
Challenges in the diagnosis of marginal zone lymphoma with symptoms of small intestinal disease: a case report and scoping review of the literature
Introduction
The gastrointestinal (GI) tract is the most frequent extranodal site for non-Hodgkin lymphoma (NHL) involvement (1,2). Primary small intestinal lymphoma is a rare condition, accounting for 15–20% of gastrointestinal lymphomas (3,4). Small intestinal marginal zone B cell lymphoma (MZL) of mucosa-associated lymphoid tissue (MALT) is an even less common disease accounting for about 15–30% of primary small bowel lymphomas (5-8). In the literature the term “intestinal MALT-lymphoma” is often used synonymously for “intestinal MZL”. In this article we use the term “small intestinal MZL” to describe MALT-lymphoma/ MZL of the small bowel. There seems to be a strong geographic variation: it is the second most common subtype (after diffuse large B cell lymphoma, not otherwise specified, DLBCL-NOS) in Asia, the third most common subtype (after DLBCL-NOS and Burkitt lymphoma) in India and the fourth most common subtype (after DLBCL-NOS, follicular and Burkitt lymphoma) in the USA (6,9-12). The reasons for these epidemiological differences are unclear but may be related to environmental factors and genetic predisposition.
Intestinal MZL occurs predominately in patients older than 50 years and can present with abdominal pain, weight loss, bleeding, obstruction or non-specific gastrointestinal symptoms (13). A correct diagnosis is of increasing importance since intestinal MZL generally responds well to therapy. Despite the possibility of relapses, patients have a good overall prognosis with a 5- and 10-year survival rate of around 75–85% (6,14,15).
Diagnosis of small intestinal MZL remains challenging since most tumors are out of the reach of standard gastroscopy and colonoscopy. Video capsule endoscopy is widely used to diagnose small bowel diseases because it is non-invasive and easy to perform, while balloon-assisted enteroscopy also allows for diagnostic tissue sampling in the deep portions of the jejunum and ileum. Despite advances in both techniques, assessment of the small intestine for verification or exclusion of small intestinal lymphoma remains difficult.
The best diagnostic approach in a patient with suspected small intestinal MZL is unclear. Scarcity of cases renders large diagnostic case series challenging. Furthermore, changes in lymphoma classification over time limit direct comparison of many existing studies. Moreover, in many case series, intestinal MZL is only reported together with other NHL. Finally, small intestinal MZL case series frequently focus on treatment and outcome (14-16) but much less on the diagnostic workup. For instance, the endoscopic appearance of small intestinal MZL remains insufficiently characterized and descriptions are limited to either polypoid or ulcerative lesions in existing case series (6,13).
We describe the challenging diagnostic process in a patient with intestinal MZL, including endoscopy, bone marrow biopsy, 2-18F-fluorodeoxyglucose-positron emission tomography/computed tomography (2-18F-FDG-PET/CT) and surgery. We also performed a scoping literature search, which identified 52 additional case reports with small intestinal lymphoma. These results provide a detailed view on the diagnostic efforts frequently necessary for successful diagnosis of small intestinal MZL. The present study was undertaken to examine the extent, range, and nature of research activity related to this topic, to summarize the available research findings and to identify gaps in the existing literature. We chose to systematically search the literature for a scoping rather than for a narrative review because of the scarcity of existing research evidence and the lack of previous large cohort studies or extensive reviews in regard to this rare disorder. We present the following article in accordance with the PRISMA-ScR and the CARE reporting checklists (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-74/rc).
Case presentation
A 57-year-old patient was referred to our outpatient clinic in January 2019 with a 10-year history of postprandial epigastric and lower abdominal pain. Symptoms had aggravated over the last three years with additional bloating, borborygmi and intolerance to milk and dairy products. He reported three significant acute exacerbations during the ten weeks prior to the current presentation characterized by severe abdominalgia preceded by pruritus and urticaria on arms and thighs two days before the onset of pain. There was a tendency towards constipation with otherwise normal bowel movements without visible blood. The patient’s appetite was decreased with a weight loss of 6 kg within the last 6 months. He denied fever or chills. Medical history was remarkable for appendectomy without a family history of gastrointestinal malignancy. Physical examination revealed diffuse lower abdominal tenderness on palpation. Laboratory tests showed mild inflammation (C-reactive protein, CRP 30 mg/L), slight lymphopenia and moderately elevated fecal calprotectin levels (120 and 245 mg/kg in two consecutive measurements, respectively, Table 1). The serum lactate dehydrogenase levels were normal (401 U/L). Serological tests for HCV, HBV and HIV, anti-transglutaminase, anti-nuclear, antineutrophil cytoplasmic and anti-saccharomyces cerevisiae antibodies were negative. Serum immunoglobulin M (IgM) level was elevated (7.56 g/L, normal 0.4–2.3) with an IgM-κ monoclonal gammopathy in serum immunofixation electrophoresis and free κ-light chains in urine electrophoresis.
Table 1
| Blood parameters | Value | Reference values |
|---|---|---|
| Hemoglobin (g/L) | 135 | 135–168 |
| White blood count (109/L) | 5.16 | 3–10.5 |
| Neutrophils | 3.96 | 1.6–7.4 |
| Monocytes | 0.37 | 0.2–0.93 |
| Eosinophils | 0.11 | 0.02–0.4 |
| Basophiles | 0.01 | 0–0.15 |
| Lymphocytes | 0.69 | 1.1–3.5 |
| Platelets (g/L) | 182 | 150–450 |
| Albumin (g/L) | 43 | 35–52 |
| Protein (g/L) | 66.1 | 64–83 |
| C-reactive protein (mg/L) | 30 | <5 |
| Lactate dehydrogenase (U/L) | 401 | <480 |
| IgG (g/L) | 8.9 | 7–16 |
| ΙgM (g/L) | 7.56 | 0.4–2.3 |
| IgA (g/L) | 1.71 | 0.7–4 |
| Free kappa light chains (mg/L) | 42.7 | 6.7–22.4 |
| Calprotectin (mg/kg) | 120.1 | <50 |
| ANA titer | Borderline negative | <1:80 |
| ANCA titer | Negative | <1:80 |
| ASCA IgA (units/mL) | 11 | <20 |
| ASCA IgG (units/mL) | 14 | <20 |
| Tissue transglutaminase IgA (CU) | <1.9 | <20 |
| Complement C3 (g/L) | 1.47 | 0.9–1.8 |
| Complement C4 (g/L) | 0.26 | 0.1–0.4 |
| β2 microglobulin (mg/L) | 2.05 | 0.8–2.2 |
| sTNF-R1 (pg/mL) | 1,614 | – |
| IL-6 (pg/mL) | 10 | <7 |
| Rheumatoid factor IgA (IU/mL) | 6.2 | <14 |
| Rheumatoid factor IgM (IU/mL) | 1.6 | <3.5 |
| HBsAg | Negative | |
| HBsAb | Positive | |
| HBcAb | Negative | |
| HIV 1+2 | Negative | |
| Anti-HCV | Negative |
Ig, immunoglobulin; ANA, antinuclear antibodies; ANCA, antineutrophil cytoplasmic antibodies; ASCA, anti-Saccharomyces cerevisiae antibodies; sTNF-R1, soluble tumor necrosis factor-receptor 1; IL-6, interleukin-6; HBsAg, hepatitis B surface antigen; HBsAb, hepatitis B surface antibody; HBcAb, hepatitis B core antibody; HIV, human immunodeficiency virus; HCV, hepatitis C virus; CU, chemiluminescent units; IU, international units.
Upper gastrointestinal endoscopy and ileocolonoscopy revealed no abnormalities, except for an isolated aphthous lesion in the terminal ileum. Biopsies were unremarkable without evidence of Whipple’s disease or ongoing infection with Helicobacter pylori. An abdominal computed tomography (CT) scan revealed a marked, approximately 6 cm long, segmental jejunal wall thickening with adjacent mesenteric fat stranding, discrete wall thickening of the cecum and terminal ileum with mild mesenteric lymphadenopathy (≤8 mm in diameter) and mild splenomegaly (craniocaudal diameter 13.3 cm). Contrast-enhanced CT scan of neck and chest did not reveal any lesions suspicious for lymphoma or tuberculosis and a magnetic resonance (MR) enteroclysis did not confirm any intestinal pathology.
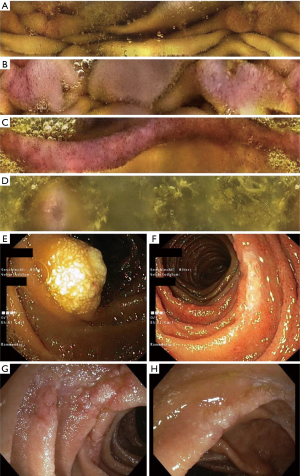
For small intestinal workup, we performed video capsule endoscopy (VCE, CapsoCam Plus®, CapsoVision, Inc. Saratoga, CA, USA). VCE demonstrated aphthous erosions and denuded mucosal areas in the distal duodenum, focal erythema, edematous folds and white tipped villi in the proximal jejunum, circumferential mucosal edema and erythema in the proximal ileum and patchy erythema and isolated aphthous erosions in the distal ileum (Figure 1). Push enteroscopy and subsequent single-balloon enteroscopy revealed areas of whitish, denuded, granular mucosa and focal lymphangiectasia in the proximal jejunum, 20 cm distal to the Treitz ligament, with slightly edematous mucosa between lesions. Another focus with edema and superficial erosions was seen approximately 50 cm beyond the ligament of Treitz. Similar lesions were also found at 100 and 130 cm distal to the duodenojejunal flexure, respectively. Histology revealed focal, moderate to severe active mucosal inflammation with erosions and numerous dilated lymph vessels (lymphangiectasias) but no concrete signs of malignancy despite extensive workup, which included immunohistochemical and molecular investigation of the biopsies. Indeed, polymerase chain reaction for heavy and light chains (IgH and IgK PCR; BIOMED2) was performed rather early on, once suspicion for lymphoma became relevant. However, the results of these tests showed a polyclonal pattern. In view of the rather low quantity of infiltrating B-cells within the biopsies, however, even a small clonal population may have not been picked up: in this setting, negative results do not exclude minimal lymphoma infiltration.
Flow cytometry of peripheral blood and of the bone marrow aspirate was suspicious for a lymphoproliferative disorder. B-lymphocytes showed a restriction for κ-light chains and were positive for CD19, CD20, CD22, CD23, CD81, FMC7, CD79b and CD25 but showed no expression of CD5, CD10, CD103 and CD11c suggestive for a lymphoplasmocytic lymphoma or MZL. An additional bone marrow biopsy did not reveal any significant or pathological infiltration of lymphocytes suggestive of a B-cell lymphoma.
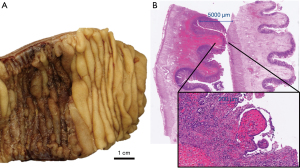
An empiric treatment with ciprofloxacin and metronidazole only briefly relieved intestinal symptoms. Due to progressive abdominal pain and continuing, relevant weight loss, the patient underwent diagnostic laparoscopy in September 2019 with resection of a narrowed, hyperemic and stiff jejunal segment 30 cm distal to the ligament of Treitz with thickened mesentery. Histology showed lamina propria fibrosis, ischemic-like mucosal alterations, lymphatic and vascular congestion and ulcerations but no evidence of lymphoma (Figure 2).
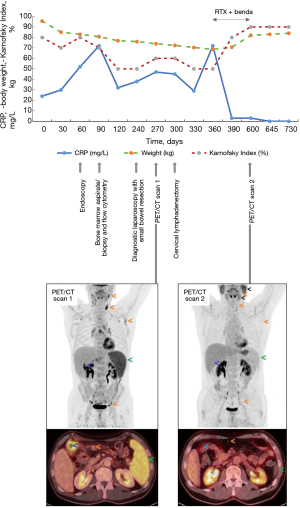
A subsequent 2-18F-FDG-PET/CT showed enlarged, moderately hypermetabolic lymph nodes on both sides of the diaphragm, splenomegaly with diffuse increased uptake and a circumscribed, hypermetabolic wall thickening of a small bowel loop in the right upper abdomen (attributable either to lymphoma or to the recent surgery), with slight prestenotic luminal dilatation (PET/CT scan 1 in Figure 3).
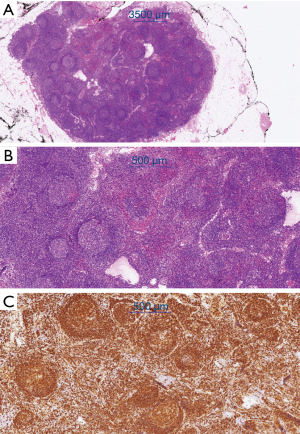
Resection of a metabolically active cervical lymph node in November 2019 confirmed partial involvement by a marginal zone lymphoma. The histological examination revealed a focal marginal zone-like growth pattern of clonal B-lymphocytes (as detected by clonal immunoglobulin heavy chain/IgH rearrangement) with a so-called “null phenotype” (no expression of CD5, Cyclin D1, CD23, bcl-6 and CD10) (Figure 4). Digital droplet polymerase chain reaction (PCR) did not detect the presence of a MYD88/L265P mutation, effectively excluding lymphoplasmacytic lymphoma in this context. We could not formally distinguish between primary intestinal MZL and nodal MZL with secondary spread to the small bowel. Assuming that the small bowel was indeed affected by the lymphoma, there would be stage IV disease according to the Lugano classification.
In the presence of pronounced symptoms including primarily postprandial abdominal pain and a cumulative weight loss of 25 kg, immunochemotherapy with rituximab (375 mg/m2 intravenous on day 1) and bendamustine (90 mg/m2 intravenous on days 1 and 2) for six 28-day cycles (from January to June 2020) was initiated. Treatment resulted in complete resolution of symptoms and the patient regained the lost weight. A 2-18F-FDG-PET/CT after six cycles showed complete morphologic and metabolic response with a Deauville Score of 1 (PET/CT scan 2 in Figure 3). Nine months after completion of treatment the patient remains free of gastrointestinal symptoms and in excellent general health (Figure 3). All procedures performed in the present study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of the case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Scoping literature review
Search strategy, inclusion and exclusion criteria and data extraction
We systematically searched PubMed, Embase and Ovid MEDLINE® databases in accordance with the PRISMA-ScR guidelines, identifying relevant case reports (Figure 5). For PubMed (Figure 6), 5 primary search terms (B-cell marginal zone lymphoma, MALT, small intestine, endoscopy, balloon enteroscopy) combined using “AND” were used freely and as controlled vocabulary (MeSH). The search was limited to the last 25 years, i.e., the time from the 1st of January 1995 to the 31st of December 2020. Only publications involving humans and published in English or German language were included. We considered only case reports published in full text (including letters to the editor but excluding abstracts). Reviews of cases previously published in the literature were excluded to avoid case duplication. The final search was performed on the 31st of December 2020. Additional studies were identified by reviewing the references cited in each of the relevant articles. The same search criteria were applied for the other two databases (Embase and Ovid MEDLINE®). The review was not registered, a protocol was not prepared.
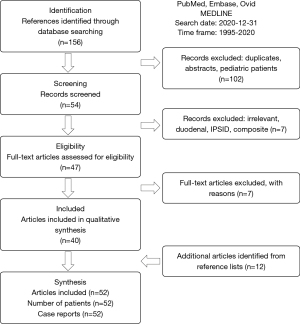
The search in the 3 databases yielded 156 initial references. This number was reduced to 47 after eliminating duplicates, reports concerning pediatric patients (<18 years of age), patients with duodenal MALT lymphoma and immunoproliferative small intestinal disease (IPSID, due to their rarity and peculiar etiopathogenesis), patients with more than one lymphoma diagnosis and those that were considered clearly irrelevant. After reviewing the full texts, 40 articles were included. Reviewing the list of references identified 12 additional publications.
Data extraction was independently performed by two authors using a basic charting form that has been iteratively refined and expanded during the course of the study, oriented towards better understanding of the clinical characteristics, endoscopic manifestations and disease outcome of small bowel MZL. We originally extracted and recorded the following data: first author, year of publication, patient characteristics (gender, age at diagnosis), disease manifestation, endoscopic findings, diagnostic modalities, treatment and outcome. Data such as country of origin, time from onset of symptoms, presence of paraproteinemia, PET/CT scanning and location of small bowel involvement have been extracted at a later stage of our research. Disagreements over study inclusion were resolved by consensus.
Staging of marginal zone lymphomas was heterogeneous with use of either a modified Ann Arbor classification, the Lugano staging system, the Revised European American Lymphoma classification (REAL), or no staging at all. Therefore, the extracted data regarding staging are not reported in detail in the present review.
Results
Our scoping literature search identified 52 additional case reports, resulting in 53 cases including the current presentation. Most cases (28/53, 52.8%) were from Asia, followed by Europe (15/53, 28.3%) and North America (7/53, 13.2%). The remaining cases were from Africa (2/53, 3.8%) and the Middle East (1/53, 1.9%).
The demographic and clinical characteristics, diagnostic and treatment modalities and outcomes of the 53 patients with intestinal MALT lymphoma are shown in detail in Table 2 and are summarized in Table 3.
Table 2
| First author/year | Age | Sex | Location of GI involvement | Bone marrow involvement | Systemic involvement* | Histological confirmation (specimen type) | Clinical presentation | Symptom duration | Treatment modalities | Outcome (follow up in months) |
|---|---|---|---|---|---|---|---|---|---|---|
| Caulet S 1995 (17) | 47 | F | Jejunoileal junction | No | Unknown | Resection specimen | Peritonitis (intestinal perforation) | Acute onset | Small bowel resection | – |
| Wegmann T 1995 (18) | 44 | F | Distal jejunum | Not specified | Unknown | Resection specimen | Abdominal pain, night sweating, arthralgias, weight loss | 1 year/ 3 months |
Small bowel resection | – |
| Brueck M 2001 (19) | 67 | M | Distal ileum | No | No | Resection specimen | Small bowel (ileal) obstruction | 4 days | Small bowel resection plus radio- and chemotherapy (CHOP) | – |
| Kim KW 2001 (20) | 55 | M | Proximal jejunum | Not specified | Unknown | Resection specimen | Lower abdominal pain, association with tuberculous enteritis | Several years | Small bowel resection plus anti-tuberculous medication plus chemotherapy (CHOP) | Regression (12 months) |
| Keung YK 2003 (21) | 49 | F | Proximal jejunum | No | No | Resection specimen | Recurrent abdominal pain (small bowel obstruction) | 18 months | Small bowel resection plus H. pylori eradication therapy plus chemotherapy (CVP) | Regression |
| Saito T 2004 (22) | 65 | F | Ileum | Yes | Yes | On autopsy | Abdominal pain, vomiting, hematochezia, cryoglobulinemia | Not specified | Conservative treatment | Death |
| Yoshida N 2004 (23) | 72 | M | Distal ileum | Not specified | Unknown | Resection specimen | Occasional episodes of hematochezia | 2 years | Small bowel resection | Regression |
| Chim CS 2004 (24) | 60 | M | Jejunum | No | Unknown | Resection specimen | Abdominal pain not related to meals/intermittent tarry stools | 2 years/ 9 months |
Small bowel resection plus chemotherapy (FND) | Regression |
| Ohmatsu H 2005 (25) | 59 | M | Small intestine (not further specified) | Not specified | Unknown | Resection specimen | Recurrent vomiting and weight loss/association with Mycosis fungoides | 6 months/ 15 years |
Small bowel resection plus radiation therapy | Regression (24 months) |
| Pintérová Kolesárová M 2005 (26) | 42 | M | Proximal Jejunum |
No | No | Mucosal biopsy specimen | Asymptomatic (known Crohn’s disease) | 10 years (Crohn’s) | Immunochemotherapy (R-CVP followed by RTX) | – |
| Ke TY 2006 (27) | 67 | F | Middle-distal jejunum | Not specified | Unknown | Resection specimen | Intermittent abdominal pain/tarry stools due to bleeding Dieulafoy’s lesion | Months (not specified)/ 1 day |
Small bowel resection | Regression (12 months) |
| Ohashi S 2006 (28) | 61 | F | Terminal ileum | Not specified | No | Mucosal biopsy specimen | Asymptomatic (positive FOBT) | – | Chemotherapy (not specified) | – |
| Gößmann H 2006 (29) | 75 | F | Jejunum | Not specified | Unknown | Resection specimen | Abdominal pain | 12 years | Small bowel resection | Regression (10 months) |
| Hirata N 2007 (30) | 50 | F | Duodenum, jejunum, ileum | No | No | Mucosal biopsy specimen | Asymptomatic (diffuse thickening of small bowel wall on CT) | – | Immunochemotherapy (R-CHOP) plus H. pylori eradication therapy | Regression (60 months) |
| Tai CM 2008 (31) | 41 | M | Jejunum | Not specified | Unknown | Mucosal biopsy specimen | Chronic diarrhea and weight loss | 3 years | Chemotherapy (not specified) | Death |
| Marks DJ 2009 (32) | 56 | F | Middle ileum | No | No | Resection specimen | Melena | Several hours | Small bowel resection plus immunochemotherapy (R-CVP) | – |
| Storey R 2009 (33) | 59 | M | Proximal ileum | No | No | Resection specimen | Intermittent abdominal pain following meals | 18 months | Small bowel resection | – |
| Yoneda K 2010 (34) | 75 | M | Jejunum | Not specified | Unknown | Mucosal biopsy specimen | Bloating, abdominal distension and diarrhea (small bowel obstruction) | 2 months | H. pylori eradication therapy plus immunochemotherapy (R-CHOP) | – |
| Wong KF 2010 (35) | 78 | M | Distal ileum | Yes | Yes | Mesenteric lymph node | Iron deficiency anemia, cryoglobulinemia | Not specified | Conservative treatment | Death |
| Dolak W 2011 (36) | 70 | M | Jejunum | Not specified | Unknown | Mucosal biopsy specimen | Not specified | – | – | Progression (36 months) |
| Kim DY 2011 (37) | 66 | M | Ileum | Yes | Yes | Resection Specimen |
Abdominal pain, monoclonal gammopathy | – | Small bowel resection plus immunochemotherapy (R-CHOP) | Regression |
| Park S 2011 (38) | 62 | M | Jejunum, ileum | No | Unknown | Resection Specimen |
Abdominal distension and pain, association with amyloidosis | 5 years | Small bowel resection plus immunochemotherapy (R-CVP) | Regression |
| Murino A 2012 (39) | 58 | M | Proximal jejunum | Not specified | Unknown | Mucosal biopsy specimen | Weight loss, colicky upper abdominal pain associated with eating (small bowel intussusception) | 2 months | Immunochemotherapy (R-CVP followed by R-CHOP) | Regression |
| Yanai S 2012 (40) | 62 | M | Ileum | Not specified | No | Resection specimen | Recurrent abdominal pain (small bowel obstruction) | Not specified | Immunotherapy (RTX) | Regression (24 months) |
| Terada T 2013 (41) | 34 | F | Entire ileum | Not specified | No | Mucosal biopsy specimen | Abdominal pain and melena | Not specified | Low dose chemotherapy (not specified) | – |
| Chen CT 2013 (9) | 51 | M | Middle jejunum | Not specified | Unknown | Mucosal biopsy specimen | Intermittent abdominal pain, positive FOBT | Months (not specified) | – | – |
| Liang S 2013 (42) | 65 | F | Middle ileum | Not specified | Unknown | Mucosal biopsy specimen | Recurrent hematochezia | Not specified | Chemotherapy (not specified) | – |
| Koc G 2013 (43) | 67 | M | Distal jejunum | Not specified | Unknown | Resection specimen | Rectal bleeding, weight loss | 6 months | Small bowel resection | – |
| Pyo JH 2013 (44) | 67 | M | Middle ileum | Not specified | No | Resection specimen | Recurrent abdominal pain/hematochezia | 3 years/3 days | Small bowel resection | Regression (9 months) |
| Nadatani Y 2014 (45) | 78 | F | Middle jejunum | Not specified | No | Mucosal biopsy specimen | Anemia and melena | 8 months | Small bowel resection | Regression (12 months) |
| Tsukamoto A 2014 (46) | 73 | F | Jejunum, ileum | No | No | Mucosal biopsy specimen | Abdominal discomfort, appetite loss, weight loss, PLE | Not specified | Immunochemotherapy (R-CHOP) | Regression |
| Nael A 2014 (47) | 44 | M | Middle ileum | No | No | Resection specimen | Meckel diverticulitis with acute small bowel obstruction | – | Small bowel resection plus immunochemotherapy (RTX+bendamustine) | Regression |
| Fukushima M 2014 (48) | 78 | F | Proximal ileum | Yes | Yes | Resection specimen | Tarry stools | – | Small bowel resection plus immunochemotherapy (R-CHOP followed by RTX) | Regression (12 months) |
| Dhull AK 2014 (49) | 55 | M | Ileum | Not specified | Unknown | Resection specimen | Abdominal pain, anorexia, vomiting, constipation (small bowel obstruction) | 2.5 years | Small bowel resection plus chemotherapy (CHOP) | Regression (12 months) |
| Albahadili MA 2015 (50) | 32 | F | Jejunum | Not specified | Unknown | Resection specimen | Abdominal distension, abdominal pain and constipation (small bowel obstruction with perforation) | 3 days | Small bowel resection | – |
| Srinivasan AP 2015 (51) | 22 | M | Ileum | Not specified | Unknown | Resection specimen | Vomiting and abdominal distension (small bowel obstruction) | 1 year | Small bowel resection (antituberculous therapy?) | – |
| Terry M 2015 (52) | 81 | F | Small intestine (not further specified) | Not specified | Yes | Resection specimen | Epigastric pain, vomiting (small bowel obstruction) | 2 months | Small bowel resection (declined chemotherapy) | – |
| Kinkade Z 2015 (53) | 58 | M | Distal ileum | No | No | Resection specimen | Small bowel (ileal) obstruction | 1 month | Small bowel resection plus immunotherapy (RTX) | Regression (6 months) |
| Mastalier B 2015 (54) | 53 | M | Terminal ileum, appendix, mesentery, omentum, abdominal wall | Not specified | Yes | Resection specimen | Abdominal pain | 15 years | Small bowel resection, excision of mesenteric tumors, omentectomy and appendectomy plus immunochemotherapy (R-CHOP) |
Regression (36 months) |
| Indrawati Y 2016 (55) | 80 | M | Terminal ileum | Not specified | Unknown | Resection specimen | Right lower abdominal pain, anorexia, weight loss | 3 months | Small bowel resection, right hemicolectomy (no information on chemotherapy available) | – |
| Stanek N 2016 (56) | 47 | M | Jejunum | Yes | Yes | Bone marrow biopsy | Diffuse postprandial abdominal pain, nausea, diarrhea, PLE | 15 years | Immunochemotherapy (RTX+bendamustine) | Regression (20 months) |
| Inada R 2016 (57) | 60 | F | Distal ileum | Yes | Yes | Resection specimen | Tarry stools with severe anemia | Not specified | Small bowel resection plus immunotherapy (RTX) | – |
| Rosat A 2016 (58) | 45 | F | Middle ileum | No | Unknown | Resection specimen | Abdominal pain, weight loss, chronic constipation/ ileal perforation associated to a phytobezoar | 1 year/1 day | Small bowel resection plus immunotherapy (RTX) | Regression (24 months) |
| Ezejiofor IF 2017 (59) | 27 | M | Jejunum | Not specified | Unknown | Resection specimen | Loin pain | 2 months | Small bowel resection plus chemotherapy (CHOP) | Regression |
| Nehme F 2018 (60) | 67 | F | Ileum | No | No | Resection specimen | Small bowel (ileal) obstruction | 2 months | Small bowel resection | Regression (6 months) |
| Shen KN 2018 (61) | 50 | M | Jejunum, proximal ileum | Not specified | No | Resection specimen | Recurrent abdominal pain and vomiting (small bowel obstruction), melena and intestinal perforation | 2 years/3 months | Small bowel resection plus immunochemotherapy (R-CHOP) |
Partial remission |
| Chehl N 2018 (62) | 59 | F | Middle jejunum | Not specified | No | Mucosal biopsy specimen | Epigastric pain, bloating and flatulence | 2 weeks | – | – |
| Suparman AS 2019 (63) | 38 | F | Jejunum | Not specified | Unknown | Resection specimen | Acute abdomen (jejunal perforation) | 2 days | Small bowel resection | Death |
| Cai ZS 2019 (64) | 67 | M | Jejunum | Not specified | No | Resection specimen | Intermittent abdominal pain, easy satiety, postprandial vomiting/weight loss (small bowel obstruction) | 6 months/1 month | Small bowel resection | Regression (60 months) |
| Muqri F 2019 (65) | 67 | M | Terminal ileum | No | Yes | Resection specimen | Small bowel (ileal) volvulus | 1 month | Small bowel resection, right hemicolectomy plus immunochemotherapy (RTX + bendamustine) | – |
| Bennani A 2019 (66) | 50 | M | Terminal ileum | Not specified | Unknown | Resection specimen | Right lower quadrant abdominal pain, weight loss, diarrhea alternating with constipation | 9 months | Ileocolectomy plus immunochemotherapy (RTX+chlorambucil) | Regression (10 months) |
| Stundiene I 2020 (67) | 50 | M | Stomach, jejunum | Not specified | Unknown | Resection specimen | Malaise and melena/weight loss, abdominal pain | Acute onset/1 year | Small bowel resection plus immunochemotherapy (R-CHOP) | Regression |
| Our case (Markopoulos K 2022) | 57 | M | Jejunum, ileum | Yes | Yes | Cervical lymph node | Abdominal pain/bloating, lactose intolerance/urticaria, weight loss | 10 years/3 years/3 months | Immunochemotherapy (RTX + bendamustine) | Regression (6 months) |
*, Systemic involvement was considered to be present if stage IV was documented. CHOP, cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone; CT, computed tomography; CVP, cyclophosphamide, vincristine, prednisone; FND, fludarabine, mitoxantrone, dexamethasone; FOBT, fecal occult blood test; MALT, mucosa-associated lymphoid tissue; PLE, protein-losing enteropathy; R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone; RTX, rituximab; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone.
Table 3
| Variables | Value |
|---|---|
| Age median [range], years (n=53) | 59 [22–81] |
| Sex (n=53) | |
| Male | 32 (60.4%) |
| Female | 21 (39.6%) |
| Clinical presentation (n=52) | |
| Abdominal pain | 19 (36.5%) |
| Small bowel obstruction | 16 (30.8%) |
| Gastrointestinal bleeding | 12 (23%) |
| Weight loss | 12 (23%) |
| Acute abdomen (perforation) | 5 (9.6%) |
| Asymptomatic | 5 (9.6%) |
| Diarrhea | 1 (1.9%) |
| Protein losing enteropathy | 1 (1.9%) |
| Symptom duration (days, n=36) | |
| Median [IQR] | 265.5 [44–822] |
| Tumor location (n=51) | |
| Ileum | 24 (47%) |
| Terminal ileum | 8 (15.7%) |
| Jejunum | 21 (41.2%) |
| Jejunum and ileum | 6 (11.8%) |
| Specimen type (n=52) | |
| Bowel resection specimen | 36 (69.2%) |
| Mucosal biopsy specimen | 13 (25%) |
| Excised lymph nodes | 2 (3.9%) |
| Bone marrow biopsy | 1 (1.9%) |
| Bone marrow involvement (n=22) | |
| Absent | 15 (68.2%) |
| Present | 7 (31.8%) |
| Paraproteinemia (n=11) | |
| Present | 7 (63.6%) |
| IgM κ | 4 (57.1%) |
| IgM λ | 2 (28.6%) |
| IgA λ | 1 (14.3%) |
| Absent | 4 (36.4%) |
| H. pylori status (n=23) | |
| Negative | 17 (74%) |
| Positive | 6 (26%) |
| PET scan (n=15) | |
| Compatible with lymphoma involvement | 9 (60%) |
| Compatible with inflammation | 1 (6.7%) |
| Normal findings | 5 (33.3%) |
| Systemic involvement (n=53) | |
| Present | 10 (18.8%) |
| Absent | 18 (34%) |
| Unknown | 25 (47.2%) |
| Treatment modality (n=48) | |
| Surgical resection plus CT | 18 (37.5%) |
| Surgical resection alone | 16 (33.3%) |
| CT alone | 12 (25%) |
| Surgical resection plus RT | 1 (2.1%) |
| Surgical resection plus CT plus RT | 1 (2.1%) |
| Chemotherapy regimen (n=27) | |
| R-CHOP | 7 (26%) |
| CVP/CHOP | 5 (18.5%) |
| Rituximab | 5 (18.5%) |
| Rituximab + bendamustine | 3 (11.1%) |
| R-CHOP/R-CVP followed by rituximab | 2 (7.4%) |
| R-CVP | 2 (7.4%) |
| R-CVP followed by R-CHOP | 1 (3.7%) |
| FND | 1 (3.7%) |
| Rituximab + chlorambucil | 1 (3.7%) |
| Outcome (n=34) | |
| Follow up, months, median [range] | 15 [6–60] |
| Clinical remission | 28 (82.3%) |
| Partial response to therapy | 1 (3%) |
| Progression | 1 (3%) |
| Death | 4 (11.7%) |
| Cause of death (n=4) | |
| Septic shock | 2 (50%) |
| Progressive disease | 2 (50%) |
CHOP, cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone; CT, chemotherapy; CVP, cyclophosphamide, vincristine, prednisone; FND, fludarabine, mitoxantrone, dexamethasone; R-CHOP, rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin and prednisone; R-CVP, rituximab, cyclophosphamide, vincristine, prednisone; RT, radiotherapy; RTX, rituximab.
The male/female ratio was 1.5 (32 to 21). The median age at diagnosis was 59 years (range 22–81). >90% of patients presented with gastrointestinal symptoms. The most common presenting symptom was abdominal pain (36.5%), followed by obstructive symptoms (30.8%), gastrointestinal bleeding (melena/hematochezia) and weight loss (23% each). Other common presenting symptoms included acute abdomen, diarrhea and protein-losing enteropathy (PLE), whilst 9.6% of the patients were asymptomatic at the time of diagnosis. There was no significant association between tumor location (jejunum, ileum) and each of the following symptoms: abdominal pain, weight loss, small bowel obstruction and bleeding (Chi-square test).
The median lag time between onset of symptoms and diagnosis was 265 days (25th–75th percentile: 44–822 days). The most common tumor location was the ileum (47%), followed by the jejunum (41.2%) and multiple (jejunoileal) intestinal involvement (11.8%). The terminal ileum was involved in 15.7% of cases. 25% of patients in our scoping review were diagnosed by endoscopic biopsy. Remarkably, in the vast majority of patients (73.1%), MZL could only be diagnosed by surgical resection of the intestine or lymph nodes. This high number might reflect either the high frequency of emergency operations due to acute presentation in this patient cohort and/or the limitations of endoscopy in sampling of the entire small intestine. Bone marrow involvement was documented in 31.8% of the cases. A 2-18F-FDG-PET/CT was performed in 15 patients and excessive metabolic activity was seen in roughly 66% of cases. In 18.9% of cases systemic involvement was documented.
Patients were most frequently treated with surgery and chemotherapy (37.5%), followed by surgery (33.3%) or chemotherapy (25%) alone. Chemotherapy protocols most often included rituximab, cyclophosphamide, hydroxydaunorubicin, oncovin, and prednisone (R-CHOP immunochemotherapy) or rituximab, cyclophosphamide, vincristine and prednisone (R-CVP immunochemotherapy), which altogether accounted for 44.5% of treatment modalities. Rituximab was included in 77.8% of treatment protocols and was used as exclusive primary treatment (immunotherapy) in 18.5% of cases.
After a median follow-up of 15 months (range 6–60), most patients (82%) were in clinical remission. Death was reported in 11.7% of patients. Death was related to disease progression in 50% of cases, two of four patients who died from MALT lymphoma had advanced disease at diagnosis. An additional 3% of patients showed progressive disease.
Endoscopic findings in small intestinal MZL
Diagnosis of marginal zone lymphoma was established by endoscopic biopsy in 13 patients (25%). For 23 patients detailed endoscopic findings were reported: most patients received balloon enteroscopy (total of 65.2%), adjunctive investigations of the small intestine (capsule endoscopy in 17.4% or push enteroscopy in 4.3%, respectively) were reported prior to the balloon enteroscopy in some cases. The diagnosis was established by ileocolonoscopy, esophagogastroduodenoscopy (EGD) or push enteroscopy alone in 26% of the patients (Table 4).
Table 4
| Variables | Number of patients | % (n=23) |
|---|---|---|
| Endoscopic technique | ||
| Balloon enteroscopy alone | 11 | 47.8 |
| Double balloon enteroscopy | 7 | 30.4 |
| Single balloon enteroscopy | 4 | 17.4 |
| Ileocolonoscopy alone | 3 | 13 |
| VCE + balloon enteroscopy | 3 | 13 |
| Push enteroscopy alone | 2 | 8.7 |
| Ileocolonoscopy + EGD | 1 | 4.3 |
| VCE + push enteroscopy + balloon enteroscopy | 1 | 4.3 |
| Not specified | 2 | 8.7 |
| Endoscopic findings | ||
| Erosive/ulcerative lesions (total) | 19 | 82.6 |
| Multiple ulcerations/shallow erosions | 8 | 34.8 |
| Multiple ulcer scars | 4 | 17.4 |
| Single irregular ulceration | 2 | 8.7 |
| Annular stricture with superficial erosions | 2 | 8.7 |
| Single large sessile ulcerated lesion | 1 | 4.3 |
| Disordered fold pattern due to irregularly shaped ulcers | 1 | 4.3 |
| Aphthous erosions in the distal and terminal ileum | 1 | 4.3 |
| Signs of gastrointestinal bleeding | 5 | 21.7 |
| Multiple pseudo-polypoid lesions | 4 | 17.4 |
| Fine mucosal granularity/nodularity | 4 | 17.4 |
| Absence of villi in terminal ileum or jejunum (denuded mucosa) | 3 | 13 |
| Localized intestinal dilatation (pseudo-aneurysmal) | 3 | 13 |
| Luminal stricture | 3 | 13 |
| Pathological neo-vascularization in terminal ileum or jejunum | 2 | 8.7 |
| Multiple whitish nodules (focal lymphangiectasia) | 2 | 8.7 |
| Polypoid lymphangiectasia | 1 | 4.3 |
| “White small intestine” | 1 | 4.3 |
| Annular mass | 1 | 4.3 |
VCE, video capsule endoscopy; EGD, esophagogastroduodenoscopy.
Details of endoscopic findings were available for 23 intestinal MZL patients (Table 5). The most frequent finding was a pattern of erosions and ulcerations (82.6% of the patients), with single or multiple shallow or elevated ulcerative lesions, sometimes irregularly shaped or complicated with scar formation and disordered mucosal folds. Signs of GI bleeding (arterial spurting from ulcers or adherent blood clots) were reported in 21.7% of individuals. Other findings included multiple pseudopolypoid lesions (lymphomatous polyposis) in 17.4% and a pattern of focal mucosal granularity or nodularity in another 17.4% of patients. In other cases, denuded and depressed mucosal areas with absence of villi or lymphangiectasias in the form of white tipped villi or polypoid lesions resembling lymphangiomas were described. Stricturing features and segmental bowel dilatations (due to the infiltration of muscularis or myenteric nerve plexus) were detected each in 13% of the patients. More than one of the above-mentioned patterns coexisted in some patients. While ulcerations were described in the whole intestine, there was a trend towards predominance of lymphangiectasias in the jejunum, while polypoid lesions were more frequent in the ileum.
Table 5
| First author/year | Age | Sex | Distribution of findings | Endoscopic technique | Erosions/ulcers | Endoscopic findings | Clinical presentation |
|---|---|---|---|---|---|---|---|
| Yoshida N 2004 (23) | 72 | M | Ileum | DBE | + | Several ulcer scars with localized intestinal dilatation | GI-bleeding (recurrent hematochezia) |
| Pintérová Kolesárová M 2005 (26) | 42 | M | Proximal, jejunum | Push enteroscopy | + | Swollen transverse folds, absence of villi, multiple tortuous, winding vessels (pathological neo-vascularization) and erosions on the top of affected folds | Asymptomatic |
| Ohashi S 2006 (28) | 61 | F | Ileum | Ileocolonoscopy | − | Multiple polypoid lesions, absence of villi | Asymptomatic (positive FOBT) |
| Hirata N 2007 (30) | 50 | F | Duodenum, jejunum, ileum | EGD, Ileocolonoscopy | − | Multiple lymphomatous polyposis | Asymptomatic |
| Tai CM 2008 (31) | 41 | M | Jejunum | DBE | − | “White small intestine”: marbled appearance with diffuse polypoid lesions characterized by villous atrophy and superficial whitish streaks | Chronic diarrhea, weight loss |
| Yoneda K 2010 (34) | 75 | M | Jejunum | SBE | + | Shallow ulcer on the proximal side of a stricture | Diarrhea and bloating (small bowel obstruction) |
| Dolak W 2011 (36) | 70 | M | Jejunum | Push enteroscopy | − | Multiple whitish nodules | Asymptomatic |
| Murino A 2012 (39) | 58 | M | Jejunum | DBE | + | Large, sessile, ulcerated lesion | Abdominal pain, weight loss (small bowel intussusception) |
| Yanai S 2012 (40) | 62 | M | Ileum | DBE | − | Coarse, granular mucosa | Abdominal pain (small bowel obstruction) |
| Terada T 2013 (41) | 35 | F | Ileum | Ileocolonoscopy | + | Multiple tumors and ulcers of the entire ileum | Abdominal pain, GI-bleeding |
| Chen CT 2013 (9) | 51 | M | Jejunum | DBE | + | Eccentric ulcerating-scarring area | Abdominal pain and positive FOBT |
| Liang S 2013 (42) | 65 | F | Ileum | SBE | − | 4-cm long annular mass in the mid-ileum | GI-bleeding (recurrent hematochezia) |
| Koc G 2013 (43) | 67 | M | Jejunum | Enteroscopy | + | Nodular mucosal pattern and ulcerations with exudate | GI-bleeding (rectal bleeding), weight loss |
| Pyo JH 2013 (44) | 67 | M | Middle ileum | VCE, DBE | + | Arterial spurting from an irregular ulcerative lesion with severe luminal stricture | GI-bleeding (hematochezia), abdominal pain |
| Nadatani Y 2014 (45) | 78 | F | Jejunum | VCE, DBE | + | Multiple ulcerative lesions covered with coagula | GI-bleeding (melena), anemia |
| Tsukamoto A 2014 (46) | 73 | F | Jejunum, Ileum | DBE | + | Irregular nodular mucosal lesions with erosions | PLE, abdominal discomfort, weight loss |
| Fukushima M 2014 (48) | 78 | F | Proximal ileum | SBE | + | Disordered fold pattern and irregularly shaped ulcers with bleeding | GI-bleeding (melena) |
| Inada R 2016 (57) | 60 | F | Ileum | VCE, DBE | + | Large cystic dilatation of the ileum containing a shallow ulcer with blood clots | GI-bleeding (melena) |
| Chehl N 2018 (62) | 59 | F | Middle jejunum | DBE | + | Short, ulcerated stricture | Abdominal pain (small bowel obstruction) |
| Cai ZS 2019 (64) | 67 | M | Jejunum | SBE | + | Annular stricture lesion with superficial erosions, ulcerations and prestenotic dilatation | Abdominal pain (small bowel obstruction) |
| Bennani A 2019 (66) | 50 | M | Ileum | Ileocolonoscopy | + | Ulcerative and hemorrhagic lesions of the ileum and pseudopolypoid appearance | Abdominal pain, weight loss, diarrhea alternating with constipation |
| Stundiene I 2020 (67) | 50 | M | Stomach, jejunum | Enteroscopy | + | 1.5 cm ulcer in the jejunum | Abdominal pain, GI-bleeding (melena), weight loss |
| Our case (Markopoulos K 2022) | 57 | M | Jejunum, ileum | Ileocolonoscopy, push enteroscopy, VCE, SBE | + | Multiple fibrin-covered ulcerations, aphthous erosions, white villi, fine mucosal nodularity, denuded areas, circumferential erythema, polypoid lymphangiectasia, focal lymphangiectasia with diminutive whitish granules, ulcers with adherent blood clots | Abdominal pain, bloating, lactose intolerance, urticaria, weight loss |
EGD, esophagogastroduodenoscopy; DBE, double-balloon enteroscopy; FOBT, fecal occult blood test; PLE, protein-losing enteropathy; SBE, single-balloon enteroscopy; VCE, video capsule endoscopy; GI, gastrointestinal.
Discussion
Diagnosis of small intestinal lymphoma or intestinal involvement by systemic low grade B-cell lymphoma with a non-specific immunophenotype can be challenging. We present the case of a patient with presumed small intestinal MZL which, despite the almost exclusive gastrointestinal symptoms, could only be affirmatively diagnosed after staging with 2-18F-FDG-PET/CT and surgical lymph node sampling. The present study also provides a comprehensive literature overview of case reports on small intestinal MZL published after 1995. Despite several limitations, it highlights the most salient characteristics of this understudied and rare disease (6,13-15): (I) adult onset, peaking in the sixth decade of life. (II) Slight male predominance. (III) Clinical presentation with gastrointestinal symptoms in 90% of patients including abdominal pain, obstructive symptoms or GI bleeding. (IV) Ileal involvement in most cases. (V) Long diagnostic latency (>9 months in most patients). (VI) Benign disease course with low mortality and a high degree of remission (>80%) upon appropriate therapy with rituximab and/or chemotherapy.
The evaluation of the small bowel remains difficult, due to the inaccessibility by conventional endoscopy: ileocolonoscopy, EGD or push enteroscopy are not able to reach the distal jejunum and proximal ileum and abdominal imaging with CT scan or magnetic resonance enterography might fail to detect subtle initial manifestations of small intestinal lymphoma or provides unspecific findings. VCE was shown to be useful in patients with suspected small bowel tumors, including gastrointestinal lymphomas (68,69). However, enteroscopy is usually needed for confirmatory visual inspection and sampling. Notably, balloon enteroscopy yields a high rate of successful diagnosis, but it has limitations, including the need for specialized training, post-interventional abdominal pain, perforation risk and high costs. Prospective trials to comparatively evaluate these already established methods in the diagnosis of primary GI lymphomas are needed.
In past series, diagnosis of small intestinal MZL was established using endoscopy in 54% of patients (by means of up to three endoscopies) and required surgery in 44% (13). In our case series, endoscopy was diagnostic in only 25% of patients and typically balloon enteroscopy was required. However, in most small intestinal MZL cases (73%), small bowel or lymph node resection was diagnostic. In line with past data (13) our study highlights diagnostic challenges in small intestinal MZL, requiring a high degree of suspicion and perseverance.
The typical endoscopic appearance of small intestinal lymphoma including MALT lymphoma is insufficiently characterized, with limited data from case reports and retrospective studies providing some insights (6,68-71): In one case series, intestinal MZL was described as ulcerous in 67% of cases, tumorous in 27% and diffuse in 7% (6). Similarly, in a meta-analysis of 415 patients with small intestinal lymphoma, in the 97 patients with MALToma, the lymphoma was described as an ulcer or a tumor in 48% and 17% of cases, respectively (13). Our results confirm the predominant ulcerous/erosive endoscopic pattern of small intestinal lymphoma, since at least some erosive/ulcerative aspect was described in 83% of patients. A frank mass was observed less frequently; however, our review also identifies more subtle endoscopic patterns such as a denuded mucosa with villous atrophy, a granular pattern, strictures, pathological neo-vascularization and lymphangiectasias. Therefore, due to the variable presentation, a low threshold for sampling of all suspicious lesions seems adequate.
In our patient, extensive histological and molecular examination of small bowel mucosal and surgical biopsies failed to confirm clear-cut intestinal involvement by MZL. In only two other patients in the literature with small intestinal MZL, as in our patient, the diagnosis was made by sampling other organs i.e., the bone marrow or mesenteric lymph nodes. Nonetheless, the predominant clinical presentation (with pronounced abdominal pain, bloating and severe weight loss) and the typical endoscopic findings (very similar to those previously described in small intestinal MZL) were both highly suggestive of an involvement of the small bowel by malignant lymphoma. Furthermore, immunochemotherapy resulted in prompt, complete and lasting resolution of abdominal symptoms and significant weight gain, indirectly confirming the intestinal involvement by lymphoma. However, in our case the distinction between primary small bowel MZL and systemic MZL with secondary involvement of the small intestine is not possible.
More than one definition of primary intestinal lymphoma exists. Lewin’s expanded definition from 1978 requires a presentation with predominant GI symptoms and/or GI lesions secondary to mesenteric lymph node involvement (72), as in our patient. However, our patient could not be classified as an intestinal lymphoma according to the more stringent Dawson’s criteria from 1961 (73).
Failure to detect intestinal lymphoma histopathologically and using molecular pathology in our patient could be attributed to sampling errors, both endoscopically and intraoperatively, due to the patchy distribution of lesions. Furthermore, MZL presents immunohistochemically with a so-called “null phenotype”, i.e., lack of specific surface markers which could be used for immune staining. These malignant immune cells can only be detected by their growth or infiltration pattern, which would only be obvious in the presence of massive intestinal infiltration. They are easier to detect in lymph nodes where the concentration of MZL is higher than in the intestine. Therefore, minimal and patchy infiltrations by neoplastic B-lymphocytes may be almost impossible to detect in intestinal biopsy samples, despite extensive ancillary tests. Ischemic enteritis with abdominal pain and endoscopic findings at least partially similar to those in our patient, has been described in association with stenosis/occlusion of splanchnic vessels (74) and secondarily to angiocentric T-cell lymphoma of the intestine (75), but not in the context of an intestinal MZL. Alternatively, mucosal alterations and symptoms encountered in our patient could be explained by lymphatic stasis secondary to lymphoma or by other paraneoplastic effects of the lymphoma (56).
Chronic abdominal pain was the predominant symptom in our patient and was present in 36% of small intestinal MZL cases. Our patient had a significant weight loss at presentation which further deteriorated until definite therapy was established, with significant regain of weight thereafter. The presence of alarming features (76) (in our patient: new onset of symptoms at age >50 years, presence of significant weight loss and elevated inflammation markers such as CRP and fecal calprotectin) mandated the need for further investigations. Weight loss is described in 64.8% of patients with intestinal lymphoma (5) and in 17.5% of small bowel lymphoma cases (6), similar to the 23% of patients in our case series but was not a prominent feature in another case series of small intestinal MZL (14). Weight loss in cancer patients is related to inflammatory cytokines such as tumor necrosis factor, interleukin (IL)-1, IL-6 and interferon-gamma (77,78), which induce anorexia, increased energy expenditure and excess catabolism. Besides CRP and IL-6, we also observed high levels of soluble tumor necrosis factor receptor-1 (sTNFR1) (79,80), as an additional inflammatory marker. Surprisingly, despite relevant abdominal pain, no mass lesion or stricture could be demonstrated radiologically and the reason for the abdominal pain remains unclear.
In our patient, an immunoglobulin M-type paraprotein provided some evidence for the presence of a malignant lymphoma. The association of monoclonal gammopathy with B cell NHL is well-known and has been reported in up to 50% of patients with splenic MZL and in 36% of patients with extranodal MZL (81,82). Interestingly, paraproteinemia was present in 63.6% of small bowel MZL cases in our review. Notably, paraproteinemia accompanying MZL is correlated with involvement of the bone marrow, progression of the disease and tendency towards large cell transformation (83). In addition, paraproteinemia might be a useful diagnostic biomarker for malignant lymphoma in middle-aged or elderly patients with abdominal pain and alarm symptoms: after initial unsuccessful evaluation, these patients could be routinely screened for the presence of a paraprotein. However, further research is warranted to evaluate the utility of this approach and in any case, absence of a paraprotein does not exclude lymphoma.
Bone marrow was affected in only 7/22 (31.8%) of our patients with small intestinal MZL. Recent evidence has shown that the frequency of bone marrow involvement differs greatly between nodal and splenic MZL. In splenic MZL, the reported prevalence is between 90% and 100%, while for nodal MZL it is between 28% and 54%. In comparison, bone marrow involvement is present in only 4 to 44% of patients with extranodal MZL (14,84,85). Comparing bone marrow infiltration in splenic and extranodal MZL, the extent of involvement was also greater in splenic MZL (86).
In our patient, the extent of the lymphoma was only revealed by 2-18F-FDG-PET/CT; in our case series, 10/15 (66%) of patients with small intestinal MZL showed abnormal findings on 2-18F-FDG-PET/CT. In contrast to more aggressive lymphomas, MZL, follicular lymphoma and small lymphocytic lymphoma have variable rates of 2-18F-FDG-PET/CT positivity (87-90). A recent meta-analysis showed a pooled detection rate of 71% (95% confidence interval, 61–80%) (91). In another retrospective study, the sensitivity of 2-18F-FDG-PET/CT for detection of MZL was 96% and 2-18F-FDG-PET/CT outperformed CT especially for detection of extranodal lesions (92). Therefore, in case of small bowel complaints due to suspected lymphoma, bone marrow biopsy followed by 2-18F-FDG-PET/CT with guided aspiration or surgical removal of affected lymph nodes should be considered. This approach might help avoiding unnecessary interventions and achieve an early diagnosis.
Radiation therapy (RT) as central component of a multimodal treatment concept including surgery and chemotherapy has been established throughout the last 25 years as curative approach in primary therapy of intestinal lymphoma because of success in achieving disease regression, excellent local tumor control and high survival rates. In a recent large prospective study of 134 patients with indolent or aggressive intestinal lymphoma, Reinartz et al. showed that RT adapted to stage, histology and previous resection, in a multidisciplinary approach and supported by the development of modern techniques (involved site-, intensity modulated- and image guided-RT) that reduce normal tissue complication probability, is a well tolerated option with excellent clinical outcome (complete response rate of 100%, low disease specific death rate of 11.2%, relapse in 15.7% of the entire cohort) (93). In our case series, RT was included in the therapeutic protocol in only 4.2% of cases, probably reflecting the inclusion of older case reports in our study and the high heterogeneity among cases with regard to availability of RT. In our patient, systemic lymphomatous involvement (spleen, lymph nodes on both sides of the diaphragm, bone marrow) warranted a combined immunochemotherapeutic approach with no evidence of residual disease during the follow-up and therefore no need for subsequent consolidation RT.
Our review has several limitations. All publications are case reports, with no clinical trials or large case series available. There is clinical heterogeneity between patients with regard to disease duration and ethnicity. Publication bias leading to a predominant representation in the literature of rare or unusual findings is another limiting factor. The review’s nature is retrospective posing additional possibility of bias. Lastly, the follow-up time for most cases is short and long-term outcomes remain unknown for most patients.
In conclusion, the diagnosis of small intestinal MZL is often challenging, requiring a multidisciplinary approach. Patients typically present with gastrointestinal symptoms such as abdominal pain and alarm features. Establishing histological proof of MZL small intestinal involvement can be challenging due to patchy involvement and/or the “null phenotype” of MZL cells. Paraproteinemia, 2-18F-FDG-PET/CT and bone marrow biopsy with flow cytometry can provide additional crucial diagnostic information. In particular, a 2-18F-FDG-PET/CT might help to establish the diagnosis if repeated endoscopic sampling remains unsuccessful and has the potential to spare the patient more extensive interventions including intestinal surgery. However, an evidence based diagnostic algorithm has not yet been established. Vigorous diagnostic efforts should be made since upon appropriate treatment such as immunochemotherapy (94,95) most patients reach clinical remission.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA-ScR and the CARE reporting checklists. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-74/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-74/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-74/coif). EB received traveling and congress costs fees from Bayer, unrelated to the current work. BM has received traveling fees, consulting fees or speaking fees from Gilead, Given Imaging, MSD, BMS, Takeda, Novigenix, Falk, Vifor, iQONE and Novartis and has received unrestricted research grants from MSD, Nestle and BMS outside of the submitted work. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All procedures involving human participants performed in the present study were in accordance with the ethical standards of the institutional and/or national research committee(s) and with the Declaration of Helsinki (as revised in 2013). Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the editorial office of this journal.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Paryani S, Hoppe RT, Burke JS, et al. Extralymphatic involvement in diffuse non-Hodgkin's lymphoma. J Clin Oncol 1983;1:682-8. [Crossref] [PubMed]
- Groves FD, Linet MS, Travis LB, et al. Cancer surveillance series: non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst 2000;92:1240-51. [Crossref] [PubMed]
- Koch P, del Valle F, Berdel WE, et al. Primary gastrointestinal non-Hodgkin's lymphoma: I. Anatomic and histologic distribution, clinical features, and survival data of 371 patients registered in the German Multicenter Study GIT NHL 01/92. J Clin Oncol 2001;19:3861-73. [Crossref] [PubMed]
- Daum S, Ullrich R, Heise W, et al. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol 2003;21:2740-6. [Crossref] [PubMed]
- Amer MH, el-Akkad S. Gastrointestinal lymphoma in adults: clinical features and management of 300 cases. Gastroenterology 1994;106:846-58. [Crossref] [PubMed]
- Nakamura S, Matsumoto T, Takeshita M, et al. A clinicopathologic study of primary small intestine lymphoma: prognostic significance of mucosa-associated lymphoid tissue-derived lymphoma. Cancer 2000;88:286-94. [Crossref] [PubMed]
- Zucca E, Roggero E, Bertoni F, et al. Primary extranodal non-Hodgkin's lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann Oncol 1997;8:727-37. [Crossref] [PubMed]
- d'Amore F, Brincker H, Grønbaek K, et al. Non-Hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol 1994;12:1673-84. [Crossref] [PubMed]
- Chen CT, Yen HH, Wu L. Primary jejunal mucosa-associated lymphoid tissue lymphoma. QJM 2013;106:195-6. [Crossref] [PubMed]
- Sukumaran R, Nair RA, Jacob PM, et al. Anatomic distribution and histologic subtypes of primary gastrointestinal lymphomas: A retrospective analysis of 152 cases. Clin Cancer Investig J 2017;6:15-20. [Crossref]
- Arora N, Manipadam MT, Pulimood A, et al. Gastrointestinal lymphomas: pattern of distribution and histological subtypes: 10 years experience in a tertiary centre in South India. Indian J Pathol Microbiol 2011;54:712-9. [PubMed]
- Cardona DM, Layne A, Lagoo AS. Lymphomas of the gastro-intestinal tract - pathophysiology, pathology, and differential diagnosis. Indian J Pathol Microbiol 2012;55:1-16. [Crossref] [PubMed]
- Chen Y, Chen Y, Chen S, et al. Primary Gastrointestinal Lymphoma: A Retrospective Multicenter Clinical Study of 415 Cases in Chinese Province of Guangdong and a Systematic Review Containing 5075 Chinese Patients. Medicine (Baltimore) 2015;94:e2119. [Crossref] [PubMed]
- Oh SY, Kwon HC, Kim WS, et al. Intestinal marginal zone B-cell lymphoma of MALT type: clinical manifestation and outcome of a rare disease. Eur J Haematol 2007;79:287-91. [Crossref] [PubMed]
- Kim SJ, Choi CW, Mun YC, et al. Multicenter retrospective analysis of 581 patients with primary intestinal non-hodgkin lymphoma from the Consortium for Improving Survival of Lymphoma (CISL). BMC Cancer 2011;11:321. [Crossref] [PubMed]
- Li B, Shi YK, He XH, et al. Primary non-Hodgkin lymphomas in the small and large intestine: clinicopathological characteristics and management of 40 patients. Int J Hematol 2008;87:375-81. [Crossref] [PubMed]
- Caulet S, Robert I, Bardaxoglou E, et al. Malignant lymphoma of mucosa associated lymphoid tissue: a new etiology of amyloidosis. Pathol Res Pract 1995;191:1203-7. [Crossref] [PubMed]
- Wegmann T. Diffuse, right-sided pelvic pain and weight loss. Low malignancy lymphoma of the small intestine of the MALT type (mucosa-associated lymphoid tissue). Radiologe 1995;35:991-2. [PubMed]
- Brueck M, Barton M, Rauber K, et al. Ileus of the small intestine in intestinal marginal-zone B-cell lymphoma of mucoid-associated lymphoid tissue (MALT). Dtsch Med Wochenschr 2001;126:1391-5. [Crossref] [PubMed]
- Kim KW, Park SY, Lee EH, et al. Mucosa-associated lymphoid tissue (MALT) lymphoma combined with tuberculous enteritis at the same site in the jejunum. Leuk Lymphoma 2001;42:1151-5. [Crossref] [PubMed]
- Keung YK, Higgs V, Albertson DA, et al. Mucosa-associated lymhpoid tissue (MALT) lymphoma of the jejunum and Helicobacter pylori--chance association? Leuk Lymphoma 2003;44:1413-6. [Crossref] [PubMed]
- Saito T, Tamaru J, Kishi H, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) arising in the small intestine with monoclonal cryoglobulinemia. Pathol Int 2004;54:712-8. [Crossref] [PubMed]
- Yoshida N, Wakabayashi N, Nomura K, et al. Ileal mucosa-associated lymphoid tissue lymphoma showing several ulcer scars detected using double-balloon endoscopy. Endoscopy 2004;36:1022-4. [Crossref] [PubMed]
- Chim CS, Loong F, Ooi GC. Mucosa-associated lymphoid tissue (MALT) lymphoma of the jejunum: an elusive cause of recurrent upper gastrointestinal bleeding. Leuk Lymphoma 2004;45:405-7. [Crossref] [PubMed]
- Ohmatsu H, Saeki H, Fujita H, et al. Mycosis fungoides associated with intestinal mucosa-associated lymphoid tissue lymphoma. Int J Dermatol 2005;44:878-80. [Crossref] [PubMed]
- Pintérová Kolesárová M, Kopáčová M, Tyčová V, et al. Unusual endoscopic appearance of B-cell lymphoma of the small bowel in a patient with Crohn’s disease: a case report. Folia Gastroenterol Hepatol 2005;3:104-9.
- Ke TY, Chen LP, Hwang GL. Dieulafoy's lesion associated with Maltoma in jejunum: A case report. Cheng Ching Medical Journal 2006;2:34-6.
- Ohashi S, Yazumi S, Watanabe N, et al. Education and imaging. Gastrointestinal: MALT lymphoma of the terminal ileum. J Gastroenterol Hepatol 2006;21:1495. [Crossref] [PubMed]
- Gössmann H, Görlitz T, Beck A, et al. Intestinal small bowel lymphomas--diagnosis and treatment. Rontgenpraxis 2006;56:67-72. [PubMed]
- Hirata N, Tominaga K, Ohta K, et al. A case of mucosa-associated lymphoid tissue lymphoma forming multiple lymphomatous polyposis in the small intestine. World J Gastroenterol 2007;13:1453-7. [Crossref] [PubMed]
- Tai CM, Tu CH, Wu HB, et al. An unexpected cause of chronic diarrhoea. Gut 2008;57:902, 921.
- Marks DJ, Prodromou A, Silvanto A, et al. Ileal MALT lymphoma causing massive gastrointestinal haemorrhage revealed on computed tomography angiography. Postgrad Med J 2009;85:163-5. [Crossref] [PubMed]
- Storey R, Gatt M, Bradford I. Mucosa associated lymphoid tissue lymphoma presenting within a solitary anti-mesenteric dilated segment of ileum: a case report. J Med Case Rep 2009;3:6. [Crossref] [PubMed]
- Yoneda K, Takahashi H, Abe Y, et al. A mucosa-associated lymphoid tissue (MALT) lymphoma of the small intestine that was difficult to diagnose endoscopically. Endoscopy 2010;42:E175. [Crossref] [PubMed]
- Wong KF, Wong WS, Siu LL. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue presenting with cryoglobulinemia and subtle marrow infiltrate. Leuk Lymphoma 2010;51:543-5. [Crossref] [PubMed]
- Dolak W, Raderer M, Maresch J, et al. Detection of gastric MALT lymphoma spreading to the small bowel by enteroscopy. Endoscopy 2011;43:731-3. [Crossref] [PubMed]
- Kim DY, Kim YS, Huh HJ, et al. A case of monoclonal gammopathy in extranodal marginal zone B-cell lymphoma of the small intestine. Korean J Lab Med 2011;31:18-21. [PubMed]
- Park S, Cho HY, Ha SY, et al. Marginal zone B-cell lymphoma of MALT in small intestine associated with amyloidosis: a rare association. J Korean Med Sci 2011;26:686-9. [Crossref] [PubMed]
- Murino A, Despott EJ, Hansmann A, et al. A rare case of small bowel intussusception. Endoscopy 2012;44 Suppl 2 UCTN:E157-8.
- Yanai S, Nakamura S, Hirahashi M, et al. Education and imaging. Gastrointestinal: MALT lymphoma of the small bowel accompanied by NSAID-induced enteropathy. J Gastroenterol Hepatol 2012;27:1126. [Crossref] [PubMed]
- Terada T. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma) of the ileum in a 35-year-old Japanese woman. Int J Clin Exp Pathol 2013;6:951-6. [PubMed]
- Liang S, Pan Y, Wang B, et al. Complete small-bowel examination by oral single-balloon enteroscopy using the water-exchange method. Endoscopy 2013;45 Suppl 2 UCTN:E415-7.
- Koc G, Oyar O, Yazicioğlu N, et al. B-cell malt lymphoma of the small intestine. JBR-BTR 2013;96:40. [Crossref] [PubMed]
- Pyo JH, Lee BJ, Lee HJ, et al. A case of ileal mucosa-associated lymphoid tissue lymphoma accompanied by luminal stricture and arterial spurting. Korean J Gastroenterol 2013;62:365-9. [Crossref] [PubMed]
- Nadatani Y, Tanigawa T, Fukunaga S, et al. Primary small intestinal mucosa-associated lymphoid tissue lymphoma diagnosed by balloon-assisted enteroscopy. Intern Med 2014;53:2671-4. [Crossref] [PubMed]
- Tsukamoto A, Nakamura F, Nannya Y, et al. MALT lymphoma of the small bowel with protein-losing enteropathy. Int J Hematol 2014;99:198-201. [Crossref] [PubMed]
- Nael A, Wu ML, Rao PN, et al. Extranodal Marginal Zone Lymphoma Presenting within the Meckel Diverticulum as Diverticulitis: A Case Report. Case Rep Pathol 2014;2014:374814. [Crossref] [PubMed]
- Fukushima M, Ioka F, Miyajima S, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) that developed in the small intestine and carried the t(11;18)(q21;q21)/ BIRC3-MALT1 fusion gene. Tenri Medical Bulletin 2014;17:81. [Crossref]
- Dhull AK, Kaushal V, Singh S, et al. A journey into insidious world of MALT lymphoma of the ileum: from the beginning to the end. J Gastrointest Oncol 2014;5:E125-7. [PubMed]
- Albahadili MA, Hadi A, Louy H. Jejunum maltoma presented with perforation masked by dexamethazone abuse complicated by wound dehiscence managed with Ozone (O3) therapy. Int J Med Res Prof 2015;1:54-7.
- Srinivasan AP, Parijatham BO, Ganapathy H. A case of intestinal MALToma with co-existent tuberculosis and Peutz-Jeghers polyp. J Postgrad Med 2015;61:134-6. [Crossref] [PubMed]
- Terry M, Northrup M, Connors JP. Small Bowel Obstruction Due to MALT Lymphoma: Sonographic Appearance and CT Correlation. Journal of Diagnostic Medical Sonography 2015;31:173-5. [Crossref]
- Kinkade Z, Esan OA, Rosado FG, et al. Ileal mucosa-associated lymphoid tissue lymphoma presenting with small bowel obstruction: a case report. Diagn Pathol 2015;10:105. [Crossref] [PubMed]
- Mastalier B, Deaconescu V, Elaiah W, et al. Multiple Intestinal Lymphoma. Rom J Intern Med 2015;53:73-8. [Crossref] [PubMed]
- Indrawati Y. MALT lymphoma in terminal ileum a case report. Jurnal Biomedik 2016;8:58-66. [Crossref]
- Stanek N, Bauerfeind P, Herzog G, et al. Marginal Zone Lymphoma Complicated by Protein Losing Enteropathy. Case Rep Hematol 2016;2016:9351408. [Crossref] [PubMed]
- Inada R, Fukata N, Ito T, et al. A case of aneurysmal lymphoma of the small intestine. Jpn J Clin Oncol 2016;46:288-9. [Crossref] [PubMed]
- Rosat A, Sánchez JM. Perforated ileal phytobezoar revealed a MALT lymphoma. Pan Afr Med J 2016;25:16. [Crossref] [PubMed]
- Ezejiofor I, Ogbu CC, Onwukamuche ME, et al. Primary B-cell jejunal maltoma in a young adult male: A case report and review of literature. N Niger J Clin Res 2017;6:57-60.
- Nehme F, Alderson J, Nassif I. Small Bowel Obstruction Secondary to Ileal Mucosa-Associated Lymphoid Tissue Lymphoma. J Gastrointest Cancer 2018;49:207-10. [Crossref] [PubMed]
- Shen KN, Zhang Y, Zhou JL, et al. Intestinal Mucosal-Associated Lymphoid Tissue Lymphoma Mimicked Cryptogenic Multifocal Ulcerous Stenosing Enteritis. Chin Med J (Engl) 2018;131:1126-7. [Crossref] [PubMed]
- Chehl N, Kantsevoy S, Raina A. Primary Jejunal MALT Lymphoma Diagnosed Using Double Balloon Enteroscopy. American Journal of Gastroenterology 2018;113:S1235. [Crossref]
- Suparman AS, Soeselo DA, Harjanti DA. Jejunal MALT (Mucosa Associated Lymphoid Tissue) Lymphoma: A Case Report. Indonesian Journal of Cancer 2019;12:127-30. [Crossref]
- Cai ZS, Chen MJ, Chu CH, et al. Small bowel mucosa-associated lymphoid tissue lymphoma: A case report and literature review. Adv Dig Med 2019;6:73-6. [Crossref]
- Muqri F, Khan A, Naous R, et al. Small Bowel Volvulus Caused by Small Intestinal B Cell Lymphoma. J Gastrointest Surg 2019;23:2065-7. [Crossref] [PubMed]
- Bennani A, Kharrasse G, Achraf M, et al. Synchronous colonic adenoma and intestinal marginal zone B-cell lymphoma associated with Crohn's disease: a case report and literature review. BMC Cancer 2019;19:966. [Crossref] [PubMed]
- Stundiene I, Maksimaityte V, Liakina V, et al. Mucosa-associated lymphoid tissue lymphoma simulating Crohn's disease: A case report. World J Clin Cases 2020;8:1454-62. [Crossref] [PubMed]
- Flieger D, Keller R, May A, et al. Capsule endoscopy in gastrointestinal lymphomas. Endoscopy 2005;37:1174-80. [Crossref] [PubMed]
- Al-Taie O, Dietrich CG, Flieger D, et al. Is there a role for capsule endoscopy in the staging work-up of patients with gastric marginal zone B-cell lymphoma of MALT? Z Gastroenterol 2013;51:727-32. [Crossref] [PubMed]
- Yoo AY, Lee BJ, Kim WS, et al. Clinicopathological Features of Small Bowel Tumors Diagnosed by Video Capsule Endoscopy and Balloon-Assisted Enteroscopy: A Single Center Experience. Clin Endosc 2021;54:85-91. [Crossref] [PubMed]
- Vetro C, Romano A, Amico I, et al. Endoscopic features of gastro-intestinal lymphomas: from diagnosis to follow-up. World J Gastroenterol 2014;20:12993-3005. [Crossref] [PubMed]
- Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer 1978;42:693-707. [Crossref] [PubMed]
- DAWSON PJ. HARRISON CV. A clinicopathological study of benign Hodgkin's disease. J Clin Pathol 1961;14:219-31. [Crossref] [PubMed]
- Keuchel M, Kurniawan N, Baltes P. Small bowel ulcers: when is it not inflammatory bowel disease? Curr Opin Gastroenterol 2019;35:213-22. [Crossref] [PubMed]
- Hsiao CH, Lee WI, Chang SL, et al. Angiocentric T-cell lymphoma of the intestine: a distinct etiology of ischemic bowel disease. Gastroenterology 1996;110:985-90. [Crossref] [PubMed]
- American College of Gastroenterology Task Force on Irritable Bowel Syndrome. An evidence-based position statement on the management of irritable bowel syndrome. Am J Gastroenterol 2009;104:S1-35. [PubMed]
- Dhanapal R, Saraswathi T, Govind RN. Cancer cachexia. J Oral Maxillofac Pathol 2011;15:257-60. [Crossref] [PubMed]
- Burger R. Impact of interleukin-6 in hematological malignancies. Transfus Med Hemother 2013;40:336-43. [Crossref] [PubMed]
- Gough P, Myles IA. Tumor Necrosis Factor Receptors: Pleiotropic Signaling Complexes and Their Differential Effects. Front Immunol 2020;11:585880. [Crossref] [PubMed]
- Metkar SS, Naresh KN, Manna PP, et al. Circulating levels of TNF alpha and TNF receptor superfamily members in lymphoid neoplasia. Am J Hematol 2000;65:105-10. [Crossref] [PubMed]
- Wöhrer S, Streubel B, Bartsch R, et al. Monoclonal immunoglobulin production is a frequent event in patients with mucosa-associated lymphoid tissue lymphoma. Clin Cancer Res 2004;10:7179-81. [Crossref] [PubMed]
- Catovsky D, Matutes E. Splenic lymphoma with circulating villous lymphocytes/splenic marginal-zone lymphoma. Semin Hematol 1999;36:148-54. [PubMed]
- Asatiani E, Cohen P, Ozdemirli M, et al. Monoclonal gammopathy in extranodal marginal zone lymphoma (ENMZL) correlates with advanced disease and bone marrow involvement. Am J Hematol 2004;77:144-6. [Crossref] [PubMed]
- de Boer JP, Hiddink RF, Raderer M, et al. Dissemination patterns in non-gastric MALT lymphoma. Haematologica 2008;93:201-6. [Crossref] [PubMed]
- Boveri E, Arcaini L, Merli M, et al. Bone marrow histology in marginal zone B-cell lymphomas: correlation with clinical parameters and flow cytometry in 120 patients. Ann Oncol 2009;20:129-36. [Crossref] [PubMed]
- Kent SA, Variakojis D, Peterson LC. Comparative study of marginal zone lymphoma involving bone marrow. Am J Clin Pathol 2002;117:698-708. [Crossref] [PubMed]
- Luminari S, Biasoli I, Arcaini L, et al. The use of FDG-PET in the initial staging of 142 patients with follicular lymphoma: a retrospective study from the FOLL05 randomized trial of the Fondazione Italiana Linfomi. Ann Oncol 2013;24:2108-12. [Crossref] [PubMed]
- Hoffmann M, Kletter K, Becherer A, et al. 18F-fluorodeoxyglucose positron emission tomography (18F-FDG-PET) for staging and follow-up of marginal zone B-cell lymphoma. Oncology 2003;64:336-40. [Crossref] [PubMed]
- Weiler-Sagie M, Bushelev O, Epelbaum R, et al. (18)F-FDG avidity in lymphoma readdressed: a study of 766 patients. J Nucl Med 2010;51:25-30. [Crossref] [PubMed]
- Beal KP, Yeung HW, Yahalom J. FDG-PET scanning for detection and staging of extranodal marginal zone lymphomas of the MALT type: a report of 42 cases. Ann Oncol 2005;16:473-80. [Crossref] [PubMed]
- Treglia G, Zucca E, Sadeghi R, et al. Detection rate of fluorine-18-fluorodeoxyglucose positron emission tomography in patients with marginal zone lymphoma of MALT type: a meta-analysis. Hematol Oncol 2015;33:113-24. [Crossref] [PubMed]
- Carrillo-Cruz E, Marín-Oyaga VA, de la Cruz Vicente F, et al. Role of 18F-FDG-PET/CT in the management of marginal zone B cell lymphoma. Hematol Oncol 2015;33:151-8. [Crossref] [PubMed]
- Reinartz G, Molavi Tabrizi C, Liersch R, et al. Renaissance of Radiotherapy in Intestinal Lymphoma? 10-Year Efficacy and Tolerance in Multimodal Treatment of 134 Patients: Follow-up of Two German Multicenter Consecutive Prospective Phase II Trials. Oncologist 2020;25:e816-32. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl BS, et al. Randomized trial of bendamustine-rituximab or R-CHOP/R-CVP in first-line treatment of indolent NHL or MCL: the BRIGHT study. Blood 2014;123:2944-52. [Crossref] [PubMed]
- Flinn IW, van der Jagt R, Kahl B, et al. First-Line Treatment of Patients With Indolent Non-Hodgkin Lymphoma or Mantle-Cell Lymphoma With Bendamustine Plus Rituximab Versus R-CHOP or R-CVP: Results of the BRIGHT 5-Year Follow-Up Study. J Clin Oncol 2019;37:984-991. [Crossref] [PubMed]



