
Prognostic role of myeloid-derived tumor-associated macrophages at the tumor invasive margin in gastric cancer with liver metastasis (GCLM): a single-center retrospective study
Introduction
Gastric cancer (GC) is a common solid malignant tumor in China, with the fifth highest incidence rate and the third highest mortality rate (1). Distant metastasis is the main cause of poor prognosis of GC. Due to anatomy, venous return, and other characteristics, the liver is the most common organ affected by distant metastasis of GC (2,3). The overall incidence of GC liver metastases (GCLM) is 9.9–18.7%. A considerable number of advanced GC patients have synchronous liver metastasis at diagnosis, and the five-year survival rate is less than 10% (1,4).
The mechanism of GCLM is extremely complicated. The classic “seed-soil theory” proposes that tumor metastasis is the result of the interaction between seed (tumor cells) and soil (tumor microenvironment) (5). Therefore, the liver microenvironment at the invasive margin of GCLM is critical for the formation of GCLM (6,7). This is the area where normal liver parenchymal cells initially lose their differentiation and tumor cells gradually gain mesenchyme-like capabilities. These changes confer tumor cells with the characteristics of metastatic and invasive growth (8). Studies have shown that there are abundant macrophages at the invasive margin of GCLM, which may play a decisive role in metastatic tumor progression (9-12). Among them, Kupffer cells are macrophages living in hepatic sinusoids, accounting for about 35% of non-parenchymal hepatocytes in the liver (6,13). The injury and inflammation caused by tumor cells entering the liver could also recruit monocytes from bone marrow to enter the tumor, and then differentiate into mature macrophages to form myeloid-derived tumor-associated macrophages (TAMs), which play a vital role in the process of tumor proliferation and metastasis (7,11,12,14,15).
Several studies had revealed the underlying mechanisms driving liver metastasis of gastric cancer, containing the tumor-stromal crosstalk and epigenetic regulation (16-18). Whereas, there was an acutely controversial on the resection benefits of metastatic lesion, and few studies had identified the correlation between prognosis and microenvironmental contents. Since most patients have no surgical indications at the time of diagnosis, it is difficult to obtain clinical specimens and let further studies on GCLM alone. The fourth edition of the Japanese gastric cancer treatment guidelines had recommended that patients with local liver metastasis from GC could benefit from radical resection of primary GC combined with secondary liver metastases tumor, which was also confirmed by some retrospective studies (19-22). Surgical treatment is performed conditionally for patients who are suitable for resection. Moreover, there is a lack of effective indicators to predict the prognosis of patients after resection. Hence, 72 relevant surgical pathological specimens were retrospectively analyzed in this study to clarify the prognostic impact of macrophages at the invasive margin of GCLM in the liver microenvironment and whether they could be used as a potential indicator of liver metastases tumor R1 resection. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-530/rc).
Methods
Inclusion of subjects and clinical data collection
The study was a retrospective study based on a total group of 72 liver metastatic specimens collected from patients who were diagnosed with GCLM by both pathological and clinical doctors, and underwent surgical resections in the Department of Gastrointestinal Surgery, Ren Ji Hospital Affiliated to Shanghai Jiao Tong University School of Medicine, between February 2015 and December 2020. The clinical data corresponding to metastatic cases, involving age, gender, tumor location, histological types, TNM stages and pathological characteristics (vascular or nerve invasion, Ki67 and p53 levels), were collected retrospectively. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). All specimens and experimental protocols were approved by the Ethics Committee of Ren Ji Hospital [No. (2017)114]. All the participants gave informed consent before collecting specimens together with their clinical information.
Follow-up
Follow-up was performed by outpatient re-examination and telephone periodically. The following postoperative follow-up data were collected for every patient: survival status, disease treatment, laboratory test results. Overall survival (OS) was defined as the time span from the date of initial diagnosis to the date of death from any cause or the date of last known contact. Our department follows-up with patients every six months for the first five years after surgery and yearly thereafter. The follow-up period in this study was 82 months, the date of last follow-up was October, 2021.
Immunohistochemistry (IHC)
The IHC assay was performed to detect the expression of CD68, CD206, and Clec4f in GCLM tissues. We used CD68 as a marker of total macrophages population (14,15); CD206 was used as a marker of myeloid-derived TAMs derived from bone marrow mononuclear cells (9); and Clec4f was used as a marker of Kupffer cells (10). We deparaffinized and rehydrated 5 µm-thick consecutive paraffin sections from the pathology department of Ren Ji Hospital; 3% H2O2 in methanol was used to block the endogenous peroxidase activity at room temperature, followed by antigen retrieval in citrate buffer (pH 6.0) for 15 minutes. The specimens were blocked by 10% serum at 37 ℃ for 1 hour and incubated with mouse monoclonal anti-human primary antibodies against CD68, CD206, or Clec4f (CD68 applied at 1:100, Abcam, Cambridge, MA, USA; CD206 applied at 1:200, Abcam, USA; Clec4f applied at 1:200, Abcam, USA) in a humidified chamber overnight at 4 ℃. Next, the horseradish peroxidase (HRP)-labeled goat anti mouse or rabbit polyclonal antibody (DakoCytomation, Glostrup, Denmark) was dripped onto the slides, incubated at 37 ℃ for 0.5 hour, and stained with 3,3'-diaminobenzidine (DAB). Finally, the samples were counterstained by hematoxylin.
Evaluation of IHC
Macrophages densities were quantitatively estimated at the invasive margin of GCLM using the above-mentioned criteria, independently by two pathologists. One sample was screened at low magnification (×100), followed by selecting five areas with highest number of positively stained cells for further analysis. The average count of macrophage in five areas was estimated at high power (×400) magnification. The levels of immunoreactivity were scored from 0 to 3 according to the ratio of positive cells as follows: 0, <5%; 1, 5–20%; 2, 20–50%; and 3, >50%. A score of 2–3 was classified as high density and 0–1 as low density. Infiltrating macrophage densities were independently counted by two pathologists blinded to the patient’s clinical status. To confirm the reproducibility, 25% of the slides were randomly chosen and scored twice and those duplicates were evaluated in a similar manner.
Statistical analysis
All statistical analysis were performed using SPSS 24.0 software (IBM Corp., Armonk, NY, USA) for Windows and GraphPad Prism 8.0 (GraphPad Software, La Jolla, CA, USA). The correlation between CD68, CD206, or Clec4f expression and clinicopathological parameters of GCLM patients was analyzed by chi-square test and multivariate logistic regression analysis. The Kaplan-Meier method was used to calculate OS, and log-rank testing was used to compare the survival curves between different groups. Prognostic analysis was performed using univariate and multivariate Cox regression models on overall survival, where the factors age, gender, tumor location, Lauran type, TNM stage, vascular or nerve invasion, Ki67 and p53 level, tumor diameter, H classification, CD68-positive macrophages, CD206-positive macrophages and Clec4f-positive macrophages were involved into the analysis. P value <0.05 at two-sided was defined as statistically significant.
Results
Clinicopathological characteristics of 72 GCLM patients
A total of 72 patients with histologically confirmed GCLM were included in the present analysis. Among them, 55 were male and 17 were female. The proportion of patients aged ≥65 years (51.4%) was almost the same as those aged <65 years (48.6%). According to Lauren classification, 40 patients (55.6%) were diagnosed as intestinal type, 24 patients (33.3%) as diffuse type, and eight cases (11.1%) as mixed type. There was one patient in T1 stage, 2 cases (2.8%) in T2 stage, and 69 patients (95.8%) in T4 stage. Nine patients did not have lymph node metastasis, while all others had lymph node metastasis. According to the fifth edition of the Japanese “Gastric Cancer Treatment Guide” H-class system, GCLM is divided into H1, H2, or H3. The H1 level indicates only one metastatic tumor in one hepatic lobe; H2 level indicates minor metastatic tumor in two hepatic lobes (less than or equal to two metastatic tumors); and H3 level indicates two hepatic lobes have multiple scattered tumors. In this study, 45 (62.5%) patients were H1 level, 18 (25.0%) were H2 level, and the remaining nine (12.5%) were H3 level (Table 1).
Table 1
| Variables | n (%) |
|---|---|
| Age (years) | |
| ≥65 | 37 (51.4) |
| <65 | 35 (48.6) |
| Gender | |
| Male | 55 (76.4) |
| Female | 17 (23.6) |
| Tumor location | |
| Cardia | 22 (30.6) |
| Non-cardia | 50 (69.4) |
| Lauren | |
| Intestinal | 40 (55.6) |
| Diffuse | 24 (33.3) |
| Mixed | 8 (11.1) |
| T stage | |
| T1 | 1 (1.4) |
| T2 | 2 (2.8) |
| T3 | 0 (0.0) |
| T4 | 69 (95.8) |
| N stage | |
| Present | 63 (87.5) |
| Absent | 9 (12.5) |
| M stage | |
| M0 | 0 (0.0) |
| M1 | 72 (100.0) |
| Vascular invasion | |
| Present | 21 (29.2) |
| Absent | 51 (70.8) |
| Nerve invasion | |
| Present | 10 (13.9) |
| Absent | 62 (86.1) |
| Ki67 | |
| + | 13 (18.1) |
| ++ | 23 (31.9) |
| +++ | 36 (50.0) |
| p53 | |
| − | 26 (36.1) |
| + | 16 (22.2) |
| ++ | 6 (8.3) |
| +++ | 24 (33.3) |
| Tumor maximum diameter of GCLM (cm) | |
| <3.0 | 57 (79.2) |
| ≥3.0 and <6.0 | 11 (15.3) |
| ≥6.0 | 4 (5.6) |
| Tumor number of GCLM | |
| 1 | 33 (45.8) |
| 2 | 18 (25.0) |
| ≥3 | 21 (29.2) |
| H classification of GCLM | |
| H1 | 45 (62.5) |
| H2 | 18 (25.0) |
| H3 | 9 (12.5) |
−, negative; +, slightly positive; ++, moderately positive; +++, strongly positive. GCLM, gastric cancer with liver metastasis.
Expression of CD68, CD206, and Clec4f in invasive margin of GCLM
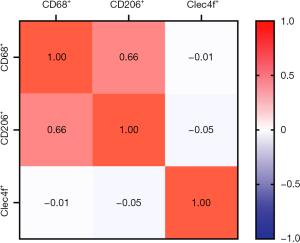
We detected the expression of CD68, CD206, and Clec4f in 72 metastatic tumor tissues at the invasive margin in the liver by IHC assay. The representative results are shown in Figure 1. At the liver metastatic invasive margin, CD68 and CD206 were highly expressed, while the expression of Clec4f was low. The count of positive tumor stromal cells is shown in Table 2. The expression of CD68 was moderately correlated with CD206 expression, and the correlation coefficient was 0.66; however, there was no obvious correlation with the expression of Clec4f. Moreover, there was no correlation between the expression of CD206 and Clec4f (Figure 2).
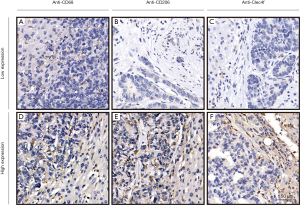
Table 2
| Variable* | Mean | SD | Median | Range |
|---|---|---|---|---|
| CD68+ macrophages | 107.2 | 53.9 | 99 | 19–232 |
| CD206+ macrophages | 98.8 | 48.2 | 93 | 16–271 |
| Clec4f+ macrophages | 64.2 | 30.3 | 54 | 10–157 |
*, numbers of CD68, CD206 and Clec4f positive macrophages in every high-power field (×400). SD, standard deviation.
Correlation between expression of CD68, CD206, Clec4f and clinicopathological parameters
The high expression of CD206 was related to the Lauren classification (P=0.041). In addition, it was also significantly related to the expression of p53 (P=0.031). The expression of CD68 and Clec4f had no significant correlation with the clinical features of GCLM (Table 3).
Table 3
| Variables | CD68+ macrophages | CD206+ macrophages | Clec4f+ macrophages | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Low (n=22) | High (n=50) | P value | Low (n=21) | High (n=51) | P value | Low (n=38) | High (n=34) | P value | |||
| Age (years) | |||||||||||
| ≥65 | 12 | 25 | 0.722 | 12 | 25 | 0.531 | 18 | 19 | 0.471 | ||
| <65 | 10 | 25 | 9 | 26 | 20 | 15 | |||||
| Gender | |||||||||||
| Male | 15 | 40 | 0.277 | 15 | 40 | 0.525 | 28 | 27 | 0.568 | ||
| Female | 7 | 10 | 6 | 11 | 10 | 7 | |||||
| Tumor location | |||||||||||
| Cardia | 9 | 13 | 0.206 | 6 | 16 | 0.815 | 12 | 10 | 0.842 | ||
| Non-cardia | 13 | 37 | 15 | 35 | 26 | 24 | |||||
| Lauren | |||||||||||
| Intestinal | 14 | 26 | 0.658 | 16 | 24 | 0.041 | 20 | 20 | 0.574 | ||
| Diffuse | 6 | 18 | 5 | 19 | 13 | 11 | |||||
| Mix | 2 | 6 | 0 | 8 | 5 | 3 | |||||
| T stage | |||||||||||
| 1 | 0 | 1 | 0.671 | 1 | 0 | 0.197 | 0 | 1 | 0.233 | ||
| 2 | 1 | 1 | 0 | 2 | 2 | 0 | |||||
| 3 | 0 | 0 | 0 | 0 | 0 | 0 | |||||
| 4 | 21 | 48 | 20 | 49 | 36 | 33 | |||||
| N stage | |||||||||||
| Present | 20 | 43 | 0.562 | 18 | 45 | 0.769 | 35 | 28 | 0.212 | ||
| Absent | 2 | 7 | 3 | 6 | 3 | 6 | |||||
| M stage | |||||||||||
| M0 | 0 | 0 | – | 0 | 0 | – | 0 | 0 | – | ||
| M1 | 22 | 50 | 21 | 51 | 38 | 34 | |||||
| Invasion (vascular & nerve) | |||||||||||
| Present | 8 | 17 | 0.846 | 10 | 15 | 0.140 | 15 | 10 | 0.371 | ||
| Absent | 14 | 33 | 11 | 36 | 23 | 24 | |||||
| Ki67 | |||||||||||
| + | 4 | 9 | 0.517 | 4 | 9 | 0.925 | 7 | 6 | 0.111 | ||
| ++ | 9 | 14 | 6 | 17 | 16 | 7 | |||||
| +++ | 9 | 27 | 11 | 25 | 15 | 21 | |||||
| p53 | |||||||||||
| − | 10 | 16 | 0.602 | 13 | 13 | 0.031 | 13 | 13 | 0.656 | ||
| + | 3 | 13 | 2 | 14 | 10 | 6 | |||||
| ++ | 2 | 4 | 1 | 5 | 4 | 2 | |||||
| +++ | 7 | 17 | 5 | 19 | 11 | 13 | |||||
| Tumor maximum diameter of GCLM (cm) | |||||||||||
| ≥6.0 | 2 | 2 | 0.309 | 3 | 1 | 0.085 | 2 | 2 | 0.987 | ||
| ≥3.0 and <6.0 | 5 | 6 | 4 | 7 | 6 | 5 | |||||
| <3.0 | 15 | 42 | 14 | 43 | 30 | 27 | |||||
| Tumor number of GCLM | |||||||||||
| 1 | 10 | 23 | 0.949 | 9 | 24 | 0.899 | 17 | 16 | 0.688 | ||
| 2 | 6 | 12 | 6 | 12 | 11 | 7 | |||||
| ≥3 | 6 | 15 | 6 | 15 | 10 | 11 | |||||
| H classification of GCLM | |||||||||||
| H1 | 11 | 34 | 0.117 | 11 | 34 | 0.500 | 25 | 20 | 0.800 | ||
| H2 | 9 | 9 | 7 | 11 | 9 | 9 | |||||
| H3 | 2 | 7 | 3 | 8 | 4 | 5 | |||||
−, negative; +, slightly positive; ++, moderately positive; +++, strongly positive. GCLM, gastric cancer with liver metastasis.
Relationship between CD68, CD206, Clec4f expression and prognosis of GCLM patients
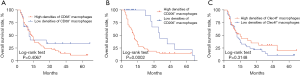
The Kaplan-Meier survival curve of patients with GCLM is shown in Figure 3. The 1-, 3-, and 5-year survival rates were 98.5%, 44.4%, and 19.3% in the CD206 low expression group, and 42.7%, 14.9%, and 14.9% in the CD206 high expression group, respectively. The OS of the CD206 low expression group was longer than that of the CD206 high expression group (P=0.0002). The survival curves of patients with low expressions of CD68 and Clec4f were shorter than those of patients with high expression, but without statistical difference.
Prognostic analysis
Univariate Cox proportional hazard regression analysis showed that CD206 was associated with the prognosis of patients with GCLM (Table 4). Multivariate regression analysis also showed that CD206 was an independent risk factor for prognosis (Table 5). Other clinical features had no significant effect on the prognosis of the patients.
Table 4
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age (years) (<65 vs. ≥65) | 0.902 | 0.517–1.574 | 0.717 |
| Gender (female vs. male) | 0.783 | 0.380–1.614 | 0.508 |
| Tumor location (cardia vs. non-cardia) | 0.892 | 0.486–1.635 | 0.711 |
| Lauran (intestinal vs. diffuse vs. mix) | 1.072 | 0.721–1.593 | 0.732 |
| T stage (T1/T2 vs. T3/T4) | 0.843 | 0.454–1.566 | 0.589 |
| N stage (absent vs. present) | 1.139 | 0.482–2.689 | 0.767 |
| Invasion (absent vs. present) | 0.672 | 0.361–1.251 | 0.210 |
| Ki67 (+/++ vs. +++) | 0.936 | 0.653–1.342 | 0.721 |
| p53 (−/+ vs. ++/+++) | 0.957 | 0.770–1.188 | 0.688 |
| Tumor maximum diameter of GCLM (cm) (<3 vs. ≥3) | 0.760 | 0.406–1.422 | 0.390 |
| H classification of GCLM (H1/H2 vs. H3) | 0.853 | 0.613–1.186 | 0.343 |
| CD68-positive macrophages (high vs. low) | 1.353 | 0.705–2.598 | 0.363 |
| CD206-positive macrophages (high vs. low) | 3.039 | 1.418–6.515 | 0.004 |
| Clec4f-positive macrophages (high vs. low) | 0.297 | 0.419–1.304 | 0.297 |
−, negative; +, slightly positive; ++, moderately positive; +++, strongly positive. GCLM, gastric cancer with liver metastasis; HR, hazard ratio.
Table 5
| Variables | HR | 95% CI | P value |
|---|---|---|---|
| Age (years) (<65 vs. ≥65) | 0.804 | 0.404–1.599 | 0.533 |
| Gender (female vs. male) | 0.945 | 0.390–2.291 | 0.900 |
| Tumor location (cardia vs. non-cardia) | 0.895 | 0.402–1.991 | 0.786 |
| Lauran (intestinal vs. diffuse vs. mix) | 0.824 | 0.514–1.321 | 0.422 |
| T stage (T1/T2 vs. T3/T4) | 0.696 | 0.336–1.439 | 0.328 |
| N stage (absent vs. present) | 1.341 | 0.493–3.645 | 0.565 |
| Invasion (absent vs. present) | 0.710 | 0.347–1.456 | 0.350 |
| Ki67 (+/++ vs. +++) | 0.967 | 0.635–1.472 | 0.876 |
| p53 (−/+ vs. ++/+++) | 0.817 | 0.625–1.067 | 0.138 |
| Tumor maximum diameter of GCLM (cm) (<3 vs. ≥3) | 1.075 | 0.539–2.144 | 0.838 |
| H classification of GCLM (H1/H2 vs. H3) | 0.857 | 0.596–1.233 | 0.406 |
| CD68-positive macrophages (high vs. low) | 0.608 | 0.240–1.542 | 0.295 |
| CD206-positive macrophages (high vs. low) | 5.276 | 1.730–16.089 | 0.003 |
| Clec4f-positive macrophages (high vs. low) | 0.871 | 0.435–1.741 | 0.695 |
−, negative; +, slightly positive; ++, moderately positive; +++, strongly positive. GCLM, gastric cancer with liver metastasis. HR, hazard ratio.
Discussion
At present, locally advanced GC accounts for 70% of all confirmed cases of GC, and GCLM is a typical manifestation of stage IV GC. The prognosis of patients with GCLM is exceedingly poor (2,20,23). Although R1 resection surgery of liver metastases tumor prolongs the survival time of patients and improves their prognosis, whether it is necessary for these patients remains controversial. The median survival time of patients after radical resection of primary GC combined with secondary liver metastases tumor has been reported to be about 16–37 months (21,22,24,25), and was 17.82 months in our study.
According to the classic theory of “seed-soil”, we hypothesized that macrophages in the liver microenvironment have a major effect on the development of GCLM. As multifunctional cells, they have different functions in the progression of liver metastasis. CD206+ myeloid-derived TAMs react with cytokines and enzymes released from different parts of the tumor to regulate tumor growth, angiogenesis, invasion or metastasis, and promote the invasion of GC cells and the secondary growth of liver metastases (9,11,26,27). As the main non-parenchymal cells in the liver, Clec4f+ Kupffer cells participate in the innate and adaptive immune responses in the initial stage of tumor development by conducting phagocytosis and promoting tumor cell apoptosis. At the later stage, they also release various cytokines to promote liver metastasis by immune escape, blood circulation enhancement, tumor cell adhesion, and proliferation. They are indispensable immune cells in the human body, which facilitate tumor growth by promoting tumor cell apoptosis and phagocytosis, and also play an anti-tumor metastasis role (6,28).
In this study, IHC was used to detect the expression of different macrophages in GCLM, and a correlation was found between macrophages and the clinicopathological characteristics of patients with GCLM. Since CD68 is a marker of macrophage population, its high expression confirmed the presence of numerous macrophages at the invasive margin of GCLM. Next, we used markers to distinguish macrophages and examine the effect of different macrophages on GCLM. The results indicated that the expression of CD206+ myeloid-derived TAMs was significantly higher than that of Kupffer cells at the invasive margin tissues of GCLM. Meanwhile, there was no correlation between the number of CD206+ TAMs and clinical features such as gender, age, TNM stage, and H class system, but there was a correlation between Lauren classification, p53, and CD206+ TAMs. The proportion of patients with high expression of CD206+ TAMs in diffuse GC was higher than that in intestinal type. Therefore, we hypothesized that because the tissue differentiation of diffuse GC was worse than that of intestinal type, diffuse GC may have stronger antigenicity and greater impact on the tumor microenvironment of GCLM, recruiting more TAMs from the myeloid system to play a role in promoting cancer, thereby leading to poor prognosis. The p53 gene is considered a tumor suppressor gene, which controls the initiation of cell cycle and regulates normal activities of cells. Its strong positive expression inhibits cancer development (29). Meanwhile, high expression of CD206 can counteract the anti-tumor effect of p53, and plays a stronger role in promoting tumor development. Hence, we explored the clinicopathological process of GCLM and found that CD206+ TAM infiltration was related to the prognosis of GCLM, suggesting that CD206+ myeloid-derived TAMs had a potential biological significance in the development of GCLM.
Since all cases in this study underwent metastatic liver tumor R1 resection, the results suggested that CD206+ myeloid-derived TAMs may determine the prognosis of patients with GCLM, which was worse in patients with high expression of CD206. Therefore, this marker could be used to predict whether patients will benefit from radical resection of primary tumor combined with R1 resection of liver metastases tumor. Biopsy of GCLM and detection of CD206 could predict whether patients with liver metastases from GC would benefit from liver metastases tumor R1 resection. Since the prognosis of patients with low expression of CD206 was better than that of patients with high expression, they could be operated on to prolong their survival time. For patients with GCLM and high expression of CD206, a sequence of conservative and palliative treatments should be undertaken to improve their prognosis, including chemotherapy, targeted therapy, immunotherapy, local therapy, and so on, so that a subset of those patients could achieve long-term survival. This study has important theoretical significance and practical value for accurately assessing the clinical prognosis of patients with GCLM and guiding clinical therapeutic schedule. However, this was a single-center retrospective study, and the research evidence was insufficient. Therefore, the findings need to be further confirmed by multi-center and large sample size prospective studies.
Acknowledgments
Funding: This work was supported by science research fund from National Natural Science Foundation of China (Nos. 8217110096, 8197100200, and 8200100943).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-530/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-530/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-530/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. All the participants gave informed consent before collecting specimens together with their clinical information. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of Ren Ji Hospital [No. (2017)114].
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Chandra R, Balachandar N, Wang S, et al. The changing face of gastric cancer: epidemiologic trends and advances in novel therapies. Cancer Gene Ther 2021;28:390-9. [Crossref] [PubMed]
- Digklia A, Wagner AD. Advanced gastric cancer: Current treatment landscape and future perspectives. World J Gastroenterol 2016;22:2403-14. [Crossref] [PubMed]
- Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021;24:1-21.
- Clark AM, Ma B, Taylor DL, et al. Liver metastases: Microenvironments and ex-vivo models. Exp Biol Med (Maywood) 2016;241:1639-52. [Crossref] [PubMed]
- Cancer Metastasis Rev 1989;8:98-101. [PubMed]
- Dou L, Shi X, He X, et al. Macrophage Phenotype and Function in Liver Disorder. Front Immunol 2019;10:3112. [Crossref] [PubMed]
- Paolillo M, Schinelli S. Extracellular Matrix Alterations in Metastatic Processes. Int J Mol Sci 2019;20:4947. [Crossref] [PubMed]
- Pernot S, Terme M, Radosevic-Robin N, et al. Infiltrating and peripheral immune cell analysis in advanced gastric cancer according to the Lauren classification and its prognostic significance. Gastric Cancer 2020;23:73-81. [Crossref] [PubMed]
- Genin M, Clement F, Fattaccioli A, et al. M1 and M2 macrophages derived from THP-1 cells differentially modulate the response of cancer cells to etoposide. BMC Cancer 2015;15:577. [Crossref] [PubMed]
- Kinoshita M, Uchida T, Sato A, et al. Characterization of two F4/80-positive Kupffer cell subsets by their function and phenotype in mice. J Hepatol 2010;53:903-10. [Crossref] [PubMed]
- Nawaz A, Aminuddin A, Kado T, et al. CD206+ M2-like macrophages regulate systemic glucose metabolism by inhibiting proliferation of adipocyte progenitors. Nat Commun 2017;8:286. [Crossref] [PubMed]
- Tan-Garcia A, Lai F, Sheng Yeong JP, et al. Liver fibrosis and CD206+ macrophage accumulation are suppressed by anti-GM-CSF therapy. JHEP Rep 2020;2:100062. [Crossref] [PubMed]
- Phillips NC. Kupffer cells and liver metastasis. Optimization and limitation of activation of tumoricidal activity. Cancer Metastasis Rev 1989;8:231-52. [Crossref] [PubMed]
- Gottfried E, Kunz-Schughart LA, Weber A, et al. Expression of CD68 in non-myeloid cell types. Scand J Immunol 2008;67:453-63. [Crossref] [PubMed]
- Tabas I, Bornfeldt KE. Macrophage Phenotype and Function in Different Stages of Atherosclerosis. Circ Res 2016;118:653-67. [Crossref] [PubMed]
- Li Q, Zhu CC, Ni B, et al. Lysyl oxidase promotes liver metastasis of gastric cancer via facilitating the reciprocal interactions between tumor cells and cancer associated fibroblasts. EBioMedicine 2019;49:157-71. [Crossref] [PubMed]
- Xia X, Zhang Z, Zhu C, et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat Commun 2022;13:1017. [Crossref] [PubMed]
- Yue B, Song C, Yang L, et al. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol Cancer 2019;18:142. [Crossref] [PubMed]
- Dittmar Y, Altendorf-Hofmann A, Rauchfuss F, et al. Resection of liver metastases is beneficial in patients with gastric cancer: report on 15 cases and review of literature. Gastric Cancer 2012;15:131-6. [Crossref] [PubMed]
- Garancini M, Uggeri F, Degrate L, et al. Surgical treatment of liver metastases of gastric cancer: is local treatment in a systemic disease worthwhile? HPB (Oxford) 2012;14:209-15. [Crossref] [PubMed]
- Guner A, Son T, Cho I, et al. Liver-directed treatments for liver metastasis from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer 2016;19:951-60. [Crossref] [PubMed]
- Oki E, Tokunaga S, Emi Y, et al. Surgical treatment of liver metastasis of gastric cancer: a retrospective multicenter cohort study (KSCC1302). Gastric Cancer 2016;19:968-76. [Crossref] [PubMed]
- D'Angelica M, Gonen M, Brennan MF, et al. Patterns of initial recurrence in completely resected gastric adenocarcinoma. Ann Surg 2004;240:808-16. [Crossref] [PubMed]
- Qiu JL, Deng MG, Li W, et al. Hepatic resection for synchronous hepatic metastasis from gastric cancer. Eur J Surg Oncol 2013;39:694-700. [Crossref] [PubMed]
- Uggeri F, Ripamonti L, Pinotti E, et al. Is there a role for treatment-oriented surgery in liver metastases from gastric cancer? World J Clin Oncol 2020;11:477-94. [Crossref] [PubMed]
- Wu K, Lin K, Li X, et al. Redefining Tumor-Associated Macrophage Subpopulations and Functions in the Tumor Microenvironment. Front Immunol 2020;11:1731. [Crossref] [PubMed]
- Li W, Zhang X, Wu F, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis 2019;10:918. [Crossref] [PubMed]
- Hepatology 2021;74:2633-51. [Crossref] [PubMed]
- Gasco M, Crook T. p53 family members and chemoresistance in cancer: what we know and what we need to know. Drug Resist Updat 2003;6:323-8. [Crossref] [PubMed]
(English Language Editor: J. Jones)


