
Survival comparison of stage IIA rectal cancer with or without neoadjuvant therapy: a SEER database analysis with propensity score matching
Introduction
Colorectal cancer is one of the most common malignancies in the world, with the third-highest incidence and death rate among all malignancies (1,2). Rectal cancer accounts for approximately 30% of all colorectal cancer cases (1). The treatment has evolved with the introduction of neoadjuvant radiotherapy and chemotherapy before total mesorectal resection (TME) surgery (3). Currently, neoadjuvant chemoradiotherapy (CRT), TME, and adjuvant chemotherapy are the primary recommended treatments for patients with locally advanced rectal cancer (LARC) (4). Among them, neoadjuvant CRT has been widely accepted as a therapeutic method to reduce tumor stage, increase resectability, and reduce the local recurrence rate of patients with LARC (5). However, this success in improving survival comes at the cost of a significant reduction in quality of life (6). The National Comprehensive Cancer Network (NCCN) Guidelines recommend neoadjuvant and adjuvant therapy for patients at high risk of local recurrence, including stage II (T3–4, N0, tumor invasion of the myenteric layer or further) and stage III (N+, no distant metastases) (7). The European Society of Medical Oncology (ESMO) guidelines first recommended in 2013 that the treatment of rectal cancer should be stratified according to the risk of recurrence. For patients with low-risk rectal cancer, including early T3N0, and no significant invasion of the vasculature or rectal mesentery, surgical treatment can be considered directly (8). In addition, some studies have indicated that neoadjuvant CRT only reduces the risk of local recurrence but not distant metastasis (9), and fails to improve long-term survival and retention of the anal sphincter (10). Therefore, the need to complete neoadjuvant CRT for patients with different stages of LARC, such as stage IIA (T3N0M0), has generated some new controversies in different guidelines and literature.
Hence, our primary objective was to investigate the impact of neoadjuvant CRT on the prognosis of patients with stage IIA rectal cancer and to explore whether neoadjuvant CRT is necessary for these patients. Here, we performed an analysis comparing survival and clinicopathological differences between patients with stage IIA rectal cancer who underwent surgery alone and those who completed neoadjuvant CRT, applying a propensity score matching (PSM) method based on the information in the U.S. Surveillance, Epidemiology, and End Results (SEER) database. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-166/rc).
Methods
Patient selection
The SEER database comprises 18 cancer registries covering 27.8% of the U.S. population and collects and provides data on cancer clinicopathology, morbidity, and survival data. We extracted demographic and clinicopathological data of patients diagnosed with rectal cancer between January 2010 and December 2015 from the SEER database [database name: Incidence-SEER Research (Plus) Data, 18 Registries, Nov 2020 Sub (2000–2018)]. Patients who met the following criteria were included: (I) clear pathological diagnosis of rectal malignant tumors; (II) diagnosis time was from January 2010 to December 2015; (III) the American Joint Committee on Cancer (AJCC) stage was IIA; (IV) surgery has been completed definitely; (V) neoadjuvant therapy has been specified in the CRT database; and (VI) known time of survival and cause of death. Patients with the following criteria were excluded: (I) borderline tumors of the rectum; (II) local tumor resection only; (III) neoadjuvant therapy is not clear in the CRT database; (IV) colorectal cancer diagnosed by death certificate or autopsy; (V) other concurrent or multiple malignant tumors.
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since the data of this project were all extracted from the SEER database and did not involve patient safety, privacy, and postoperative follow-up, no informed consent and no ethics committee approval were required.
Result measurement
Information on the following clinicopathological variables was extracted from the SEER database: sex, age, year of diagnosis, race, site of lesion, type of pathology, histological grade, TNM stage according to AJCC, number of lymph nodes harvested intraoperatively (LNH), whether radiotherapy or chemotherapy was administered and in sequence with surgery, carcinoembryonic antigen (CEA), tumor deposits (TD), perineural invasion (PNI), tumor size, follow-up time, tumor-specific deaths, and deaths from other causes. The primary outcomes were overall survival (OS) and cancer-specific survival (CSS). OS was defined as the time from diagnosis to death from any cause. CSS was defined as the time from diagnosis to cancer-specific death, with the data on death from other causes or survival at the end of follow-up being censored.
Statistical analysis
A logistic regression model was applied for PSM (3:1 matching), with a matching tolerance of 0.02 and without replacement. Survival curves were plotted using the Kaplan-Meier method, survival differences were assessed using the log-rank test, and multivariate analysis was performed using the Cox proportional risk model to generate adjusted hazard ratios (HRs) and 95% confidence intervals (CIs). Statistical significance was set at P<0.05 for a two-tailed test. All statistical analyses were performed using IBM SPSS Statistics 26.0 and R 4.1.0. All survival curves were plotted using GraphPad Prisms 8.2.1.
Results
We screened and analyzed 14,505 patients with stage IIA rectal cancer from the database. Among them, 8,708 patients underwent surgery only, and 5,797 underwent perioperative therapy, including neoadjuvant and/or adjuvant therapy. When cases with incomplete data have been excluded, 3,976 patients underwent surgery alone, 1,055 patients underwent neoadjuvant CRT + surgery, 547 patients underwent neoadjuvant CRT + surgery + adjuvant chemotherapy, and 276 patients underwent surgery + adjuvant chemotherapy. Of these patients, the median follow-up time was 58 months (interquartile range, 41–78 months). A total of 1,675 (28.6%) patients died at the end of follow-up, and 939 patients (16.0%) died specifically from rectal cancer.
Confounding factors including age, sex, race, grade, LNH, TD, CEA, PNI and tumor size were adjusted. Before PSM, the surgery group had older patients (P=0.02), and more LNH (P<0.01) compared to the neoadjuvant CRT + surgery group. After PSM, 1,055 matched pairs were created, and the distributions of variables were balanced (Table 1). Before PSM, the surgery group had more female (P=0.02), older patients (P<0.01), more LNH (P<0.01), and more larger tumors (P=0.01) compared to the neoadjuvant CRT + surgery + chemotherapy group. After PSM, 547 matched pairs were created, and the distributions of variables were balanced (Table 2). Before PSM, the surgery + chemotherapy group had more female (P<0.01), higher grade (P<0.01), more LNH (P<0.01), and more larger tumors (P=0.01) compared to the neoadjuvant CRT + surgery + chemotherapy group. After PSM, 237 matched pairs were created, and the distributions of variables were balanced (Table 3).
Table 1
| Variables | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Surgery only | Neoadjuvant CRT + surgery | P value | No. of patients | Surgery only | Neoadjuvant CRT + surgery | P value | ||
| No. of patients | 5,031 | 3,976 | 1,055 | – | 4,041 | 2,986 | 1,055 | – | |
| Sex, n (%) | 0.23 | 0.83 | |||||||
| Male | 3,088 | 2,423 (60.9) | 665 (63.0) | 2,534 | 1,869 (62.6) | 665 (63.0) | |||
| Female | 1,943 | 1,553 (39.1) | 390 (37.0) | 1,507 | 1,117 (37.4) | 390 (37.0) | |||
| Age, n (%) | 0.02 | 0.72 | |||||||
| <55 years | 1,250 | 970 (24.4) | 280 (26.5) | 1,048 | 768 (25.7) | 280 (26.5) | |||
| 55–64 years | 1,376 | 1,065 (26.8) | 311 (29.5) | 1,173 | 862 (28.9) | 311 (29.5) | |||
| ≥65 years | 2,405 | 1,941 (48.8) | 464 (44.0) | 1,820 | 1,356 (45.4) | 464 (44.0) | |||
| Race, n (%) | 0.72 | 0.81 | |||||||
| White | 4,057 | 3,197 (80.4) | 860 (81.5) | 3,319 | 2,459 (82.4) | 860 (81.5) | |||
| Black | 406 | 324 (8.1) | 82 (7.8) | 299 | 217 (7.3) | 82 (7.8) | |||
| Other | 568 | 455 (11.4) | 113 (10.7) | 423 | 310 (10.4) | 113 (10.7) | |||
| Grade, n (%) | 0.55 | 0.90 | |||||||
| I & II | 4,576 | 3,611 (90.8) | 965 (91.5) | 3,702 | 2,737 (91.7) | 965 (91.5) | |||
| III & IV | 455 | 365 (9.2) | 90 (8.5) | 339 | 249 (8.3) | 90 (8.5) | |||
| LNH, n (%) | <0.01 | 0.06 | |||||||
| <12 | 1,396 | 1,022 (25.7) | 374 (35.5) | 1,335 | 961 (32.2) | 374 (35.5) | |||
| ≥12 | 3,635 | 2,954 (74.3) | 681 (64.5) | 2,706 | 2,025 (67.8) | 681 (64.5) | |||
| TD, n (%) | 0.33 | 0.74 | |||||||
| Positive | 143 | 112 (2.8) | 31 (2.9) | 111 | 80 (2.7) | 31 (2.9) | |||
| Negative | 4,888 | 3,864 (97.2) | 1,024 (97.1) | 3,930 | 2,906 (97.3) | 1,024 (97.1) | |||
| CEA, n (%) | 0.44 | 1.00 | |||||||
| Positive | 2,041 | 1,602 (40.3) | 439 (41.6) | 1,680 | 1,241 (41.6) | 439 (41.6) | |||
| Negative | 2,990 | 2,374 (59.7) | 616 (58.4) | 2,361 | 1,745 (58.4) | 616 (58.4) | |||
| PNI, n (%) | 0.33 | 0.66 | |||||||
| Positive | 387 | 314 (7.9) | 73 (6.9) | 266 | 193 (6.5) | 73 (6.9) | |||
| Negative | 4,644 | 3,662 (92.1) | 982 (93.1) | 3,775 | 2,793 (93.5) | 982 (93.1) | |||
| Tumor size, n (%) | 0.08 | 0.89 | |||||||
| ≤6 cm | 4,080 | 3,204 (80.6) | 876 (83.0) | 3,348 | 2,472 (82.8) | 876 (83.0) | |||
| >6 cm | 951 | 772 (19.4) | 179 (17.0) | 693 | 514 (17.2) | 179 (17.0) | |||
PSM, propensity score matching; CRT, chemoradiotherapy; LNH, lymph node harvested; TD, tumor deposit; CEA, carcinoembryonic antigen; PNI, perineural invasion.
Table 2
| Variables | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Surgery only | Neoadjuvant CRT + surgery + CT | P value | No. of patients | Surgery only | Neoadjuvant CRT + surgery + CT | P value | ||
| No. of patients | 4,523 | 3,976 | 547 | – | 2,150 | 1,603 | 547 | – | |
| Sex, n (%) | 0.02 | 0.79 | |||||||
| Male | 2,784 | 2,423 (60.9) | 361 (66.0) | 1,407 | 1,046 (65.3) | 361 (66.0) | |||
| Female | 1,739 | 1,553 (39.1) | 186 (34.0) | 743 | 557 (34.7) | 186 (34.0) | |||
| Age, n (%) | <0.01 | 0.80 | |||||||
| <55 years | 1,164 | 970 (24.4) | 194 (35.5) | 762 | 568 (35.4) | 194 (35.5) | |||
| 55–64 years | 1,251 | 1,065 (26.8) | 186 (34.0) | 710 | 524 (32.7) | 186 (34.0) | |||
| ≥65 years | 2,108 | 1,941 (48.8) | 167 (30.5) | 678 | 511 (31.9) | 167 (30.5) | |||
| Race, n (%) | 0.90 | 0.94 | |||||||
| White | 3,637 | 3,197 (80.4) | 440 (80.4) | 1,736 | 1,296 (80.8) | 440 (80.4) | |||
| Black | 366 | 324 (8.1) | 42 (7.7) | 158 | 116 (7.2) | 42 (7.7) | |||
| Other | 520 | 455 (11.4) | 65 (11.9) | 256 | 191 (11.9) | 65 (11.9) | |||
| Grade, n (%) | 0.34 | 0.89 | |||||||
| I & II | 4,115 | 3,611 (90.8) | 504 (92.1) | 1,976 | 1,472 (91.8) | 504 (92.1) | |||
| III & IV | 408 | 365 (9.2) | 43 (7.9) | 174 | 131 (8.2) | 43 (7.9) | |||
| LNH, n (%) | <0.01 | 0.66 | |||||||
| <12 | 1,218 | 1,022 (25.7) | 196 (35.8) | 752 | 556 (34.7) | 196 (35.8) | |||
| ≥12 | 3,305 | 2,954 (74.3) | 351 (64.2) | 1,398 | 1,047 (65.3) | 351 (64.2) | |||
| TD, n (%) | 0.18 | 0.81 | |||||||
| Positive | 133 | 112 (2.8) | 21 (3.8) | 77 | 56 (3.5) | 21 (3.8) | |||
| Negative | 4,390 | 3,864 (97.2) | 526 (96.2) | 2,073 | 1,547 (96.5) | 526 (96.2) | |||
| CEA, n (%) | 0.38 | 0.99 | |||||||
| Positive | 1,811 | 1,602 (40.3) | 209 (38.2) | 824 | 615 (38.4) | 209 (38.2) | |||
| Negative | 2,712 | 2,374 (59.7) | 338 (61.8) | 1,326 | 988 (61.6) | 338 (61.8) | |||
| PNI, n (%) | 0.40 | 0.80 | |||||||
| Positive | 363 | 314 (7.9) | 49 (9.0) | 185 | 136 (8.5) | 49 (9.0) | |||
| Negative | 4,160 | 3,662 (92.1) | 498 (91.0) | 1,965 | 1,467 (91.5) | 498 (91.0) | |||
| Tumor size, n (%) | 0.01 | 1.00 | |||||||
| ≤6 cm | 3,669 | 3,204 (80.6) | 465 (85.0) | 323 | 1,362 (85.0) | 465 (85.0) | |||
| >6 cm | 854 | 772 (19.4) | 82 (15.0) | 1,827 | 241 (15.0) | 82 (15.0) | |||
PSM, propensity score matching; CRT, chemoradiotherapy; CT, chemotherapy; LNH, lymph node harvested; TD, tumor deposit; CEA, carcinoembryonic antigen; PNI, perineural invasion.
Table 3
| Variables | Before PSM | After PSM | |||||||
|---|---|---|---|---|---|---|---|---|---|
| No. of patients | Surgery + CT | Neoadjuvant CRT + surgery + CT | P value | No. of patients | Surgery + CT | Neoadjuvant CRT + surgery + CT | P value | ||
| No. of patients | 823 | 276 | 547 | – | 589 | 237 | 352 | – | |
| Sex | <0.01 | 0.14 | |||||||
| Male | 512 | 151 (54.7) | 361 (66.0) | 358 | 135 (57.0) | 223 (63.4) | |||
| Female | 311 | 125 (45.3) | 186 (34.0) | 231 | 102 (43.0) | 129 (36.6) | |||
| Age | 0.461 | 0.74 | |||||||
| <55 years | 299 | 105 (38.0) | 194 (35.5) | 224 | 94 (39.7) | 130 (36.9) | |||
| 55–64 years | 268 | 82 (29.7) | 186 (34.0) | 181 | 69 (29.1) | 112 (31.8) | |||
| ≥65 years | 256 | 89 (32.2) | 167 (30.5) | 184 | 74 (31.2) | 110 (31.2) | |||
| Race | 0.055 | 0.06 | |||||||
| White | 662 | 222 (80.4) | 440 (80.4) | 1736 | 188 (79.3) | 281 (79.8) | |||
| Black | 74 | 32 (11.6) | 42 (7.7) | 158 | 30 (12.7) | 28 (8.0) | |||
| Other | 87 | 22 (8.0) | 65 (11.9) | 256 | 19 (8.0) | 43 (12.2) | |||
| Grade | <0.01 | 0.69 | |||||||
| I & II | 735 | 231 (83.7) | 504 (92.1) | 534 | 213 (91.8) | 321 (92.1) | |||
| III & IV | 88 | 45 (16.3) | 43 (7.9) | 55 | 24 (10.1) | 31 (8.8) | |||
| LNH | <0.01 | 0.23 | |||||||
| <12 | 240 | 44 (15.9) | 196 (35.8) | 120 | 42 (17.7) | 78 (22.2) | |||
| ≥12 | 583 | 232 (84.1) | 351 (64.2) | 469 | 195 (82.3) | 274 (77.8) | |||
| TD | 0.52 | 0.98 | |||||||
| Positive | 35 | 14 (5.1) | 21 (3.8) | 21 | 9 (3.8) | 12 (3.4) | |||
| Negative | 788 | 262 (94.9) | 526 (96.2) | 568 | 228 (96.2) | 340 (96.6) | |||
| CEA | 0.09 | 0.99 | |||||||
| Positive | 297 | 88 (31.9) | 209 (38.2) | 186 | 75 (31.6) | 111 (31.5) | |||
| Negative | 526 | 188 (68.1) | 338 (61.8) | 403 | 162 (68.4) | 241 (68.5) | |||
| PNI | 0.80 | 0.37 | |||||||
| Positive | 76 | 27 (9.8) | 49 (9.0) | 51 | 17 (7.2) | 34 (9.7) | |||
| Negative | 747 | 249 (90.2) | 498 (91.0) | 538 | 220 (92.8) | 318 (90.3) | |||
| Tumor size | 0.01 | 0.67 | |||||||
| ≤6 cm | 679 | 214 (77.5) | 465 (85.0) | 476 | 189 (79.7) | 287 (81.5) | |||
| >6 cm | 144 | 62 (22.5) | 82 (15.0) | 113 | 48 (20.3) | 65 (18.5) | |||
PSM, propensity score matching; CRT, chemoradiotherapy; CT, chemotherapy; LNH, lymph node harvested; TD, tumor deposit; CEA, carcinoembryonic antigen; PNI, perineural invasion.
Neoadjuvant CRT + surgery vs. surgery alone
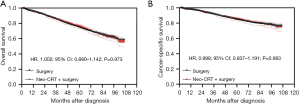
After PSM, the Kaplan-Meier analysis showed that there was no statistically significant difference in OS between patients who underwent neoadjuvant CRT + surgery and those who underwent surgery alone (P=0.973) (Figure 1A), and the 5-year OS were 75.9% (95% CI: 73.0–78.5%) and 74.5% (95% CI: 72.8–76.1%) respectively. The Kaplan-Meier analysis showed that there was no statistically significant difference in CSS between these two groups as well after PSM (P=0.983) (Figure 1B), and the 5-year CSS were 85.2% (95% CI: 82.7–87.4%) and 84.5% (95% CI: 83.0–85.9%) respectively.
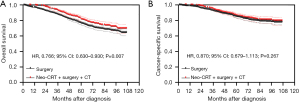
Neoadjuvant CRT + surgery + chemotherapy vs. surgery alone
After PSM, the Kaplan-Meier analysis showed that patients with stage IIA rectal cancer treated with neoadjuvant CRT + surgery + chemotherapy had better OS than those treated with surgery alone (P=0.007) (Figure 2A) and the 5-year OS were 81.6% (95% CI: 77.7–84.9%) and 76.3% (95% CI: 74.0–78.5%) respectively. The Kaplan-Meier analysis showed that there was no statistically significant difference in CSS between these two groups (P=0.267) (Figure 2B). The 5-year CSS were 86.2% (95% CI: 82.6–89.1%) and 84.9% (95% CI: 82.8–86.7%) respectively.
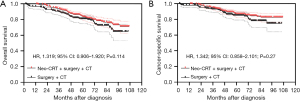
Neoadjuvant CRT + surgery + chemotherapy vs. surgery + chemotherapy
After PSM, the Kaplan-Meier analysis showed that patients with stage IIA rectal cancer treated with neoadjuvant CRT + surgery + chemotherapy had better OS than those treated with surgery + chemotherapy, but the difference was not statistically significant (P=0.114) (Figure 3A), and the 5-year OS were 83.4% (95% CI: 78.5–87.3%) and 80.8% (95% CI: 74.7–85.6%) respectively. The Kaplan-Meier analysis showed that there was no statistically significant difference in CSS between these two groups (P=0.270) (Figure 3B). The 5-year CSS were 86.7% (95% CI: 82.1–90.2%) and 85.0% (95% CI: 79.1–89.3%) respectively.
Subgroup analysis of surgery alone vs. neoadjuvant CRT + surgery + chemotherapy
The univariate and multivariate Cox proportional risk regression were used to identify the preoperative independent risk factors for patients with stage IIA rectal cancer. And we performed a subgroup analysis for OS between the surgery group and the neoadjuvant CRT + surgery + chemotherapy group according to preoperative risk factors.
The univariate Cox proportional risk regression analysis showed that factors associated with OS included sex, age at diagnosis, race, histological grade, CEA level, LNH, TD, PNI, tumor size and perioperative therapy (Table 4). Of these, male sex, age >55 years, higher histological grade, less LNH, positive TD, positive CEA, positive PNI and tumor size >6 cm were risk factors associated with a decreased OS. Neoadjuvant CRT + surgery + chemotherapy was a protective factor associated with an increased OS compared with surgery alone. The results of the multivariate Cox proportional risk regression were similar to those of the univariate analysis (Table 4).
Table 4
| Variables | Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P value | HR | 95% CI | P value | ||
| Sex | |||||||
| Female | 1.000 | Reference | 1.000 | Reference | |||
| Male | 1.241 | 1.109–1.388 | <0.001 | 1.247 | 1.127–1.380 | <0.001 | |
| Age | |||||||
| <55 years | 1.000 | Reference | 1.000 | Reference | |||
| 55–64 years | 1.429 | 1.180–1.731 | <0.001 | 1.381 | 1.166–1.635 | <0.001 | |
| ≥65 years | 2.723 | 2.402–3.086 | <0.001 | 3.286 | 2.842–3.800 | <0.001 | |
| Race | |||||||
| White | 1.000 | Reference | 1.000 | Reference | |||
| Black | 1.152 | 0.966–1.375 | 0.115 | 1.220 | 1.032–1.441 | 0.02 | |
| Other | 0.810 | 0.695–0.945 | 0.007 | 0.823 | 0.696–0.973 | 0.023 | |
| Grade | |||||||
| I & II | 1.000 | Reference | 1.000 | Reference | |||
| III & IV | 1.551 | 1.278–1.882 | <0.001 | 1.359 | 1.174–1.572 | <0.001 | |
| CEA | |||||||
| Negative | 1.000 | Reference | 1.000 | Reference | |||
| Positive | 1.560 | 1.394–1.746 | <0.001 | 1.464 | 1.329–1.613 | <0.001 | |
| PNI | |||||||
| Negative | 1.000 | Reference | 1.000 | Reference | |||
| Positive | 1.782 | 1.443–2.202 | <0.001 | 1.449 | 1.238–1.696 | <0.001 | |
| TD | |||||||
| Negative | 1.000 | Reference | 1.000 | Reference | |||
| Positive | 2.660 | 1.894–3.735 | <0.001 | 1.984 | 1.601–2.458 | <0.001 | |
| LNH | |||||||
| ≤12 | 1.000 | Reference | 1.000 | Reference | |||
| >12 | 0.783 | 0.693–0.885 | <0.001 | 0.805 | 0.726–0.892 | <0.001 | |
| Size | |||||||
| ≤6 cm | 1.000 | Reference | 1.000 | Reference | |||
| >6 cm | 1.283 | 1.112–1.480 | <0.001 | 1.310 | 1.163–1.474 | <0.001 | |
| Perioperative therapy | |||||||
| Neoadjuvant CRT + surgery | 1.000 | Reference | 1.000 | Reference | |||
| Surgery alone | 1.038 | 0.917–1.175 | 0.664 | 1.041 | 0.918–1.180 | 0.533 | |
| Neoadjuvant CRT + surgery + CT | 0.672 | 0.550–0.821 | 0.001 | 0.726 | 0.584–0.903 | 0.004 | |
| Surgery + CT | 0.766 | 0.595–0.986 | 0.039 | 0.836 | 0.632–1.105 | 0.208 | |
OS, overall survival; HR, hazard ratio; 95% CI, 95% confidence interval; CEA, carcinoembryonic antigen; PNI, perineural invasion; TD, tumor deposit; LNH, lymph node harvested; CRT, chemoradiotherapy; CT, chemotherapy.
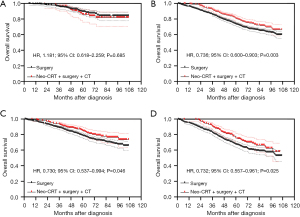
Based on multivariate Cox regression analysis, we defined age >55 years, grade III/IV, positive CEA and tumor size >6 cm as preoperative high-risk factors. Patients without these four high-risk factors were considered to be in the low-risk group and those containing at least one high-risk factor were considered to be in the high-risk group. After PSM, in the low-risk group, the Kaplan-Meier analysis showed that there was no statistically significant difference in OS between patients who underwent neoadjuvant CRT + surgery + chemotherapy and those who underwent surgery alone (P=0.685) (Figure 4A), and the 5-year OS was 85.8% (95% CI: 76.0–91.8%) and 89.4% (95% CI: 84.7–92.7%) respectively. In the high-risk group, the Kaplan-Meier analysis showed that patients treated with neoadjuvant CRT + surgery + chemotherapy had better OS than those treated with surgery alone (P=0.003) (Figure 4B). Moreover, patients treated with neoadjuvant CRT + surgery + chemotherapy had better OS than those treated with surgery alone in either the subgroup containing one high-risk factor (P=0.046) (Figure 4C) or the subgroup containing more than one high-risk factor (P=0.025) (Figure 4D).
Discussion
In the last two decades, multimodal treatment including neoadjuvant CRT has been widely used for LARCs (11). However, this treatment strategy has an important disadvantage, namely the high risk of intraoperative or postoperative complications. The adverse reactions of neoadjuvant CRT may lead to a financial burden and a decline in quality of life, and delay follow-up treatment (12). Therefore, for some LARCs in earlier stages, such as stage IIA, whether neoadjuvant therapies should be completed is controversial in different studies and literature. Some studies have suggested that patients with T3N0 and T1-3N1-2 rectal cancer judged by imaging should receive neoadjuvant CRT (7). Benson et al. pointed out that in patients with cT3/T4 or lymph node-positive rectal cancer, preoperative therapy reduces local recurrence and improves tumor prognosis according to the NCCN guidelines (13). There is growing evidence that patients with LARC benefit more from neoadjuvant CRT than surgery alone or postoperative CRT in terms of local control rates and sphincter protection (14). However, some studies have indicated that T3N0 rectal cancer patients can control local recurrence at a relatively low level with surgery alone, suggesting that these patients may not need neoadjuvant CRT (15). Although neoadjuvant CRT significantly reduced the risk of local recurrence in LARCs, the benefit of local control did not translate into a survival benefit (9). Therefore, it remains controversial whether neoadjuvant CRT provides sufficient benefit to outweigh the side effects and inconvenience of treatment for patients with stage IIA rectal cancer (15).
Our results showed that there was no significant difference in either OS or CSS between patients who underwent neoadjuvant CRT + surgery and those who underwent surgery alone. However, patients with stage IIA rectal cancer treated with neoadjuvant CRT + surgery + chemotherapy had better OS than those treated with surgery alone. But the CSS of the neoadjuvant CRT + surgery + chemotherapy group did not show superiority than that of the surgery alone group. Therefore, our study concluded that for patients with stage IIA rectal cancer, neoadjuvant CRT + surgery is not effective in improving the long-term survival of patients. But neoadjuvant CRT + surgery + chemotherapy can improve the OS of stage IIA patients.
However, do all patients with stage IIA rectal cancer need neoadjuvant and adjuvant treatment? What patients could be exempt from perioperative therapy? As we know, the severe toxicity associated with neoadjuvant CRT, including complications such as structural fractures of the lumbar spine, sexual and urinary dysfunction, proctitis, significant increases in defecation times, and incontinence (16), presents a significant obstacle and distress to the patient’s subsequent recovery and quality of survival.
The ESMO guidelines for the treatment of rectal cancer recommend a neoadjuvant treatment strategy based on multiple factors such as tumor location, circumferential resection margin (CRM), and clinical stage (17). This is specifically indicated for patients with low-risk rectal cancer who meet the following criteria: early T3N0, tumor invasion <5 mm, no significant invasion of the mesorectal fascia (MRF), no neurovascular invasion, and other factors; direct surgical treatment can be considered, and adjuvant treatment such as chemotherapy could be considered if postoperative pathology suggests including high-risk factors (8). Lin et al. also stated that the risk of colorectal cancer depends on the lymph node metastasis, positive CRM and distal resection margin (18). Skancke et al. noted that the short distal margin, lymph node metastasis, and positive CRM were significant predictors of local recurrence, distant metastasis, and OS (19). The prognosis of T3 patients with infiltration of more than 5 mm is significantly worse than that of patients with infiltration of less than 5 mm, and neoadjuvant CRT is usually required (7). Studies have shown that CRM is an important independent prognostic factor for local recurrence and long-term survival (20,21). Neoadjuvant radiotherapy should be considered if the distance between the tumor and rectal mesenteric fascia is predicted to be <1 mm on preoperative magnetic resonance imaging (MRI) (22). Studies have shown that in early, favorable cases (e.g., some early cT3N0), TME surgery alone is appropriate (15). For middle and lower stage II and III rectal cancer, preoperative radiotherapy or CRT can reduce the local recurrence rate without improving OS (23). Lavryk et al. showed that the distance between the tumor and anal verge was an independent prognostic parameter (24). Therefore, these factors could be considered when assessing the need for neoadjuvant therapy in stage IIA patients. Unconditional application of neoadjuvant CRT in low-risk patients was likely to result in overtreatment (18). Our study also performed a subgroup analysis to explore the effects of neoadjuvant and adjuvant therapy in different risk groups. The results showed that neoadjuvant CRT + surgery + chemotherapy did not significantly improve OS in the low-risk subgroup, but significantly improved OS in the high-risk subgroup regardless of the number of preoperative risk factors. Because we need to judge whether to use neoadjuvant treatment preoperatively, we chose risk factors among the preoperative independent factors, rather than postoperative ones.
For adjuvant treatment, we only included the most commonly used adjuvant chemotherapy. Baek et al. stated that in patients with low-lying rectal cancer, adjuvant CRT reduced local recurrence rates (25). In contrast, de Paula et al. showed that adjuvant CT did not prolong OS compared with adjuvant chemotherapy for T3N0 patients (26). We compared the patients who received neoadjuvant CRT + surgery + chemotherapy and those who received surgery + chemotherapy, the results showed that although there was no statistical difference in OS and CSS between the two groups, the OS of the neoadjuvant CRT + surgery + chemotherapy group was significantly higher than that of the surgery + chemotherapy group. In addition, multivariate analysis showed that OS in the neoadjuvant CRT + surgery + chemotherapy group was statistically superior to that in the neoadjuvant CRT + surgery group and the surgery alone group, while the surgery + chemotherapy group was not. Therefore, we think that the long-term survival of the surgery + chemotherapy group may be better than that of the neoadjuvant CRT + surgery group and the surgery alone group, but the long-term survival of the neoadjuvant CRT + surgery + chemotherapy group is much better.
The strength of this study is the large sample size available from the SEER database, which contains a wide range of information on neoadjuvant and adjuvant therapy for analysis and comparison, and increases the persuasiveness of this study. However, this study has some limitations. First, the SEER database only covers 27.8% of the US population, and many patients migrated in and out of SEER registry areas. So, there may be some bias in the inclusion and selection of data. Second, some key data that may affect the outcome of prognosis cannot be obtained from the SEER database, like underreported and incomplete data regarding adjuvant therapy and unrecorded variables, for example, (I) CRM. Although we did not have access to CRM data, its role as an independent postoperative risk factor did not provide guidance on whether to perform neoadjuvant therapy. (II) Depth of tumor invasion into the MRF. MRF, which obtained by pelvic MRI, may provide some guidance on neoadjuvant treatment for rectal cancer. (III) Where the tumor is located. Third, SEER database only includes the final pathologic stage, which may include patients who had a higher clinical stage, received neoadjuvant treatment, and then were downstaged at the time of resection. However, patients with higher stage usually have a poorer prognosis. If the results containing these patients were still significantly different, this would indicate the robustness of the results. Forth, patients with insufficient information were excluded, and it is unclear whether the results apply to them. Finally, this was a retrospective cohort study with a lower level of evidence than the randomized controlled trials. However, the application of PSM to reduce the influence of confounding factors can also increase the persuasiveness of the study.
Conclusions
In conclusion, there is no evidence that neoadjuvant CRT + surgery can significantly improve OS and CSS compared to surgery alone in patients with stage IIA rectal cancer. Compared to surgery alone, neoadjuvant CRT + surgery + chemotherapy can improve OS, but only in patients with preoperative risk factors. So, we suggest that patients with no preoperative high-risk factor may be considered for surgery alone, patients with preoperative risk factors need to be considered for neoadjuvant CRT + surgery + chemotherapy to improve OS.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-166/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-166/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-166/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuing that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Since the data of this project were all extracted from the SEER database and did not involve patient safety, privacy, and postoperative follow-up, no informed consent and no ethics committee approval were required.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7-30. [Crossref] [PubMed]
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Jin J, Tang Y, Hu C, et al. Multicenter, Randomized, Phase III Trial of Short-Term Radiotherapy Plus Chemotherapy Versus Long-Term Chemoradiotherapy in Locally Advanced Rectal Cancer (STELLAR). J Clin Oncol 2022;40:1681-92. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology. Rectal Cancer, Version 1. 2021.
- Quezada-Diaz FF, Smith JJ. Neoadjuvant Therapy for Rectal Cancer. Surg Oncol Clin N Am 2022;31:279-91. [Crossref] [PubMed]
- Glynne-Jones R, Harrison M, Hughes R. Challenges in the neoadjuvant treatment of rectal cancer: balancing the risk of recurrence and quality of life. Cancer Radiother 2013;17:675-85. [Crossref] [PubMed]
- Li Y, Wang J, Ma X, et al. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci 2016;12:1022-31. [Crossref] [PubMed]
- Maguire A, Sheahan K. Controversies in the pathological assessment of colorectal cancer. World J Gastroenterol 2014;20:9850-61. [Crossref] [PubMed]
- De Caluwé L, Van Nieuwenhove Y, Ceelen WP. Preoperative chemoradiation versus radiation alone for stage II and III resectable rectal cancer. Cochrane Database Syst Rev 2013;CD006041. [Crossref] [PubMed]
- Wang QX, Li SH, Zhang X, et al. Identification of locally advanced rectal cancer with low risk of local recurrence. PLoS One 2015;10:e0117141. [Crossref] [PubMed]
- Weiser MR, Zhang Z, Schrag D. Locally advanced rectal cancer: time for precision therapeutics. Am Soc Clin Oncol Educ Book 2015;e192-6. [Crossref] [PubMed]
- Lin J, Peng J, Qdaisat A, et al. Severe weight loss during preoperative chemoradiotherapy compromises survival outcome for patients with locally advanced rectal cancer. J Cancer Res Clin Oncol 2016;142:2551-60. [Crossref] [PubMed]
- Benson AB, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018;16:874-901. [Crossref] [PubMed]
- Tural D, Selcukbiricik F, Yıldız Ö, et al. Preoperative versus postoperative chemoradiotherapy in stage T3, N0 rectal cancer. Int J Clin Oncol 2014;19:889-96. [Crossref] [PubMed]
- Zhang C, Zhao S, Wang X. A Prognostic Nomogram for T3N0 Rectal Cancer After Total Mesorectal Excision to Help Select Patients for Adjuvant Therapy. Front Oncol 2021;11:698866. [Crossref] [PubMed]
- Joye I, Haustermans K. Early and late toxicity of radiotherapy for rectal cancer. Recent Results Cancer Res 2014;203:189-201. [Crossref] [PubMed]
- Glimelius B, Tiret E, Cervantes A, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24:vi81-8. [Crossref] [PubMed]
- Lin Y, Lin H, Xu Z, et al. Comparative Outcomes of Preoperative Chemoradiotherapy and Selective Postoperative Chemoradiotherapy in Clinical Stage T3N0 Low and Mid Rectal Cancer. J Invest Surg 2019;32:679-87. [Crossref] [PubMed]
- Skancke M, Schoolfield C, Umapathi B, et al. Minimally Invasive Surgery for Rectal Adenocarcinoma Shows Promising Outcomes Compared to Laparotomy, a National Cancer Database Observational Analysis. J Laparoendosc Adv Surg Tech A 2019;29:218-24. [Crossref] [PubMed]
- Taylor FG, Quirke P, Heald RJ, et al. Preoperative magnetic resonance imaging assessment of circumferential resection margin predicts disease-free survival and local recurrence: 5-year follow-up results of the MERCURY study. J Clin Oncol 2014;32:34-43. [Crossref] [PubMed]
- de Paul TR, Augestad KM, Kiran RP, et al. Management of the positive pathologic circumferential resection margin in rectal cancer: A national cancer database (NCDB) study. Eur J Surg Oncol 2021;47:296-303. [Crossref] [PubMed]
- Liu Q, Luo D, Cai S, et al. Circumferential resection margin as a prognostic factor after rectal cancer surgery: A large population-based retrospective study. Cancer Med 2018;7:3673-81. [Crossref] [PubMed]
- Glynne-Jones R, Wyrwicz L, Tiret E, et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017;28:iv22-40. [Crossref] [PubMed]
- Lavryk OA, Manilich E, Valente MA, et al. Neoadjuvant chemoradiation improves oncologic outcomes in low and mid clinical T3N0 rectal cancers. Int J Colorectal Dis 2020;35:77-84. [Crossref] [PubMed]
- Baek JY, Yu JI, Park HC, et al. Determining whether postoperative chemoradiotherapy is required in patients with pathologic T3N0 rectal cancer with negative resection margin. Int J Colorectal Dis 2020;35:2239-48. [Crossref] [PubMed]
- de Paula TR, Gorroochurn P, Kiran RP, et al. Does Adjuvant Chemotherapy Improve Survival in T3N0 Rectal Cancer? An Evaluation of Use and Outcomes from the National Cancer Database (NCDB). J Gastrointest Surg 2020;24:1188-91. [Crossref] [PubMed]


