 2012;366:687-96. [Crossref] [PubMed]
- Alberts DS, Martínez ME, Roe DJ, et al. Lack of effect of a high-fiber cereal supplement on the recurrence of colorectal adenomas. Phoenix Colon Cancer Prevention Physicians' Network. N Engl J Med 2000;342:1156-62. [Crossref] [PubMed]
- Choi WS, Han DS, Eun CS, et al. Three-year colonoscopy surveillance after polypectomy in Korea: a Korean Association for the Study of Intestinal Diseases (KASID) multicenter prospective study. Intest Res 2018;16:126-33. [Crossref] [PubMed]
- Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology 2012;143:844-57. [Crossref] [PubMed]
- Fairley KJ, Li J, Komar M, et al. Predicting the risk of recurrent adenoma and incident colorectal cancer based on findings of the baseline colonoscopy. Clin Transl Gastroenterol 2014;5:e64. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Serviddio G, et al. Factors Associated With Recurrence of Advanced Colorectal Adenoma After Endoscopic Resection. Clin Gastroenterol Hepatol 2016;14:1148-54.e4. [Crossref] [PubMed]
- van Heijningen EM, Lansdorp-Vogelaar I, Kuipers EJ, et al. Features of adenoma and colonoscopy associated with recurrent colorectal neoplasia based on a large community-based study. Gastroenterology 2013;144:1410-8. [Crossref] [PubMed]
- Facciorusso A, Di Maso M, Serviddio G, et al. Development and validation of a risk score for advanced colorectal adenoma recurrence after endoscopic resection. World J Gastroenterol 2016;22:6049-56. [Crossref] [PubMed]
- Jang ES, Kim JW, Jung YJ, et al. Clinical and endoscopic predictors of colorectal adenoma recurrence after colon polypectomy. Turk J Gastroenterol 2013;24:476-82. [Crossref] [PubMed]
- Haq S, Ali S, Mohammad R, et al. The complexities of epidemiology and prevention of gastrointestinal cancers. Int J Mol Sci 2012;13:12556-72. [Crossref] [PubMed]
- Fagunwa IO, Loughrey MB, Coleman HG. Alcohol, smoking and the risk of premalignant and malignant colorectal neoplasms. Best Pract Res Clin Gastroenterol 2017;31:561-8. [Crossref] [PubMed]
- Huang X, Fan Y, Zhang H, et al. Association between serum HbA1c levels and adenomatous polyps in patients with the type 2 diabetes mellitus. Minerva Endocrinol 2015;40:163-7. [PubMed]
- Ottaviano LF, Li X, Murray M, et al. Type 2 diabetes impacts colorectal adenoma detection in screening colonoscopy. Sci Rep 2020;10:7793. [Crossref] [PubMed]
- Rinaldi S, Cleveland R, Norat T, et al. Serum levels of IGF-I, IGFBP-3 and colorectal cancer risk: results from the EPIC cohort, plus a meta-analysis of prospective studies. Int J Cancer 2010;126:1702-15. [Crossref] [PubMed]
- Taniguchi L, Higurashi T, Uchiyama T, et al. Metabolic factors accelerate colorectal adenoma recurrence. BMC Gastroenterol 2014;14:187. [Crossref] [PubMed]
- Fossi S, Bazzoli F, Ricciardiello L, et al. Incidence and recurrence rates of colorectal adenomas in first-degree asymptomatic relatives of patients with colon cancer. Am J Gastroenterol 2001;96:1601-4. [Crossref] [PubMed]
- Xi X, Fu Z, Liu T, et al. Establishment and Verification of Scoring System for Colorectal Adenoma Recurrence. Risk Manag Healthc Policy 2021;14:4545-52. [Crossref] [PubMed]
- Saiken A, Gu F. Lifestyle and lifestyle-related comorbidities independently associated with colorectal adenoma recurrence in elderly Chinese people. Clin Interv Aging 2016;11:801-5. [Crossref] [PubMed]
- Chen Y, Wang W, Zhang X, et al. Prognostic significance of combined preoperative platelet-to-lymphocyte ratio and lymphocyte-to-monocyte ratio in patients undergoing surgery with stage IB non-small-cell lung cancer. Cancer Manag Res 2018;10:5411-22. [Crossref] [PubMed]
- Şahin AB, Cubukcu E, Ocak B, et al. Low pan-immune-inflammation-value predicts better chemotherapy response and survival in breast cancer patients treated with neoadjuvant chemotherapy. Sci Rep 2021;11:14662. [Crossref] [PubMed]
- Ohtani H. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human colorectal cancer. Cancer Immun 2007;7:4. [PubMed]
- Kim JH, Cho KI, Kim YA, et al. Elevated Neutrophil-to-Lymphocyte Ratio in Metabolic Syndrome Is Associated with Increased Risk of Colorectal Adenoma. Metab Syndr Relat Disord 2017;15:393-9. [Crossref] [PubMed]
- Jablonska E, Piotrowski L, Jablonski J, et al. VEGF in the culture of PMN and the serum in oral cavity cancer patients. Oral Oncol 2002;38:605-9. [Crossref] [PubMed]
- Paik KY, Lee IK, Lee YS, et al. Clinical implications of systemic inflammatory response markers as independent prognostic factors in colorectal cancer patients. Cancer Res Treat 2014;46:65-73. [Crossref] [PubMed]
- Mano Y, Shirabe K, Yamashita Y, et al. Preoperative neutrophil-to-lymphocyte ratio is a predictor of survival after hepatectomy for hepatocellular carcinoma: a retrospective analysis. Ann Surg 2013;258:301-5. [Crossref] [PubMed]
- Chua W, Charles KA, Baracos VE, et al. Neutrophil/lymphocyte ratio predicts chemotherapy outcomes in patients with advanced colorectal cancer. Br J Cancer 2011;104:1288-95. [Crossref] [PubMed]
- Bibbins-Domingo KU.S. Preventive Services Task Force. Aspirin Use for the Primary Prevention of Cardiovascular Disease and Colorectal Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med 2016;164:836-45. [Crossref] [PubMed]
- Palumbo JS, Talmage KE, Massari JV, et al. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood 2005;105:178-85. [Crossref] [PubMed]
- Mei Y, Liu H, Sun X, et al. Plasma fibrinogen level may be a possible marker for the clinical response and prognosis of patients with breast cancer receiving neoadjuvant chemotherapy. Tumour Biol 2017;39:1010428317700002. [Crossref] [PubMed]
- Zhang Y, Cao J, Deng Y, et al. Pretreatment plasma fibrinogen level as a prognostic biomarker for patients with lung cancer. Clinics (Sao Paulo) 2020;75:e993. [Crossref] [PubMed]
- Thurner EM, Krenn-Pilko S, Langsenlehner U, et al. The association of an elevated plasma fibrinogen level with cancer-specific and overall survival in prostate cancer patients. World J Urol 2015;33:1467-73. [Crossref] [PubMed]
- Zhang Y, Liu N, Liu C, et al. High Fibrinogen and Platelets Correlate with Poor Survival in Gastric Cancer Patients. Ann Clin Lab Sci 2020;50:457-62. [PubMed]
- Li M, Wu Y, Zhang J, et al. Prognostic value of pretreatment plasma fibrinogen in patients with colorectal cancer: A systematic review and meta-analysis. Medicine (Baltimore) 2019;98:e16974. [Crossref] [PubMed]
- Erdogan S, Yilmaz FM, Yazici O, et al. Inflammation and chemerin in colorectal cancer. Tumour Biol 2016;37:6337-42. [Crossref] [PubMed]
- Allin KH, Bojesen SE, Nordestgaard BG. Inflammatory biomarkers and risk of cancer in 84,000 individuals from the general population. Int J Cancer 2016;139:1493-500. [Crossref] [PubMed]
- Tang L, Liu K, Wang J, et al. High preoperative plasma fibrinogen levels are associated with distant metastases and impaired prognosis after curative resection in patients with colorectal cancer. J Surg Oncol 2010;102:428-32. [Crossref] [PubMed]


