
Anti-OJ antibody-positive anti-synthetase syndrome with repeated arthritis, fever, and recurrent liver cancer: a case report
Introduction
Anti-aminoacyl transfer RNA synthetase is one of the most common types of specific autoantibodies in patients with polymyositis/dermatomyositis (PM/DM) (1). These patients often present with a fever, arthritis, Raynaud’s phenomenon, myopathy, mechanic’s hand, and interstitial lung disease (ILD), called anti-synthetase syndrome (ASS). Based on the different types of anti-synthetase antibodies, there are different ASS subtypes. To date, 10 anti-synthetase antibodies have been found (2,3), among which the anti-jo-1 antibody is the most common anti-synthetase antibody (approximately 20–30% of PM/DM patients) (4). Anti-isoleucyl-transfer RNA synthetase (anti-OJ) antibodies are found in <5% of PM/DM patients (5). Patients with different clinical subtypes of ASS have different anti-synthetase antibodies and different clinical manifestations, chest imaging findings, and prognoses, among other characteristics (6). Currently, there are still few clinical reports on anti-OJ antibody-positive ASS. In this article, we report a case of an anti-OJ antibody-positive ASS, with severe multi joint pain, high fever and increased inflammatory indicators, but occult myositis. The patient was misdiagnosed for more than 1 year and recurrent hepatocellular carcinoma was confirmed 1 year after the diagnosis of ASS. This clinical manifestation of an anti-OJ antibody-positive ASS has not been reported before. We report this case in order to raise awareness to the disease. For patients with recurrent unexplained arthritis accompanied by fever, the possibility of ASS should be considered. We present the following case in accordance with the CARE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-720/rc).
Case presentation
A 75-year-old male, retired worker, was admitted to our hospital complaining of recurrent joint pain for more than 1 year, recurrent fever for 6 months, and recurrence of joint pain and fever for 1 week. Both large and small joints were involved, such as the left ankle, the 3rd metacarpophalangeal joint of the right hand, the right shoulder, and the left wrist. The joint pain affected the patient’s activity.
A year before, the patient had repeatedly visited an orthopaedic clinic and intermittently took etoricoxib. Some 6 months before the current presentation, the patient was admitted to our hospital with a fever. A complete blood count showed an increased white blood cell (WBC) count of 30,500/µL (neutrophils: 90.1%) with mild anaemia (haemoglobin: 11.9 g/dL), and a platelet count of 194×109/L. The laboratory results further showed the following inflammation markers: C-reactive protein (CRP): 140.79 mg/dL; erythrocyte sedimentation rate (ESR): 34 mm/h; procalcitonin (PCT): 0.48 ng/mL; and ferritin: 460.8 ng/mL. Tests for rheumatoid factors and cyclic citrullinated peptide immunoglobulin G antibodies and the anti-nuclear antibody titre were negative.
Computed tomography (CT) of the chest and abdomen revealed postoperative changes indicating liver cancer. A colour ultrasound showed that the heart was normal. During the hospital stay, the patient had repeated episodes of fever and joint pain. The patient underwent a bone marrow puncture and biopsy, and the results were normal. A dual-energy CT for gout indicated a few urate crystals deposited in the bilateral radiocarpal joints, but the blood uric acid levels were normal. Infectious fever and gout were considered, and the patient was successively treated with piperacillin/tazobactam sodium [4.5 g quaque (q) 8 h intravenously for 11 days], imipenem (1.0 g q8 h intravenously for 6 days), linezolid (0.6 g q12 h orally for 10 days), and loxoprofen (60 mg q12 h orally for 3 days). The patient improved and was discharged on the 24th day of hospitalization.
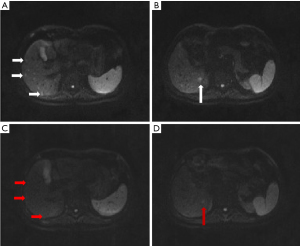
Some 2 months before the current presentation, the patient was admitted to our hospital again with similar symptoms. The laboratory results were as follows: WBC count: 16,600/µL (neutrophils: 92.1%); CRP: 97.3 mg/L; ESR: 79 mm/h; and PCT: 0.09 ng/mL. The results of the human leucocyte antigen-B27, tuberculin, and Tuberculosis infection T cell spot test (T-SPOT-TB) tests were negative. An abdominal CT indicated multiple new low-density foci in the liver and enhanced magnetic resonance imaging (MRI) of the upper abdomen showed multiple scattered enhanced nodules in the liver, indicating that metastasis should be considered (see Figure 1A,1B). However, positron emission tomography–CT showed that inflammatory lesions should be considered first. The patient was treated with levofloxacin (0.5 g qd intravenously for 5 days) and etoricoxib (60 mg q12 h orally for 7 days). The patient was discharged on the 7th day of hospitalization.
The patient had a non-specific ailment, chronic viral hepatitis B, and had engaged in the long-term use of lamivudine. He had a history of HCC surgery 11 years ago and had also received 3 cycles of postoperative chemotherapy. No tumour recurrence was found in the regular re-examinations, and his last follow-up was 2 years ago. The patient had also had hypertension and type 2 diabetes for >10 years, both of which were well controlled with drugs.
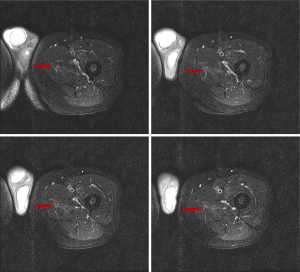
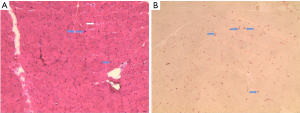
At this presentation, the physical examination showed that the skin on the right 3rd metacarpophalangeal joint was red and swollen with obvious tenderness, and there was tenderness in the right shoulder, lumbosacral region, lower back, left wrist joint, and left ankle joint. Additionally, there were also several areas of muscle tenderness, such as in the lateral muscle of the left thigh. The routine blood results were as follows: WBC count: 36,800/µL (neutrophils: 95.6%); CRP: 261.86 mg/L; and PCT: 0.11 ng/mL. Enhanced MRI of the upper abdomen was repeated, and it revealed that many of the nodules in the liver had disappeared, except for the nodule in segment VI (see Figure 1C,1D). Because of the muscle tenderness, we conducted a further physical examination and found that the patient had suffered from decreased proximal muscle strength and muscle weakness for several months. Multiple creatinine kinase/creatine kinase-myocardial band (MB) tests were normal. A myositis antibody test was completed and was positive for anti-OJ antibodies. Enhanced MRI of the left thigh and lower leg showed abnormal T2 signals in the medial thigh muscle group (see Figure 2). A muscle biopsy suggested myositis (see Figure 3). A chest high-resolution CT revealed that the patient did not have the clinical manifestations of ILD or mechanic’s hand.
The patient was eventually diagnosed with ASS and treated with prednisone (30 mg/day for 10 days). The treatment was stopped by the patient following remission, after which the condition remained in remission. The patient intermittently took antipyretic analgesics and had occasional joint pain and fever.
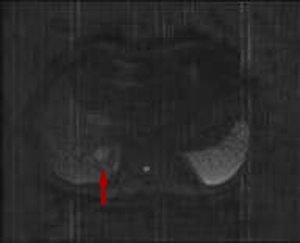
After 1 year of follow-up, enhanced MRI of the upper abdomen showed a mass occupying the right hepatic lobe (segment VI) that had become enlarged, and recurrence was considered (see Figure 4). Postoperative pathology showed HCC. After the operation, the patient’s joint pain, fever, and muscle weakness improved. At present, the patient has been followed up for nearly 3 years and has not been treated with drugs for ASS, his general condition is good, and he has no interstitial pneumonia (see Figure 5). All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Hwa Mei Hospital, University of Chinese Academy of Sciences (IRB number: PJ-KY-NBEY-2021-101-01). Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent form is available for review by the Editor of this journal.
Discussion
ASS is a heterogenous, rare group of systemic autoimmune diseases that manifest as an inflammatory myopathy. In 2010, Connors et al. (7) formally proposed the diagnostic criteria for ASS; that is, positive for serum anti-synthetase antibody and at least 1 of the following clinical manifestations: Raynaud’s phenomenon, arthritis, ILD, fever, or mechanic’s hand.
In this case, the patient had no imaging manifestations of interstitial pneumonia, and the condition was mainly characterized by multiple episodes of arthritis and fever. Clinically, the joint pain was severe, and the myositis was insidious. Thus, the patient’s symptoms differed to those detailed in previous reports. Marie et al. (8) proposed that the incidence of ILD is higher among patients with ASS without anti-JO-1 antibodies than those with ASS with anti-JO-1 antibodies. Ge et al. (9) found that 90% of anti-OJ antibody-positive patients in China had ILD. Thus, the diagnosis of this case was extremely difficult. Finally, the patient was transferred to the Departments of Orthopaedics, Infectious Medicine, and Rheumatology, among others, resulting in a misdiagnosis for >1 year. We also found that ASS can cause a significant increase in inflammatory markers, which has not been reported previously.
The association between PM/DM and malignant diseases has been reported and confirmed (10,11). Studies have found that ASS and interstitial pneumonia have a negative correlation with cancer-associated myositis (12-14), but there are also cases of various ASS-related diseases combined with malignant tumours (15,16). In this case, myositis, arthritis, and fever were manifestations of paraneoplastic syndrome. It is possible that the patient’s specific immune system was immature before HCC resection, so there was no autoimmune response against the joints or muscles, even in the presence of anti-OJ antibodies against HCC cell antigens. However, memory B cells retain the ability to generate an immune response to these liver cancer antigens. As the cancer recurs, the B-cell production of OJ antibodies is triggered; the subsequent number of antibodies may be high enough to cause autoimmune inflammation of the joints and muscle tissues expressing OJ-like antigens (17).
ASS usually has a poor prognosis, depending on the involvement of interstitial pneumonia (18). Some patients positive for anti-OJ antibodies have a good prognosis (19-21). In this case, the patient was not treated with drugs after tumour resection, and the disease remained in remission.
We reported the 1st case of anti-OJ antibody-positive ASS associated with HCC recurrence in which the patient presented with recurrent arthritis, fever, significantly elevated inflammatory markers, and occult myositis. We have also gained some clinical experience in the diagnosis and treatment of ASS: (I) When the patient's joint pain and fever are prominent, muscle pain and muscle weakness are often neglected, and it is necessary to carefully ask the medical history and physical examination. (II) For patients with recurrent unexplained arthritis accompanied by fever, we should consider ASS, and myositis antibody tests should be performed if necessary. (III) Patients with a history of tumours should be monitored for tumour recurrence. (IV) The symptoms of ASS secondary to tumor can be completely relieved after anti-tumor treatment.
Acknowledgments
Funding: This work was supported by Key Medical Subjects of Joint Construction Between Provinces and Cites (Infectious Diseases), China (Grant No. 2016-S04); the Key Discipline Foundation of Hwa Mei Hospital, University of Chinese Academy of Sciences (Grant No.2020ZDXK03); Medical Scientific Research Foundation of Zhejiang Province, China (Grant No. 2019KY598). The funding bodies supported the publication fees but had no influence on the diagnosis and treatment of the patient, or the writing of manuscript.
Footnote
Reporting Checklist: The authors have completed the CARE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-720/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-720/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Hwa Mei Hospital, University of Chinese Academy of Sciences (IRB number: PJ-KY-NBEY-2021-101-01). Written informed consent was obtained from the patient for the publication of this case report and any accompanying images. A copy of the written consent form is available for review by the Editor of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Lazarou IN, Guerne PA. Classification, diagnosis, and management of idiopathic inflammatory myopathies. J Rheumatol 2013;40:550-64. [Crossref] [PubMed]
- Witt LJ, Curran JJ, Strek ME. The Diagnosis and Treatment of Antisynthetase Syndrome. Clin Pulm Med 2016;23:218-26. [Crossref] [PubMed]
- Chatterjee S, Prayson R, Farver C. Antisynthetase syndrome: not just an inflammatory myopathy. Cleve Clin J Med 2013;80:655-66. [Crossref] [PubMed]
- Jiang M, Dong X, Zheng Y. Clinical characteristics of interstitial lung diseases positive to different anti-synthetase antibodies. Medicine (Baltimore) 2021;100:e25816. [Crossref] [PubMed]
- Noguchi E, Uruha A, Suzuki S, et al. Skeletal Muscle Involvement in Antisynthetase Syndrome. JAMA Neurol 2017;74:992-9. [Crossref] [PubMed]
- Cavagna L, Trallero-Araguás E, Meloni F, et al. Influence of Antisynthetase Antibodies Specificities on Antisynthetase Syndrome Clinical Spectrum Time Course. J Clin Med 2019;8:2013. [Crossref] [PubMed]
- Connors GR, Christopher-Stine L, Oddis CV, et al. Interstitial lung disease associated with the idiopathic inflammatory myopathies: what progress has been made in the past 35 years? Chest 2010;138:1464-74. [Crossref] [PubMed]
- Marie I, Josse S, Decaux O, et al. Comparison of long-term outcome between anti-Jo1- and anti-PL7/PL12 positive patients with antisynthetase syndrome. Autoimmun Rev 2012;11:739-45. [Crossref] [PubMed]
- Ge YP, Zhang YL, Shu XM, et al. Clinical characteristics of anti-isoleucyl-tRNA synthetase antibody associated syndrome and comparison with different patient cohorts. Clin Exp Rheumatol 2022;40:625-30. [Crossref] [PubMed]
- Yang SH, Chang C, Lian ZX. Polymyositis and dermatomyositis - challenges in diagnosis and management. J Transl Autoimmun 2019;2:100018. [Crossref] [PubMed]
- Zanframundo G, Tripoli A, Cometi L, et al. One year in review 2020: idiopathic inflammatory myopathies. Clin Exp Rheumatol 2021;39:1-12. [Crossref] [PubMed]
- Ponyi A, Constantin T, Garami M, et al. Cancer-associated myositis: clinical features and prognostic signs. Ann N Y Acad Sci 2005;1051:64-71. [Crossref] [PubMed]
- Li Y, Jia X, Sun X, et al. Risk factors for cancer-associated myositis: A large-scale multicenter cohort study. Int J Rheum Dis 2021;24:268-73. [Crossref] [PubMed]
- Rozelle A, Trieu S, Chung L. Malignancy in the setting of the anti-synthetase syndrome. J Clin Rheumatol 2008;14:285-8. [Crossref] [PubMed]
- Betteridge ZE, Priest L, Cooper RG, et al. Investigation of myositis and scleroderma specific autoantibodies in patients with lung cancer. Arthritis Res Ther 2018;20:176. [Crossref] [PubMed]
- Fukui S, Kobayashi K, Fujita Y, et al. Anti-EJ Antibody-positive Anti-synthetase Syndrome Associated with Retroperitoneal Sarcoma. Intern Med 2020;59:2071-6. [Crossref] [PubMed]
- Casciola-Rosen L, Nagaraju K, Plotz P, et al. Enhanced autoantigen expression in regenerating muscle cells in idiopathic inflammatory myopathy. J Exp Med 2005;201:591-601. [Crossref] [PubMed]
- Liu H, Xie S, Liang T, et al. Prognostic factors of interstitial lung disease progression at sequential HRCT in anti-synthetase syndrome. Eur Radiol 2019;29:5349-57. [Crossref] [PubMed]
- Kunimasa K, Arita M, Nakazawa T, et al. The clinical characteristics of two anti-OJ (anti-isoleucyl-tRNA synthetase) autoantibody-positive interstitial lung disease patients with polymyositis/dermatomyositis. Intern Med 2012;51:3405-10. [Crossref] [PubMed]
- Kapoor A, Vaidyan P, Jalil B, et al. Novel case of anti-synthetase syndrome. Eur J Rheumatol 2018;5:275-7. [Crossref] [PubMed]
- Vulsteke JB, Satoh M, Malyavantham K, et al. Anti-OJ autoantibodies: Rare or underdetected? Autoimmun Rev 2019;18:658-64. [Crossref] [PubMed]



