
Collagen XV mediated the epithelial-mesenchymal transition to inhibit hepatocellular carcinoma metastasis
Introduction
Hepatocellular carcinoma (HCC) is the third most lethal malignancy, causing nearly 600,000 deaths each year worldwide (1). Unfortunately, the incidence of HCC has been rising in recent decades (2). Although radiofrequency thermal ablation, surgical resection, orthotopic liver transplantation and sorafenib have improved the prognosis of patients with HCC, postsurgical recurrence and drug resistance reduce patient satisfaction (3). Furthermore, immunosurveillance introduced immune checkpoint blockades, or anti-PD1 antibody therapy showed a minimal response and sometimes resulted in drug resistance (4). The progression of HCC involved the dysregulation of extracellular matrix (ECM) that provided more than passive physical support to hold neoplastic cells. The cells sensed the signal from the ECM so that affected the cell proliferate and invasion by means of versatile surface receptors (5).
Collagen XV is a nonfibrillar collagen with multiple interruptions (6). Collagen XV was lost prior to tumour metastasis in several organs located in the basement membrane (BM) or the basement zone, which suggested collagen XV played a role in maintaining BM integrity and preventing the migration of tumour cells through this barrier (7). Some studies demonstrated that collagen XV had antiangiogenic and antitumoral properties and played a vital role in tissue homeostasis in the liver, kidney, eye and central nervous system (8). However, in colonic adenocarcinomas (9) and cervical carcinoma (10), collagen XV was negatively related to tumorigenicity and metastasis.
Epithelial–mesenchymal transition (EMT) is closely associated with the cell invasive ability and poor prognosis (11). EMT-inducing transcription factors (EMT-TFs), involving Snail, ZEB1 and Twist1, activated the expression of genes that promoted the mesenchymal cell state and repressed the epithelial state (12,13). The expression of E-cadherin (epithelial markers) was markedly decreased, while N-cadherin, vimentin and fibronectin (mesenchymal markers) were upregulated during EMT, which led to the detachment of epithelial cells from each other, undermining the basement membrane, and reorganizing cell-cell and cell-extracellular matrix interactions. As a result, the cells gained motility (13,14).
Discoidin domain receptor 1 (DDR1), a membrane receptor tyrosine kinase (RTK), not only activated classical growth factor tyrosine kinase receptors rapidly and transiently but also interacted with collagen (15). DDR1, with roles in malignant transformation, cell proliferation, EMT, invasive processes, and the response to chemotherapy, was upregulated in fibrosis and carcinoma occurring in different organs, such as skin hypertrophic scars, idiopathic pulmonary fibrosis, and liver cirrhosis due to various causes (15,16). The previous research showed that a significant increase in DDR1 was accompanied by the promotion of EMT and glutamine metabolism in HCC cells (17).
In our study, the existed databases showed the positive effect of Col15a1 for survival. Overexpression of collagen XV inhibited cell metastasis and reversed the EMT phenotype in HepG2 and HCCLM3 cells in vitro. Collagen XV inhibited pulmonary and liver metastasis in the HCCLM3 mouse model. Additionally, we provided evidence that collagen XV targeted DDR1 to regulate EMT. We present the following article in accordance with the ARRIVE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-299/rc).
Methods
Survival analysis
Kaplan-Meier plotter (http://kmplot.com/analysis/) with existed mRNA databases about several cancer including HCC, an online tool, was applied to evaluate the effect of Col15a1 for survival and prognostic of patients.
Cell culture
Human HepG2 and HCCLM3 cells were bought from the Shanghai Institute of Biochemistry and Cell Biology (Shanghai, China). These cell lines were maintained in DMEM (SparkJade, Shandong, China) with adjunct of 10% foetal bovine serum (FBS) (Gibco, Gran Island, NY, USA), 1% penicillin and 1% streptomycin (Solarbio, Beijing, China) as. Cells were cultured at 37 ℃ in 5% CO2.
Animal study
Five-weeks-old female BALB/c nude mice purchased from the Southern Mode Company (Shanghai, China) were used for subcutaneous xenograft and metastasis assays. For subcutaneous xenografts, 2×106 HCCLM3 cells were subcutaneously injected into the right armpits of mice (n=5 per group). The mice were observed within 6 weeks for tumour formation. The tumour volume was measured and recorded using the formula: V = 0.5 × length × width2 every week. The tumour metastasis model was established via injecting 1×106 HCCLM3 cells into blood of nude mice and metastases was assessed in the liver and lung by H&E staining after 6 weeks via tail vein injection.
Collagen XV expression construct and cell transfection
The full-length human Col15a1 cDNA (NM_001855) was inserted at the BamHI/AgeI sites in p-GV492, which was purchased from JiKai Company (Shanghai, China); empty p-GV492 was used as a control. Cells were transfected with the lentiviruses at an MOI of 5×108 TU/mL in the presence of 5 µg/mL polybrene (Hanheng, Shanghai, China) and were selected for successful transfection with 1 µg/mL puromycin (Hanheng, Shanghai, China).
Cell viability assay
HepG2, HCCLM3, and the corresponding cell lines overexpressing collagen XV were seeded in 96-well plates at the same quantity. 10 µL of CCK solution (Dojindo, Kumamoto, Japan) was added to the cells at a ratio of CCK-8 solution: medium =10:100. After incubation for 4 h, the optical density (OD) values were measured at 450 nm with the multifunctional microplate reader (BioTek, Winooski, VT, USA).
Real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR) analysis
Total RNA from hepatocellular lines was extracted using an RNA extraction kit (SparkJade, Shandong, China) according to the manufacturer’s instructions, and cDNA was synthesized using reverse transcriptase (ABClonal, Wuhan, China). A SYBR Green PCR kit (ABClonal, Wuhan, China) was used to quantify relative mRNA levels via the 2−ΔΔCT method. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as internal reference. Sequences for the real-time PCR primers are shown in Table S1.
Western blot analysis
The cells were lysed with RIPA lysis buffer (Solarbio, Beijing, China), and the extracted proteins were quantified using a bicinchoninic acid (BCA) protein assay kit (Solarbio, Beijing, China). The proteins in equal amounts (40 µg) were separated by 10% SDS–PAGE and transferred to PVDF membranes (Millipore, Bedford, MA, USA). The primary antibodies were added to bind the corresponding protein overnight at 4 ℃: anti-E-cadherin (1:1,000), anti-N-cadherin (1:1,000), anti-Snail (1:1,000), anti-Slug (1:1,000), anti-vimentin (1:1,000), anti-matrix metalloproteinase (MMP) 9 (1:1,000) and anti-DDR1 (1:1,000), which were purchased from Cell Signaling Technology (Boston, MA, USA). Anti-collagen XV (1:1,000) was purchased from Affinity Biosciences (Cincinnati, OH, USA) and β-actin, as the internal control was purchased from Cell Signaling Technology (1:1,000). Subsequently, the membranes were incubated with HRP-conjugated secondary antibodies (Absin, Shanghai, China) for 1 h at room temperature and were exposed to ECL reagent (NCM Biotech, Suzhou, China). The intensity of the bands was quantified by using ImageJ software.
Cell invasion assay
To perform the colony formation assay, ~500 dispersed cells per cell line were seeded in 6-well plates and allowed to proliferate for 2 weeks. At the specified time, the colonies were fixed with 4% paraformaldehyde for 30 min and stained with 0.1% crystal violet (Beyotime, Shanghai, Chain) at room temperature for 15 min according to the established order. The number of colonies was counted under a light microscope. To perform the wound healing assay, the cells in logarithmic growth phase proliferated in 6‑well plates to reach 80–90% confluence with a line wound scratched using a 200 µL pipette tip. Then the medium was substituted with serum-free DMEM for 24 h. The scratch wounds were photographed at 0 and 24 h under a light microscope. The migration of cells was analysed using ImageJ software. The formula to calculate wound healing rate was showed below: wound healing rate = [(scratch width at 0 h) − scratch width at 24 h]/(scratch width at 0 h)]×100%. Transwell chambers with 8 µm pore sizes (Corning, MA, USA) were coated with 80 µL of 1:4 diluted Matrigel (Corning, MA, USA) and incubated at 37 ℃ for 2 h to perform Transwell invasion analysis. Approximately 2×104 cells with 300 µL serum-free DMEM were placed into the upper chamber, while 500 µL DMEM supplemented with 10% FBS was added into the lower chamber. After 48 h of incubation, the cells in the upper chamber were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet (Solarbio, Beijing, China) for 15 min at room temperature. The non-invaded cells in the upper chamber were removed with a cotton swab, and the invaded cells were counted and photographed under a light microscope. For collagen I scatter assays, 6-well plates were coated with 50 mg/mL collagen I solution from rat tails (Fushen, Shanghai, China) overnight at 4 ℃, and then 1×105 cells were plated for 48 h. The scatter phenotype was photographed utilizing a light microscope.
Immunofluorescence
HepG2, HCCLM3, and the corresponding cell lines overexpressing collagen XV were plated on 24-well plates overnight. Then, the cells were fixed with 4% paraformaldehyde for 30 min and permeabilized with 0.1% Triton-X 100 for 20 min, then incubated with 2% BSA for 2 h to block the nonspecific binding. After cells being washed with PBS, the specific protein in the cell was bound by the primary antibody overnight at 4 ℃, followed by Alexa Fluor® 594 (red) secondary antibodies for 2 h. Then, nuclei were stained with DAPI. Images were captured under a fluorescence microscope.
Immunohistochemistry
The xenografts growing on nude mice were fixed with 4% paraformaldehyde and cut into tissue slices after embedding in paraffin. After the sections were deparaffinized, they were incubated with a primary antibody against collagen XV or the endothelial component CD31 followed by the second goat anti-rabbit antibody.
Statistical analysis
Data from this study were presented as the mean ± standard deviation (SD) and were statistically analysed by one-way analysis of variance (ANOVA) using GraphPad 8.0 statistical software. A P value of <0.05 was considered to indicate a statistically significant result. All experiments were repeated at least three times.
Ethical statement
All animal experimental procedures were approved by the laboratory animal ethical commissions of Shanghai Jiao Tong University Affiliated Sixth People’s Hospital (No. DWSY2021-011), in compliance with Chinese national guidelines for the care and use of animals.
Results
Collagen XV inhibited migration in vitro
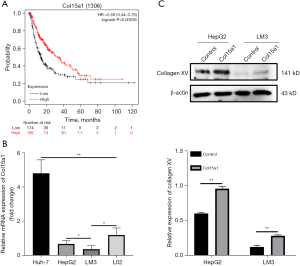
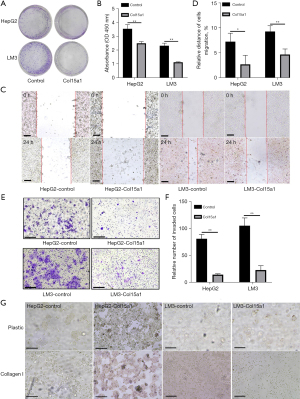
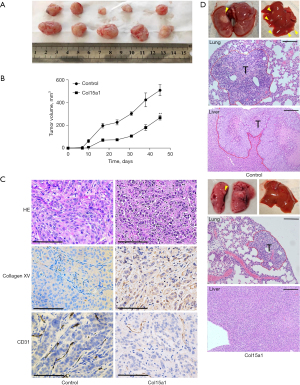
Firstly, the Kaplan–Meier plotter bioinformatics analysis platform was used to confirm the protective and prognostic value of Col15a1 where 370 liver cancer patients in total were available. The result suggested that high expression of Col15a1 was positive with favourable overall survival of patients with liver cancer (P=0.00036) (Figure 1A). To explore the effect of Col15a1 on HCC metastasis, HepG2 and HCCLM3, whose expression levels of Col15a1 were lower (Figure 1B), were two cell lines used to stably overexpress collagen XV. Overexpression of collagen XV was confirmed by western blotting (Figure 1C). Overexpression of collagen XV suppressed the formation of colonies (Figure 2A) and cell proliferation (Figure 2B). In addition, the results indicated that there was a slowed healing of scratch wounds (Figure 2C,2D) and significant decrease in the relative percentage of invaded cells (Figure 2E,2F) in the collagen XV overexpression group compared with the control group, which demonstrated that collagen XV inhibited the migratory capabilities of cancer cells in HCC. There is an assay meanes scattering to mimic the process of EMT in vitro during tumour progression (18). Our results showed the cells in control group scattered on the collagen I substrate. Oppositely, overexpression of collagen XV inhibited the scatter response (Figure 2G). However, this effect on HCCLM3 cells was not as significant as that on HepG2 cells.
Collagen XV inhibited EMT in HCC
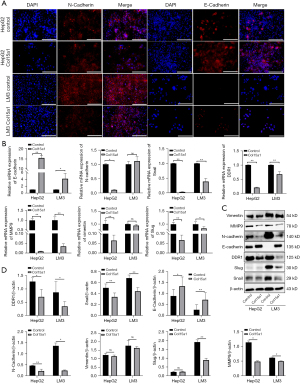
Immotile epithelial cells losing adhesion and tight junctions, become migratory mesenchymal cells during EMT in invasive front of metastatic cancer (19). Therefore, we hypothesized that collagen XV inhibited the migration and invasion of HCC cells via modulation of EMT. Immunofluorescence showed that the protein level of E-cadherin increased in HepG2 cells, while N-cadherin decreased in HCCLM3 cells overexpressing collagen XV (Figure 3A). However, the changes in E-cadherin expression in HCCLM3 cells and N-cadherin expression in HepG2 cells were not obvious, which may be attributed to the different characteristics of the cells. In HCC cell lines overexpressing collagen XV, the mRNA and protein levels of E-cadherin were significantly upregulated, while the expression of N-cadherin, vimentin and MMP9 were dramatically downregulated compared with the control group (Figure 3B-3D). Previous studies showed that Snail, a key transcriptional repressor of E-cadherin expression, and Slug, another member of the Snail family of transcription factor conferred tumour cells with cancer stem cell-like traits and promoted tumour recurrence and metastasis (13,14,19). Snail/Slug was the upstream factor that probably regulated the expression of E-cadherin and N-cadherin, and their variation trends entirely were inconsistent with the changing trend of E-cadherin and in line with that of N-cadherin (Figure 3B-3D).
Collagen XV interacts with DDR1 to regulate EMT
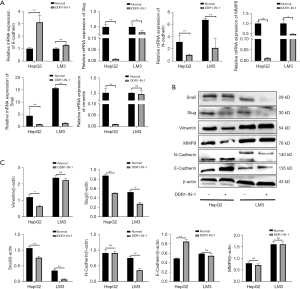
DDR1 has been proven to interact with collagen XV (18) and mediate numerous functions in cell proliferation, survival, and migration, correlating with poor prognosis in different tumour types, such as hepatocellular, breast and lung carcinoma (20,21). Conversely, DDR1 has a protective role in stability of E-cadherin at the cell membrane possibly, relieving the repression of the integrin pathway (22). In our study, the expression of collagen XV had a negative effect on DDR1. A previous study showed that the DDR1 promoter contained a putative binding site for the EMT transcription factor zinc finger E-box binding homeobox 1 (ZEB1) (23). Furthermore, Snail enhanced the expression of many mesenchymal genes, including MMP9 and ZEB1 (24). Therefore, to explore the hypothesis that DDR1 regulates the expression of Snail or/and Slug, we treated the cells with the DDR1 inhibitor DDR1-IN-1. The results showed that when DDR1 function was inhibited, the transcriptional and translational levels of Snail, Slug, N-cadherin, vimentin, MMP9 were downregulated, while the expression of E-cadherin were upregulated but the changes in expression of vimentin and MMP9 in LM3 were not obvious (Figure 4A-4C).
Collagen XV inhibited migration in vivo
To explore the effects of collagen XV in vivo, HCCLM3 cells and the corresponding cell lines overexpressing collagen XV were seeded into the right armpits of nude mice (five mice in each group). The tumours originated from HCCLM3 cells overexpressing collagen XV, whose proliferation was significantly inhibited (Figure 5A,5B). The expression of collagen XV in the overexpression group was higher than that in the control group by immunohistochemistry (Figure 5C). As the antiangiogenic effect of collagen XV was disputed (6), we detected the expression of CD31, which was a marker of vessels. The results showed that it was expressed at lower levels in the collagen XV overexpression group than in the control group (Figure 5C). Subsequently, lung metastasis models were established by injecting HCCLM3 and the corresponding cell line overexpressing collagen XV into the lateral tail vein. Six weeks later, the occurrence of metastatic tumours in the lungs and livers was confirmed by H&E staining. The results showed that overexpression of collagen XV could markedly inhibit tumour metastasis in both lung and liver (Figure 5D).
Discussion
HCC arises in a severely altered microenvironment (25). The ECM surrounding cellular structures and providing structural integrity is associated with tumour invasion and metastasis (7). In the present study, higher expression of Col15a1 showed more better survival and prognostic (Figure 1A). However, the function of this molecule in different phases of HCC is complicated. In the previous researches, the mRNA expression of Col15a1 and collagen XV in HCC were significantly upregulated compared with those in nontumoral regions in both mice and humans (26,27). Nevertheless, some research had shown that collagen XV had antitumor migration activity (10,28,29), and a homologous C-terminal domain fragment of type XV collagen had antiangiogenic activity, while whether collagen XV completely lacks inhibition of HUVEC tube formation remains controversial (6). We suspected that the expression of collagen XV increased during the process from fibrosis to HCC as a component of sinusoidal capillarization. However, as the disease continued to advance, collagen XV disappeared gradually from the basement membrane before HCC metastasized, which was negatively correlated with HCC migration. In other words, combining the cancer cells and low expression of collagen XV indicated a high probability of metastasis. Considering our results that the reduction in the relative percentage of invaded cells (Figure 2E,2F), slower healing of scratch wounds (Figure 2C,2D), smaller tumour volume formation (Figure 5A,5B) and less lung and liver metastases (Figure 5D) with collagen XV overexpression, the conclusion could be drawn that collagen XV had an antitumor migration role. Meanwhile, our results showed that collagen XV could suppress angiogenesis (Figure 5C), which was a valid way to inhibit tumour development.
Metastasis is a complex and dynamic process associated with a series of biological and pathological events (30). Many pathways have been explored in HCC metastasis, such as the Wnt10b-Wnt/β-catenin signalling pathway (31), the JAK2/STAT3 signalling pathway (32), and a variety of miRNAs associated with the progression (1). Emerging evidence has demonstrated that EMT plays a vital role in the invasion and metastasis of malignant tumours (33). During EMT, tumour progression involves many stromal, cellular, and environmental responses, including hepatocyte growth factor (HGF), transforming growth factor beta (TGF-β), MMPs and other signalling proteins in the extracellular matrix (7). Snail and Slug are critical transcription factors for the invasion and metastasis involved in EMT and are capable of repressing E-cadherin (14,19,34). In our study, when the expression of collagen XV was upregulated, E-cadherin increased along with it while Snail, N-cadherin, vimentin and MMP9 showed the opposite trend. These results indicated that collagen XV might inhibit HCC metastasis by downregulating the transcription factor Snail/Slug-mediated EMT (Figure 3). DDR1 has been shown to be overexpressed in cancers associated with proliferation and migration, such as HNSCC (35) and pancreatic cancer (18). After being activated by collagen, DDR1 triggered the activation of a number of downstream signalling pathways (36) involving the expression of proinflammatory mediators as well as matrix-degrading enzymes (35). We demonstrated that DDR1 was expressed at lower levels in HepG2 and HCCLM3 cells overexpressing collagen XV. Inhibition of the DDR1 receptor by DDRI-IN-1 downregulated the expression of Snail and Slug (Figure 4). A previous study showed that an increase in DDR2 (another RTK similar to DDR1) expressed in HCC lines promoted EMT, extended the half-life of Snail, and facilitated cell invasion and survival (25). However, the expressions of some indicators were almost unchanged after DDR1 inhibition, such as MMP9 and vimentin in LM3, which indicate that collagen XV might have other mechanisms to inhibit HCC metastasis. The role of collagen XV in HCC metastasis is a complex process which needs to be further explored.
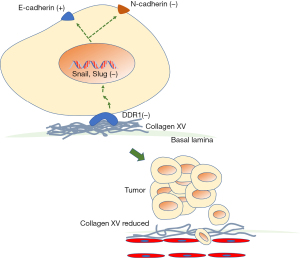
In conclusion, the present study illustrated that collagen XV, as the prognostic biomarker in HCC patients, suppressed the growth and metastasis of HCC. Furthermore, HCC metastasis was inhibited by downregulating DDR1 and affecting EMT (Figure 6). Our findings will provide a new theoretical basis for further functional, diagnostic and therapeutic research on collagen XV in HCC.
Acknowledgments
Funding: This work was supported by grants from the National Natural Science Foundation of China (No. 81770589).
Footnote
Reporting Checklist: The authors have completed the ARRIVE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-299/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-299/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-299/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. DWSY2021-011) granted by laboratory animal ethical commissions of Shanghai Jiao Tong University, in compliance with Chinese national guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Xiao Z, Wang Y, Ding H. XPD suppresses cell proliferation and migration via miR-29a-3p-Mdm2/PDGF-B axis in HCC. Cell Biosci 2019;9:6. [Crossref] [PubMed]
- McGlynn KA, Petrick JL, London WT. Global epidemiology of hepatocellular carcinoma: an emphasis on demographic and regional variability. Clin Liver Dis 2015;19:223-38. [Crossref] [PubMed]
- Gao X, Wang X, Zhang S. Bioinformatics identification of crucial genes and pathways associated with hepatocellular carcinoma. Biosci Rep 2018;38:BSR20181441. [Crossref] [PubMed]
- Huang XY, Zhang PF, Wei CY, et al. Circular RNA circMET drives immunosuppression and anti-PD1 therapy resistance in hepatocellular carcinoma via the miR-30-5p/snail/DPP4 axis. Mol Cancer 2020;19:92. [Crossref] [PubMed]
- Tahmasebi Birgani M, Carloni V. Tumor Microenvironment, a Paradigm in Hepatocellular Carcinoma Progression and Therapy. Int J Mol Sci 2017;18:405. [Crossref] [PubMed]
- Marneros AG, Olsen BR. The role of collagen-derived proteolytic fragments in angiogenesis. Matrix Biol 2001;20:337-45. [Crossref] [PubMed]
- Clementz AG, Harris A. Collagen XV: exploring its structure and role within the tumor microenvironment. Mol Cancer Res 2013;11:1481-6. [Crossref] [PubMed]
- Heljasvaara R, Aikio M, Ruotsalainen H, et al. Collagen XVIII in tissue homeostasis and dysregulation - Lessons learned from model organisms and human patients. Matrix Biol 2017;57-58:55-75. [Crossref] [PubMed]
- Amenta PS, Briggs K, Xu K, et al. Type XV collagen in human colonic adenocarcinomas has a different distribution than other basement membrane zone proteins. Hum Pathol 2000;31:359-66. [Crossref] [PubMed]
- Harris A, Harris H, Hollingsworth MA. Complete suppression of tumor formation by high levels of basement membrane collagen. Mol Cancer Res 2007;5:1241-5. [Crossref] [PubMed]
- Barasch J. Genes and proteins involved in mesenchymal to epithelial transition. Curr Opin Nephrol Hypertens 2001;10:429-36. [Crossref] [PubMed]
- Tam WL, Weinberg RA. The epigenetics of epithelial-mesenchymal plasticity in cancer. Nat Med 2013;19:1438-49. [Crossref] [PubMed]
- Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol 2019;20:69-84. [Crossref] [PubMed]
- Lamouille S, Xu J, Derynck R. Molecular mechanisms of epithelial-mesenchymal transition. Nat Rev Mol Cell Biol 2014;15:178-96. [Crossref] [PubMed]
- Rammal H, Saby C, Magnien K, et al. Discoidin Domain Receptors: Potential Actors and Targets in Cancer. Front Pharmacol 2016;7:55. [Crossref] [PubMed]
- Moll S, Desmoulière A, Moeller MJ, et al. DDR1 role in fibrosis and its pharmacological targeting. Biochim Biophys Acta Mol Cell Res 2019;1866:118474. [Crossref] [PubMed]
- Lin Y, Jin H, Wu X, et al. The cross-talk between DDR1 and STAT3 promotes the development of hepatocellular carcinoma. Aging (Albany NY) 2020;12:14391-405. [Crossref] [PubMed]
- Clementz AG, Mutolo MJ, Leir SH, et al. Collagen XV inhibits epithelial to mesenchymal transition in pancreatic adenocarcinoma cells. PLoS One 2013;8:e72250. [Crossref] [PubMed]
- Wang Y, Shi J, Chai K, et al. The Role of Snail in EMT and Tumorigenesis. Curr Cancer Drug Targets 2013;13:963-72. [Crossref] [PubMed]
- Nokin MJ, Darbo E, Travert C, et al. Inhibition of DDR1 enhances in vivo chemosensitivity in KRAS-mutant lung adenocarcinoma. JCI Insight 2020;5:137869. [Crossref] [PubMed]
- Zhao Z, Zhao S, Luo L, et al. miR-199b-5p-DDR1-ERK signalling axis suppresses prostate cancer metastasis via inhibiting epithelial-mesenchymal transition. Br J Cancer 2021;124:982-94. [Crossref] [PubMed]
- Cabodi S, del Pilar Camacho-Leal M, Di Stefano P, et al. Integrin signalling adaptors: not only figurants in the cancer story. Nat Rev Cancer 2010;10:858-70. [Crossref] [PubMed]
- Taube JH, Herschkowitz JI, Komurov K, et al. Core epithelial-to-mesenchymal transition interactome gene-expression signature is associated with claudin-low and metaplastic breast cancer subtypes. Proc Natl Acad Sci U S A 2010;107:15449-54. [Crossref] [PubMed]
- Wu WS, You RI, Cheng CC, et al. Snail collaborates with EGR-1 and SP-1 to directly activate transcription of MMP 9 and ZEB1. Sci Rep 2017;7:17753. [Crossref] [PubMed]
- Filliol A, Schwabe RF. Contributions of Fibroblasts, Extracellular Matrix, Stiffness, and Mechanosensing to Hepatocarcinogenesis. Semin Liver Dis 2019;39:315-33. [Crossref] [PubMed]
- Kimura K, Nakayama M, Naito I, et al. Human collagen XV is a prominent histopathological component of sinusoidal capillarization in hepatocellular carcinogenesis. Int J Clin Oncol 2016;21:302-9. [Crossref] [PubMed]
- Lai KK, Shang S, Lohia N, et al. Extracellular matrix dynamics in hepatocarcinogenesis: a comparative proteomics study of PDGFC transgenic and Pten null mouse models. PLoS Genet 2011;7:e1002147. [Crossref] [PubMed]
- Mutolo MJ, Morris KJ, Leir SH, et al. Tumor suppression by collagen XV is independent of the restin domain. Matrix Biol 2012;31:285-9. [Crossref] [PubMed]
- Hurskainen M, Ruggiero F, Hägg P, et al. Recombinant human collagen XV regulates cell adhesion and migration. J Biol Chem 2010;285:5258-65. [Crossref] [PubMed]
- Prahl LS, Odde DJ. Modeling Cell Migration Mechanics. Adv Exp Med Biol 2018;1092:159-87. [Crossref] [PubMed]
- Zhang S, Zhang F, Chen Q, et al. CRISPR/Cas9-mediated knockout of NSD1 suppresses the hepatocellular carcinoma development via the NSD1/H3/Wnt10b signaling pathway. J Exp Clin Cancer Res 2019;38:467. [Crossref] [PubMed]
- Wu L, Li J, Liu T, et al. Quercetin shows anti-tumor effect in hepatocellular carcinoma LM3 cells by abrogating JAK2/STAT3 signaling pathway. Cancer Med 2019;8:4806-20. [Crossref] [PubMed]
- Wang W, Wang Y, Liu M, et al. Betulinic acid induces apoptosis and suppresses metastasis in hepatocellular carcinoma cell lines in vitro and in vivo. J Cell Mol Med 2019;23:586-95. [Crossref] [PubMed]
- Nieto MA. The snail superfamily of zinc-finger transcription factors. Nat Rev Mol Cell Biol 2002;3:155-66. [Crossref] [PubMed]
- Lai SL, Tan ML, Hollows RJ, et al. Collagen Induces a More Proliferative, Migratory and Chemoresistant Phenotype in Head and Neck Cancer via DDR1. Cancers (Basel) 2019;11:1766. [Crossref] [PubMed]
- Valiathan RR, Marco M, Leitinger B, et al. Discoidin domain receptor tyrosine kinases: new players in cancer progression. Cancer Metastasis Rev 2012;31:295-321. [Crossref] [PubMed]


