
Characterization of somatic mutations and pathway alterations during hepatocellular carcinoma vascular invasion using next-generation sequencing
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant solid tumors worldwide, accounting for more than 90% of primary liver cancers, and is the fourth most common cause of cancer-related deaths (1,2). Due to factors such as hepatitis B virus infection, aflatoxin exposure, alcohol abuse, and environmental pollution, China has become the country with the highest incidence of liver cancer (about 55% of the world’s total incidence) and the largest number of deaths (3). Surgical resection and local ablation remain the most commonly used curative treatments for HCC (4,5). However, the tumor recurrence rate is as high as 70% after liver resection and 25% after liver transplantation, and the overall survival (OS) rate remains unsatisfactory, with the 5-year OS rate at 15–20% (6,7).
Vascular invasion is often associated with tumor metastasis, recurrence, and poor prognosis (8). After liver transplantation, patients with microvascular invasion (MVI) experience a more than four-fold increased risk of HCC recurrence (9). Unfortunately, preoperative imaging cannot detect potential microscopic vascular invasion (10). Therefore, identifying the molecular changes associated with vascular invasion to determine the risk of HCC recurrence is a primary task in predicting long-term prognosis. HCC has significant phenotypic and molecular heterogeneity (11). Menyhárt et al. found that the BIRC5, CDC20, and PLK1 genes could adequately predict the prognosis of Asian patients (12). Vascular invasion is often divided into macrovascular invasion and MVI, however, the molecular changes related to the extent of vascular invasion have been rarely investigated (13). Next-generation sequencing (NGS) technology can realize large-scale parallel sequencing of multiple genes, and fundamentally solve the practical problems of difficult diagnosis caused by heterogeneity of single-gene inheritance, multiple genes, and complex phenotypes.
To identify robust genes related to the prognosis of HCC, 50 patients with HCC were enrolled and screened. Patients were grouped according to the degree of vascular invasion, namely, the no vascular invasion group, the MVI group, and the macrovascular invasion group. Using high-throughput targeted gene sequencing and whole exome sequencing (WES) methods to explore the molecular mechanism changes and pathway changes related to HCC vascular invasion, this information will be useful in guiding auxiliary diagnosis and individualized treatment of Chinese HCC patients. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-556/rc).
Methods
Collection of clinical samples
Between 2018 and 2019, a total of 50 HCC patients were enrolled in this study. The median follow-up time was 26.7 months. All tumor samples were estimated for invasive tumor content by two molecular pathologists to ensure that more than 50% of the cells were tumor cells. Avascular invasion was defined as no portal vein, hepatic vein, or inferior vena cava invasion on imaging and no vascular invasion on electron microscopy. MVI refers to the observation of tumor thrombus or cancer cells under the electron microscope without invasion of large blood vessels. Macrovascular invasion refers to the formation of tumor thrombi in the portal vein and its main branches. High-depth targeted gene panel sequencing and WES were performed after passing the nucleic acid quality test. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013) (14). The protocol was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (ID of the approval: 2012-092). Written informed consent was obtained from all patients prior to inclusion in the study.
DNA extraction and NGS
DNA was extracted from serial thick sections cut from tumor samples and matched peripheral blood leukocytes as germline DNA control. The invasive tumor content was estimated by pathologists to ensure that more than 50% of cells were tumor cells. DNA was isolated from the formalin-fixed paraffin-embedded (FFPE) tissue blocks and blood samples using the DNeasy Blood and Tissue Kit (69504, QIAGEN, Venlo, Netherlands). Qubit 3.0 (Thermofisher) was used to detect the quality of the DNA. The Generic Library Construction Kit (Cat. No. M0348L, New England Biolabs, USA) and the Hybrid Capture Kit (Cat. No. 1001111E, Naonda (Nanjing) Biotechnology Co., Ltd., Nanjing) were used to capture targeted region sequences from genomic DNA and construct sequencing computer libraries. The captured libraries of targeted gene sequencing and WES were performed as paired end-readings on the Illumina NoveSeq 6000 sequencing platform.
Variant annotation analysis and visual mapping
Germline variations were identified via SpeedSeq and somatic mutations were identified using Mutect (15). Somatic indels were determined using SomaticIndelDetector (16). The high-quality somatic mutations and indels were selected. The variant data were annotated using ANNOVAR (17) and Oncotator (18), and converted to MAF files using maftools (19). The cancer driver genes were analyzed using Intogen (20), including OncodriveFM and OncodriveCLUST. Based on the fact that tumor development is an evolutionary process, detection of positive selection signals in the pattern of somatic mutation of genes has been used to identify drivers in tumor cohorts.
Statistical analysis
Progress-free survival (PFS) and overall survival (OS) were defined as the time from randomization to disease progression or last follow-up and the time from surgery to death or last follow-up, respectively. All the correlated clinical and biological variables were analyzed using the SPSS Statistics 22.0 package and the ggpubr package (21) in R software (22) by means of Fisher’s test or a non-parametric test when necessary. The Kruskal-Wallis test was used to analyze the differences in tumor mutational burden (TMB) between different groups. A P value <0.05 was considered statistically significant.
Results
Patient characteristics
Tumor tissue and corresponding blood samples were collected from 50 HCC patients at the time of diagnosis, including 46 males (92%) and 4 females (8%), with an average age of 51.7 years (range, 25–80 years). Of these, 42 patients had a history of hepatitis and 19 patients had a history of alcohol consumption. According to the Barcelona Clinic Liver Cancer (BCLC) clinical staging system, 19 cases were stage A, 8 cases were stage B, and 23 cases were stage C. There were 35 patients with vascular infiltration, including 16 cases of microvascular infiltration and 19 cases of macrovascular infiltration. The imaging or surgical samples of the other 15 patients showed no vascular infiltration under electron microscopy. In addition, 6 patients presented with extrahepatic metastases and 8 had lymph node metastases. Among the enrolled patients, 31 were treated with radical surgery and the other 19 patients were treated with medical therapy. The detailed clinical characteristics of the patients are shown in Table 1 and Table S1.
Table 1
| Characteristic | No. of cases | Proportion |
|---|---|---|
| Total number | n=50 | |
| Age, years (mean) | 51.7 [25–80] | |
| Sex | ||
| Male | 46 | 92% |
| Female | 4 | 8% |
| History of hepatitis | 42 | 84% |
| The history of drinking | ||
| Yes | 19 | 38% |
| No | 31 | 62% |
| BCLC staging | ||
| A | 19 | 38% |
| B | 8 | 16% |
| C | 23 | 46% |
| Vascular invasion | ||
| Microvascular | 16 | 32% |
| Macrovascular | 19 | 38% |
| Avascular | 15 | 30% |
| Extrahepatic metastasis | ||
| Yes | 6 | 12% |
| No | 44 | 88% |
| Lymphatic metastasis | ||
| Yes | 8 | 16% |
| No | 42 | 84% |
| Therapy method | ||
| Excision | 31 | 62% |
| Medication | 19 | 38% |
BCLC, Barcelona Clinic Liver Cancer.
The occurrence of vascular invasion affects the prognosis of hepatocellular patients
According to the degree of vascular invasion of HCC, the 50 patients were divided into the macrovascular invasion group, the MVI group, or the avascular invasion group. Combined with clinical prognostic information, Mantel-Cox tests found that there were significant differences in PFS and OS among the three groups, (P=0.0026 and P=0.0009, respectively) (Figure 1). The prognosis of patients with macrovascular invasion was significantly worse than that of patients with MVI and those with no vascular invasion.

Characterization of somatic mutations associated with vascular invasion and tumor mutation burden (TMB)
A total of 50 tumor tissue sections and matched blood DNA samples were collected, of which, 28 underwent whole-exome sequencing and 22 underwent targeted gene sequencing. We successfully analyzed all sections and matched blood second-generation sequencing data using an analysis pipeline of whole exome and targeted sequencing, with an average depth of 203.0X for whole exomes and 572.3X for targeted sequencing samples. A total of 762 somatic mutations were detected by comparing significant changes in non-reference alleles in tumor and control groups (Table S2). Missense mutations were the most common type of mutations, along with frame shift deletion, in frame insertion, frame deletion, and so on. Patient P50 had the most SNVs, followed by patient P23. The top 30 somatic gene mutations are listed according to mutation frequency. The three genes with the highest mutation frequency were TP53 (28%), CTNNB1 (14%), and KMT2B (10%). Mutations in CTNNB1, CMT2B, NF1, ZNF595, and KMT2D were found in the avascular invasion group and the MVI group, but not in the macrovascular invasion group. Conversely, mutations in KIAA2026, ALMS1, FGFR3, LRP1B, and PRDM1 were detected in the macrovascular invasion group and the MVI group, but not in the avascular invasion group (Figure 2A).
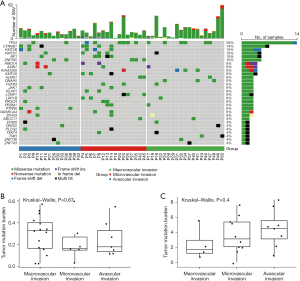
Additionally, the TMB in the whole-exome and target gene sequencing data was calculated using the number of nonsynonymous mutations occurring in somatic cells. Overall, there were large differences in the TMB values across samples within each group. However, there were no significant differences in the TMB among the avascular invasion group, the MVI group, and the macrovascular invasion group (WES data, P=0.67; targeted gene panel, P=0.4; Figure 2B,2C).
Candidate biomarkers associated with prognosis in HCC
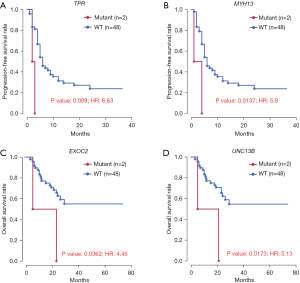
Combining the recurrence and survival status of patients, 10 genes were found to be significantly associated with OS or PFS (P<0.05). Among these, 5 genes, including ZNF763, FAM71B, ZNF616, TPR, and MYH13, were significantly associated with both OS and PFS (Table 2). As shown in Figures 3,4 significant genes were selected for survival curve analysis.
Table 2
| Symbol | Gene name | OS, P (HR) | PFS, P (HR) |
|---|---|---|---|
| ZNF616 | Zinc finger protein 616 | 5.51E-12 (Jeny101) | 7.21E-5 (17.5) |
| MYH13 | Myosin heavy chain 13 | 7.21E-7 (20.5) | 0.0137 (5.8) |
| FAM71B | Golgi associated RAB2B interactor family member 3 | 5.84E-4 (9.04) | 7.21E-5 (17.5) |
| ZNF763 | Zinc finger protein 763 | 0.00231 (7.3) | 7.21E-5 (17.5) |
| TPR | Translocated promoter region, nuclear basket protein | 0.0085 (6.03) | 0.00898 (6.63) |
| BLM | BLM RecQ like helicase | 0.00246 (7.81) | 0.304 (2.01) |
| UNC13B | Unc-13 homolog B | 0.0173 (5.13) | 0.424 (1.73) |
| CPLANE1 | Ciliogenesis and planar polarity effector complex subunit 1 | 0.0179 (5.05) | 0.401 (1.89) |
| EXOC2 | Exocyst complex component 2 | 0.0362 (4.45) | 0.0506 (4.62) |
| PRRC2B | Proline rich coiled-coil 2B | 0.05 (4.09) | 0.285 (2.32) |
PFS, progression-free survival; OS, overall survival.

Alternations in signaling pathways in vascular invasion
To further characterize the signaling pathway changes in HCC during vascular invasion, vascular invasion patients with SNV binding were divided into three gene clusters by whole exons and targeted sequencing, including an avascular invasion cluster [150], a MVI cluster [190], and a macrovascular invasion cluster [310]. Venn diagrams revealed mutations in TP53, KMT2C, AXIN1, and ZFHX3 in all three groups. In addition to the crossover gene clusters between groups, there were many genes unique to each cluster, such as 266 mutations specific to the macrovascular invasion group (Figure 4A).
The three sets of gene clusters were collectively enriched in epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor resistance, phosphatidylinositol 3-kinase (PI3K)-Akt signaling pathway, and Rap1 signaling pathway. The thyroid hormone signaling pathway was enriched in both the macrovascular invasion and MVI clusters, but not in the avascular invasion clusters. In addition, MVI clusters were independently enriched in the insulin signaling pathway and the Fanconi anemia pathway compared to the MVI cluster and the avascular invasion cluster (Figure 4B-4D).
Evaluation of the prognostic value of vascular invasion-related genes
In this study, a total of 35 patients had evidence of vascular invasion, of which, 19 patients presented with macrovascular invasion. Combined with the patient’s gene mutation information, a total of 15 genes were found to be associated with vascular invasion. Among these genes, PTPRS showed statistical significance (P=0.023; Table 3), with no mutation found in the vascular invasion group, but high mutation frequency in the non-vascular invasion group.
Table 3
| Symbol | Gene name | No. of vascular invasion | No. of avascular invasion | P value |
|---|---|---|---|---|
| PTPRS | Protein tyrosine phosphatase receptor type S | 0 | 3 | 0.023214286 |
| TP53 | Tumor protein p53 | 12 | 2 | 0.178697863 |
| KIAA2026 | KIAA2026 | 4 | 0 | 0.302431611 |
| CTNNB1 | Catenin beta 1 | 4 | 3 | 0.414986124 |
| ALMS1 | ALMS1 centrosome and basal body associated protein | 3 | 0 | 0.544642857 |
| DNAH5 | Dynein axonemal heavy chain 5 | 3 | 0 | 0.544642857 |
| FGFR3 | Fibroblast growth factor receptor 3 | 3 | 0 | 0.544642857 |
| LONP1 | Lon peptidase 1, mitochondrial | 3 | 0 | 0.544642857 |
| LRP1B | LDL receptor related protein 1B | 3 | 0 | 0.544642857 |
| PRDM1 | PR/SET domain 1 | 3 | 0 | 0.544642857 |
| KMT2B | Lysine methyltransferase 2B | 3 | 2 | 0.629311484 |
| ZNF595 | Zinc finger protein 595 | 3 | 2 | 0.629311484 |
| KMT2C | Lysine methyltransferase 2C | 4 | 1 | 1 |
| KMT2D | Lysine methyltransferase 2D | 3 | 1 | 1 |
| NF1 | Neurofibromin 1 | 4 | 1 | 1 |
Discussion
Vascular invasion in patients with HCC during diagnosis and treatment often predicts poor prognosis or an increased risk of recurrence later in treatment (23). Vascular invasion can be classified as MVI or macrovascular invasion (13). Macrovascular invasion is the result of the evolution of MVI (24). Previous studies have predominantly focused on the impact of the incidence of vascular invasion on tumor recurrence or prognosis (25-27). Guo and Jiang found that overexpression of matrix metalloproteinase 12 (MMP12) is associated with poor prognosis and vascular invasion in HCC, these data suggest that MMP12 may have potential as a therapeutic target and biomarker in HCC (28). However, few studies have examined gene mutations or signaling pathways during vascular invasion. The ability to recognize molecular changes associated with vascular invasion in advance and identify patients at higher risk of recurrence will aid clinical decision-making in the field of HCC treatment.
A total of 50 HCC patients were included in this study, including 19 with macrovascular invasion, 16 with MVI, and 15 with avascular invasion (Table 1 and Table S1). Similar to a previous study (29), our patients were grouped according to the above vascular invasion status. Analysis of the prognosis and recurrence information of patients revealed significant differences in OS and PFS among the different vascular invasion groups (Figure 1). Subsequently, the mutation information of all patients was analyzed through NGS and bioinformatics analysis technology. A total of 762 SNVs were identified (Table S2). Among them, tumor suppressor gene TP53 and proto-oncogene CTNNB1 showed the highest mutation frequency (Figure 2A). The TMB can indirectly reflect the ability and extent of tumor production of neoantigens, and has been shown to predict the efficacy of immunotherapy for a variety of tumors (30). The TMB values can also reflect the potential of tumor neoantigens in tumors, which are closely related to DNA repair defects (31). Mismatch repair deficient (dMMR) and high microsatellite instability (MSI-H) patients have higher TMB in a variety of tumors (32,33). A study has shown that the TMB obtained from gene panels is overestimated compared to WES data (34), hence, in this study, we calculated the TMB value of the WES and gene panel according to the data source (Figure 2B,2C). The macrovascular invasion subgroup analysis of the IMbrave150 study in 2021 showed that atezolizumab combined with bevacizumab was still beneficial in PFS and OS, with a median OS of 24.0 months in the Chinese subgroup (27,35). However, we found no significant difference in the TMB values between the vascular invasion groups, suggesting that the degree of vascular invasion cannot be used as a basis for immunotherapy in HCC, although more data are needed to support this. In addition, with the development of drug therapy, HCC patients who are initially suitable for surgical resection may also benefit from neoadjuvant therapy, and further definitive evidence is required.
A total of 10 genes were found to be associated with prognostic or recurrence factors for HCC, including EXOC2, PRRC2B, PTPRS, and other genes (Table 2). Mutations in EXOC2 cause defects in human brain development (36). The protein encoded by EXOC2 is part of the extracellular vesicle complex and is critical for the polarized targeting of extracellular vesicles to specific docking sites on the plasma membrane. This protein has been shown to interact with the Ral subfamily of GTPase to mediate exocytosis by anchoring vesicles to the plasma membrane (37). PRRC2B, which plays an important role in the formation of head and neck tumors, activates RNA binding activity and is involved in cell differentiation (38-40). In addition, the PTPRS gene is significantly related to vascular invasion (Table 3). The protein encoded by this gene is a member of the protein tyrosine phosphatase (PTP) family, which contains an extracellular domain, a transmembrane segment, and two tandem catalytic domains (41). PTP is thought to be a signaling molecule regulating a variety of cellular processes, including cell growth, differentiation, mitotic cycle, and oncogenic transformation (42). Although the above genes have been reported to be involved in tumor formation, transformation, and cancer cell differentiation, whether the mutation of these genes in hepatocytes is the dominant factor affecting hepatocellular carcinogenesis or driving the occurrence of vascular invasion has not been proved.
Mutations or differences in the expression of single or multiple genes often lead to perturbation of molecular signals in signaling pathways, including hormones, growth factors, cytokines, neurotransmitters, and other small-molecule compounds. The results of the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis showed that when hepatocytes became cancerous, the three signaling pathways including EGFR tyrosine kinase inhibitor resistance, PI3K-Akt signaling pathway, and Rap1 signaling pathway, were significantly altered compared to normal cells (43,44). However, when MVI occurs in HCC, thyroid hormone receives interference from exogenous molecules or dysregulation of the thyroid hormone signaling pathway, which further affects angiogenesis and cancer cell proliferation (45). In the macrovascular invasion group, mutant genes were additionally enriched in the Fanconi anemia pathway and the insulin signaling pathway. Aberrations in the Fanconi anemia pathway are associated with genomic instability and tumorigenesis in a variety of human cancers, especially breast cancer (46). In addition, Fanconi anemia patients are susceptible to human papillomavirus (HPV)-related malignancies, suggesting that loss of Fanconi anemia pathway activity promotes tumorigenesis (47). In physiological functions, insulin mainly regulates the metabolism of glucose and lipids through the insulin signaling cascade (48). However, activation of the insulin receptor (INSR) in cancer cells triggers a signaling cascade of the PI3K-Akt pathway, which promotes cell proliferation and glycolysis, while inhibiting apoptosis (49). This is also one of the reasons for the high incidence of cancer in diabetic patients (50). In addition, due to the limitation of the small sample size, further studies are warranted to further verify the above results.
Conclusions
This investigation demonstrated that patients with macrovascular invasion, MVI, and avascular invasion had significantly different somatic mutations and significant differences in prognosis and risk of recurrence. However, no significant differences in TMB were found between the vascular invasion groups. A total of 10 prognostic-related candidate biomarkers were identified, as well as one oncogene that was significantly associated with vascular invasion. Differences were found in both somatic gene mutations and signaling pathways, indicating that the molecular mechanism of tumor tissue changes at different stages of vascular invasion. Understand the molecular changes associated with the vascular invasion process will aid clinical decision-making in the field of HCC treatment.
Acknowledgments
The authors wish to thank the Shanghai Tongshu Biotechnology Co., Ltd. for technical support.
Funding: The study was supported by the Guangdong Basic and Applied Basic Research Foundation (Grant 2020A1515110941, 2021A1515011015, and 2021A1515220100).
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-556/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-556/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-556/coif). All authors report that this work has received technical support from Shanghai Tongshu Biotechnology Co., Ltd. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the Ethics Committee of Nanfang Hospital, Southern Medical University (ID of the approval: 2012-092). Written informed consent was obtained from all patients prior to inclusion in the study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Qu J, Luo M, Zhang J, et al. A paradoxical role for sestrin 2 protein in tumor suppression and tumorigenesis. Cancer Cell Int 2021;21:606. [Crossref] [PubMed]
- Zhou J, Wang W, Li Q. Potential therapeutic targets in the tumor microenvironment of hepatocellular carcinoma: reversing the protumor effect of tumor-associated macrophages. J Exp Clin Cancer Res 2021;40:73. [Crossref] [PubMed]
- Jin M, Yu Q, Liu Y, et al. Safety and Efficacy of Physical Thermal Ablation Combined Sorafenib for Hepatocellular Carcinoma: A Meta-analysis. J Clin Transl Hepatol 2021;9:149-59. [Crossref] [PubMed]
- Yang JD, Hainaut P, Gores GJ, et al. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol 2019;16:589-604. [Crossref] [PubMed]
- Hu B, Yang XB, Sang XT. Development and Verification of the Hypoxia-Related and Immune-Associated Prognosis Signature for Hepatocellular Carcinoma. J Hepatocell Carcinoma 2020;7:315-30. [Crossref] [PubMed]
- He Y, Hu B, Zhu C, et al. A Novel Multimodal Radiomics Model for Predicting Prognosis of Resected Hepatocellular Carcinoma. Front Oncol 2022;12:745258. [Crossref] [PubMed]
- Cao J, Yang X, Li J, et al. Screening and Identifying Immune-Related Cells and Genes in the Tumor Microenvironment of Bladder Urothelial Carcinoma: Based on TCGA Database and Bioinformatics. Front Oncol 2019;9:1533. [Crossref] [PubMed]
- Mokdad AA, Singal AG, Marrero JA, et al. Vascular Invasion and Metastasis is Predictive of Outcome in Barcelona Clinic Liver Cancer Stage C Hepatocellular Carcinoma. J Natl Compr Canc Netw 2017;15:197-204. [Crossref] [PubMed]
- Iwatsuki S, Dvorchik I, Marsh JW, et al. Liver transplantation for hepatocellular carcinoma: a proposal of a prognostic scoring system. J Am Coll Surg 2000;191:389-94. [Crossref] [PubMed]
- Mínguez B, Hoshida Y, Villanueva A, et al. Gene-expression signature of vascular invasion in hepatocellular carcinoma. J Hepatol 2011;55:1325-31. [Crossref] [PubMed]
- Wu T, Dong X, Yu D, et al. Natural product pectolinarigenin inhibits proliferation, induces apoptosis, and causes G2/M phase arrest of HCC via PI3K/AKT/mTOR/ERK signaling pathway. Onco Targets Ther 2018;11:8633-42. [Crossref] [PubMed]
- Menyhárt O, Nagy Á, Győrffy B. Determining consistent prognostic biomarkers of overall survival and vascular invasion in hepatocellular carcinoma. R Soc Open Sci 2018;5:181006. [Crossref] [PubMed]
- Wang L, Jin YX, Ji YZ, et al. Development and validation of a prediction model for microvascular invasion in hepatocellular carcinoma. World J Gastroenterol 2020;26:1647-59. [Crossref] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA 2013;310:2191-4. [Crossref] [PubMed]
- Cibulskis K, Lawrence MS, Carter SL, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol 2013;31:213-9. [Crossref] [PubMed]
- DePristo MA, Banks E, Poplin R, et al. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 2011;43:491-8. [Crossref] [PubMed]
- Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res 2010;38:e164. [Crossref] [PubMed]
- Ramos AH, Lichtenstein L, Gupta M, et al. Oncotator: cancer variant annotation tool. Hum Mutat 2015;36:E2423-9. [Crossref] [PubMed]
- Mayakonda A, Lin DC, Assenov Y, et al. Maftools: efficient and comprehensive analysis of somatic variants in cancer. Genome Res 2018;28:1747-56. [Crossref] [PubMed]
- Rubio-Perez C, Tamborero D, Schroeder MP, et al. In silico prescription of anticancer drugs to cohorts of 28 tumor types reveals targeting opportunities. Cancer Cell 2015;27:382-96. [Crossref] [PubMed]
- Kassambara A, Kassambara MA. Package ‘ggpubr’. R package version 01 2020;6.
- Team RC. R: A language and environment for statistical computing. 2013.
- Zhang X, Jiang D, Yang S, et al. BAP31 Promotes Tumor Cell Proliferation by Stabilizing SERPINE2 in Hepatocellular Carcinoma. Front Cell Dev Biol 2020;8:607906. [Crossref] [PubMed]
- Yan H, Wang X, Zhou D, et al. Dynamic Nomogram for Predicting Macrovascular Invasion of Patients with Unresectable Hepatocellular Carcinoma after Transarterial Chemoembolization. J Cancer 2022;13:1914-22. [Crossref] [PubMed]
- Nitta H, Allard MA, Sebagh M, et al. Prognostic Value and Prediction of Extratumoral Microvascular Invasion for Hepatocellular Carcinoma. Ann Surg Oncol 2019;26:2568-76. [Crossref] [PubMed]
- Lei Z, Li J, Wu D, et al. Nomogram for Preoperative Estimation of Microvascular Invasion Risk in Hepatitis B Virus-Related Hepatocellular Carcinoma Within the Milan Criteria. JAMA Surg 2016;151:356-63. [Crossref] [PubMed]
- Zhang D, Wei Q, Wu GG, et al. Preoperative Prediction of Microvascular Invasion in Patients With Hepatocellular Carcinoma Based on Radiomics Nomogram Using Contrast-Enhanced Ultrasound. Front Oncol 2021;11:709339. [Crossref] [PubMed]
- Guo ZY, Jiang LP. Matrix metalloproteinase 12 (MMP12) as an adverse prognostic biomarker of vascular invasion in hepatic cell carcinoma. Eur Rev Med Pharmacol Sci 2022;26:2238-49. [PubMed]
- Amado V, Rodríguez-Perálvarez M, Ferrín G, et al. Selecting patients with hepatocellular carcinoma for liver transplantation: incorporating tumor biology criteria. J Hepatocell Carcinoma 2019;6:1-10. [Crossref] [PubMed]
- Lobo J, Jerónimo C, Henrique R. Targeting the Immune system and Epigenetic Landscape of Urological Tumors. Int J Mol Sci 2020;21:829. [Crossref] [PubMed]
- Zhang N, Li P, Wu X, et al. Analysis of Threshold Changes of Tumor Mutation Burden of Gastric Cancer and Its Relationship with Patients' Prognosis. J Oncol 2021;2021:9026610. [Crossref] [PubMed]
- Shimozaki K, Hayashi H, Tanishima S, et al. Concordance analysis of microsatellite instability status between polymerase chain reaction based testing and next generation sequencing for solid tumors. Sci Rep 2021;11:20003. [Crossref] [PubMed]
- Li L, Rao X, Wen Z, et al. Implications of driver genes associated with a high tumor mutation burden identified using next-generation sequencing on immunotherapy in hepatocellular carcinoma. Oncol Lett 2020;19:2739-48. [Crossref] [PubMed]
- Hatakeyama K, Nagashima T, Urakami K, et al. Tumor mutational burden analysis of 2,000 Japanese cancer genomes using whole exome and targeted gene panel sequencing. Biomed Res 2018;39:159-67. [Crossref] [PubMed]
- Mohr R, Özdirik B, Lambrecht J, et al. From Liver Cirrhosis to Cancer: The Role of Micro-RNAs in Hepatocarcinogenesis. Int J Mol Sci 2021;22:1492. [Crossref] [PubMed]
- Van Bergen NJ, Ahmed SM, Collins F, et al. Mutations in the exocyst component EXOC2 cause severe defects in human brain development. J Exp Med 2020;217:e20192040. [Crossref] [PubMed]
- Hazelett CC, Yeaman C. Sec5 and Exo84 mediate distinct aspects of RalA-dependent cell polarization. PLoS One 2012;7:e39602. [Crossref] [PubMed]
- Xie C, Zhou M, Lin J, et al. EEF1D Promotes Glioma Proliferation, Migration, and Invasion through EMT and PI3K/Akt Pathway. Biomed Res Int 2020;2020:7804706. [Crossref] [PubMed]
- Manjur ABMK, Lempiäinen JK, Malinen M, et al. BCOR modulates transcriptional activity of a subset of glucocorticoid receptor target genes involved in cell growth and mobility. J Steroid Biochem Mol Biol 2021;210:105873. [Crossref] [PubMed]
- The International Cancer Congress, Part D: Research and treatment. Proceedings of the 13th International Cancer Congress, September 8-15, 1982, Seattle, Washington. Progress in clinical and biological research 1983:1-521.
- Huang Y, Zhang Y, Ge L, et al. The Roles of Protein Tyrosine Phosphatases in Hepatocellular Carcinoma. Cancers (Basel) 2018;10:82. [Crossref] [PubMed]
- Muskiewicz DE, Uhl GR, Hall FS. The Role of Cell Adhesion Molecule Genes Regulating Neuroplasticity in Addiction. Neural Plast 2018;2018:9803764. [Crossref] [PubMed]
- Chen Z, Chen P, Wu H, et al. Evaluation of Naringenin as a Promising Treatment Option for COPD Based on Literature Review and Network Pharmacology. Biomolecules 2020;10:1644. [Crossref] [PubMed]
- Zhao CH, Qu L, Zhang H, et al. Identification of breast cancer-related circRNAs by analysis of microarray and RNA-sequencing data: An observational study. Medicine (Baltimore) 2019;98:e18042. [Crossref] [PubMed]
- Liu YC, Yeh CT, Lin KH. Molecular Functions of Thyroid Hormone Signaling in Regulation of Cancer Progression and Anti-Apoptosis. Int J Mol Sci 2019;20:4986. [Crossref] [PubMed]
- Fang CB, Wu HT, Zhang ML, et al. Fanconi Anemia Pathway: Mechanisms of Breast Cancer Predisposition Development and Potential Therapeutic Targets. Front Cell Dev Biol 2020;8:160. [Crossref] [PubMed]
- Spriggs CC, Laimins LA. FANCD2 Binds Human Papillomavirus Genomes and Associates with a Distinct Set of DNA Repair Proteins to Regulate Viral Replication. mBio 2017;8:02340-16. [Crossref] [PubMed]
- Sommerfeld MR, Müller G, Tschank G, et al. In vitro metabolic and mitogenic signaling of insulin glargine and its metabolites. PLoS One 2010;5:e9540. [Crossref] [PubMed]
- Wang Y, Wan X, Hao Y, et al. NR6A1 regulates lipid metabolism through mammalian target of rapamycin complex 1 in HepG2 cells. Cell Commun Signal 2019;17:77. [Crossref] [PubMed]
- Choi Y, Kim TY, Oh DY, et al. The Impact of Diabetes Mellitus and Metformin Treatment on Survival of Patients with Advanced Pancreatic Cancer Undergoing Chemotherapy. Cancer Res Treat 2016;48:171-9. [Crossref] [PubMed]


