
Survival and prognostic factors for postoperative primary appendiceal cancer: a retrospective cohort study based on the Surveillance, Epidemiology, and End Results database
Introduction
Primary appendiceal cancer is a rare malignant tumor of the digestive system, the incidence of which has been increasing in recent years (1). Between 2000 and 2016, the incidence of malignant appendiceal cancer increased by 232% and 292% in the United States and Canada, respectively (2). Primary appendiceal cancer shares a common embryonic origin with colon cancer (3). Owing to the abundance of enteroendocrine cells in the appendix wall, carcinoids are the most common histological type of appendiceal cancer, accounting for more than half of all such malignancies, and are discovered in 7 out of every 1,000 appendectomy specimens (3,4). Primary appendiceal cancer is rarely diagnosed before surgery (5), and it is usually discovered accidentally, in specimens resected for acute appendicitis (6). Due to its lack of characteristic clinical features, primary appendiceal cancer may be misdiagnosed as ovarian cancer in the absence of pathological testing (7). Surgery remains the best curative treatment for cancers of the appendix and allows for the most accurate diagnosis (8). Most studies on appendiceal tumors are isolated case reports (9-12), and there is a lack of clinical trials or meta-analyses with large samples. In 2018, Li et al. conducted a retrospective study of 50 cases of low-grade appendiceal mucinous neoplasms (LAMN) and found when LAMN was treated surgically with resection of the primary site in early stage disease or with pushing invasion, there was an excellent prognosis and expanded surgical procedures were unnecessary (13). Another study showed that for patients with appendiceal mucinous neoplasm misdiagnosed as ovarian cancer, the median peritoneal cancer index (PCI) and lactate dehydrogenase (LDH) levels were identified as independent predictors of poor overall survival (OS) (14). These studies focused on a single pathogenic kind of appendiceal tumor, and neither explored the prognostic influence of chemotherapy. In previous studies based on data from the Surveillance, Epidemiology, and End Results (SEER) database, they focused on the incidence of appendiceal cancer and the impact of different pathological types on survival, rather than on the impact of treatment on survival (1,15). The influence of many factors, such as age, tumor size, and grade, on the survival of postoperative patients remains largely undetermined, and whether adjuvant treatment can prolong patient survival after appendectomy is also controversial. Therefore, in this study, we aimed to analyze data from the SEER database to identify the prognostic factors of patients with primary appendix cancer after surgery and provide more guidance for clinical practice. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-454/rc).
Methods
Data source and patients
This study is a retrospective cohort analysis of freely accessible data retrieved from the Incidence-SEER Research Plus Data, Nov 2020 Sub (1975–2018) (https://seer.cancer.gov/). Patients from between 2004 and 2015 were selected to obtain survival information. The follow-up deadline is November 2020. Patients who met the following criteria were included in the study: (I) a diagnosis of primary appendiceal cancer; (II) an International Classification of Diseases for Oncology (ICD-O)-3 behavior code of malignant; and (III) a history of appendix surgery. Patients with incomplete survival information were excluded. Figure 1 shows patient selection flow chart.
The following information was retrieved from the database: age at diagnosis; sex; race; marital status; tumor size, stage, histologic type, and grade; chemotherapy status; OS and cancer-special survival. According to the ICD-O V.3, there are five histological types of primary appendiceal cancer: colonic-type adenocarcinoma (8140, 8144, 8210, 8211, 8255, 8261, 8262, 8263, 8323, 8440, 8461, 8550, 8574), mucinous adenocarcinoma (8470, 8480, 8481), neuroendocrine neoplasm (8013, 8041, 8246), signet ring cell carcinoma (SRCC) (8490), and malignant carcinoid (8240, 8241, 8243, 8244, 8245, 8249). The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Statistical analysis
OS was defined as the time from surgery to the date of death or the last follow-up. Cancer-specific survival (CSS) was defined as the time from surgery to the date of cancer-related death. Survival curves, according to different factors, were obtained by Kaplan-Meier method. Median survival time and 5-year OS rate were estimated with the Kaplan-Meier method. Independent prognostic factors were determined by univariate and multivariate analyses using the Cox proportional-hazards regression model. All factors acquired from SEER database are included in the Cox multifactor analysis. Patients were divided into two categories, those who received chemotherapy and those who did not receive chemotherapy or did not know whether to receive chemotherapy. The chi-square test was used to compare differences between the two groups. The annual percentage change (APC) was calculated using the weighted least squares method (https://seer.cancer.gov/). The SPSS 26.0 statistical package (IBM Corporation, Armonk, NY, USA) and GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA) were used for all statistical analyses. Statistical significance was set at two-sided P<0.05.
Results
The age-adjusted incidence of appendiceal cancer
The overall age-adjusted incidence rate from 2004 to 2015 was 0.92 cases per 100,000 person-year. The annual percentage change was 5.07% (95% CI: 4.35–5.79, P<0.05). Figure 2 presents the annual age-adjusted incidence between 2004 and 2015.
Patient characteristics
A total of 2,891 cases which met the selection criteria were included in the study. Patients were divided into the following age groups: under 35 years old (n=392, 13.6%), 35 to 69 years old (n=1,918, 66.3%), and over 69 years old (n=581, 20.1%). There were more female (54.1%) than male patients. White was the largest racial group, accounting for 82.3% of patients, and most patients were married (n=1661, 57.5%). Malignant carcinoid was the most common histologic type (42.9%), followed by mucinous adenocarcinoma (25.6%), colonic-type adenocarcinoma (21.4%), SRCC (5.6%), and neuroendocrine neoplasm (4.4%). Among the 2197 (76%) cases with an available differentiation degree, 971 cases (33.6%) were well differentiated (grade I), 793 cases (27.4%) were moderately differentiated (grade II), 376 cases (13.0%) were poorly differentiated (grade III), and 57 cases (2.0%) were undifferentiated (grade IV). In terms of disease stage, localized, regional, and distant tumors accounted for 45.7%, 29.9%, and 24.4% of all cases, respectively. Of the 2,891 patients included in the study, 881 (30.5%) received chemotherapy.
The proportion of patients who received chemotherapy differed significantly according to age (P<0.001), race (P=0.019), marital status (P<0.001), tumor size (P<0.001), histologic type (P<0.001), pathologic grade (P<0.001), and cancer stage (P<0.001). The majority of patients who received chemotherapy were aged 35 to 69 years old (80.8%), were White (87.8%), had histologic colonic-type or mucinous adenocarcinoma (72.4%), had tumors of grade II or III (58.8%)), and had distant metastasis (54.4%). Table 1 shows the detailed patient information.
Table 1
| Characteristics | All patients, n (%) | Chemotherapy, n (%) | No chemotherapy, n (%) | P value |
|---|---|---|---|---|
| Age at diagnosis, years | <0.001 | |||
| <35 | 392 (13.6) | 31 (3.5) | 361 (18.0) | |
| 35–69 | 1,918 (66.3) | 712 (80.8) | 1,206 (60.6) | |
| >69 | 581 (20.1) | 138 (15.7) | 443 (22.0) | |
| Sex | 0.891 | |||
| Female | 1,563 (54.1) | 478 (54.3) | 1,085 (54.0) | |
| Male | 1,328 (45.9) | 403 (45.7) | 925 (46.0) | |
| Race | 0.019 | |||
| Black | 262 (9.1) | 77 (8.7) | 185 (9.2) | |
| White | 2,379 (82.3) | 729 (82.7) | 1,650 (82.1) | |
| Other | 218 (7.5) | 73 (8.3) | 145 (7.2) | |
| Unknown | 32 (1.1) | 2 (0.2) | 30 (1.5) | |
| Marital status | <0.001 | |||
| Single | 610 (21.1) | 114 (12.9) | 496 (24.7) | |
| Married | 1,661 (57.5) | 595 (67.5) | 1,066 (53.0) | |
| Other | 620 (21.4) | 172 (19.5) | 448 (22.3) | |
| Tumor size, cm | <0.001 | |||
| <3 | 1,356 (46.9) | 209 (23.7) | 1,147 (57.1) | |
| ≥3 | 845 (29.2) | 403 (45.7) | 442 (22.0) | |
| Unknown | 690 (23.9) | 269 (30.5) | 421 (20.9) | |
| Histologic type | <0.001 | |||
| Colonic-type adenocarcinoma | 620 (21.4) | 269 (30.5) | 351 (17.5) | |
| Mucinous adenocarcinoma | 741 (25.6) | 369 (41.9) | 372 (18.5) | |
| Neuroendocrine neoplasm | 126 (4.4) | 8 (0.9) | 118 (5.9) | |
| SRCC | 163 (5.6) | 103 (11.7) | 60 (3.0) | |
| Malignant carcinoid | 1,241 (42.9) | 132 (15.0) | 1,109 (55.2) | |
| Grade | <0.001 | |||
| I | 971 (33.6) | 178 (20.2) | 793 (39.5) | |
| II | 793 (27.4) | 291 (33.0) | 502 (25.0) | |
| III | 376 (13.0) | 227 (25.8) | 149 (7.4) | |
| IV | 57 (2.0) | 40 (4.5) | 17 (0.8) | |
| Unknown | 694 (24.0) | 145 (6.5) | 549 (27.3) | |
| Stage | <0.001 | |||
| Distant | 706 (24.2) | 479 (54.4) | 227 (11.3) | |
| Localized | 1,321 (45.7) | 92 (10.4) | 1,229 (61.1) | |
| Regional | 864 (29.9) | 310 (35.1) | 554 (27.6) |
SRCC, signet ring cell carcinoma.
Survival analysis
The Kaplan-Meier log-rank test indicated that age (Figure 3A), race (Figure 3B), tumor size (Figure 3C), marital status (Figure 3D), grade (Figure 3E), histologic type (Figure 3F), stage (Figure 3G), and chemotherapy status (Figure 3H) were related to OS. Furthermore, the analysis showed that chemotherapy (P=0.055, Figure 3I) failed to extend the survival time of patients with distant metastases. For patients who received chemotherapy, the median OS was 65 months and the 5-year OS rate was 51.9%, which were poorer outcomes than those of patients who did not receive chemotherapy or whose chemotherapy status was unknown (median OS: not reached, 5-year OS: 78.9%). Table 2 shows more detailed survival data.
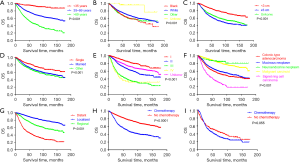
Table 2
| Variable | Median survival (months) | 5-year OS rate (%) |
|---|---|---|
| Age at diagnosis, years | ||
| <35 | – | 94.0 |
| 35–69 | – | 73.2 |
| >69 | 55.0 | 47.5 |
| Race | ||
| Black | 113.0 | 63.6 |
| White | 172.0 | 97.7 |
| Other | – | 63.8 |
| Unknown | – | 96.3 |
| Marital status | ||
| Single | – | 92.2 |
| Married | 167 | 96.9 |
| Other | 140 | 91.1 |
| Tumor size, cm | ||
| <3 | – | 97.2 |
| ≥3 | 88.0 | 93.0 |
| Unknown | 140.0 | 91.6 |
| Histologic type | ||
| Colonic-type adenocarcinoma | 89.0 | 90.8 |
| Mucinous adenocarcinoma | 122 | 92.9 |
| Neuroendocrine neoplasm | – | 84.6 |
| SRCC | 38.0 | 63.2 |
| Malignant carcinoid | – | 96.6 |
| Grade | ||
| I | – | 95.9 |
| II | 125.0 | 93.3 |
| III | 39.0 | 85.1 |
| IV | 32.0 | 34.2 |
| Unknown | – | 74.7 |
| Stage | ||
| Distant | 41.0 | 70.6 |
| Localized | – | 92.9 |
| Regional | 172.0 | 86.5 |
| Chemotherapy | ||
| Yes | 65.0 | 51.9 |
| No/unknown | – | 78.9 |
OS, overall survival; SRCC, signet ring cell carcinoma.
OS and CSS were analyzed using univariate and multivariable Cox proportional-hazards models. Cox regression univariate analysis showed that age, race, marital status, tumor size, histologic type, grade, and stage were all significant risk factors for OS (P≤0.001). Remarkably, treatment with chemotherapy was associated with a worse prognosis. The univariate analysis of CSS produced the same results. In multivariate OS analysis, age, race, histologic type, grade, stage, and chemotherapy status were all independent prognostic indicators. Moreover, these factors were also found to be independent predictors of CSS. Compared with that for Black patients, the risk of death for White patients was decreased by 31.8% (HR =0.682; 95% CI: 0.532–0.874). Also, chemotherapy (HR =1.321; 95% CI: 1.111–1.570) was revealed to be an independent indicator of poor prognosis. More detailed results from the univariate and multivariable analyses are shown in Tables 3,4.
Table 3
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at diagnosis, years | <0.001 | <0.001 | |||
| <35 | Ref | Ref | |||
| 35–69 | 4.569 (3.128–6.674) | <0.001 | 2.147 (1.442–3.197) | <0.001 | |
| >69 | 10.828 (7.368–15.912) | <0.001 | 5.259 (3.485–7.937) | <0.001 | |
| Sex | 0.436 | ||||
| Female | Ref | NI | |||
| Male | 1.051 (0.928–1.190) | 0.436 | |||
| Race | 0.001 | 0.026 | |||
| Black | Ref | Ref | |||
| White | 0.768 (0.626–0.943) | 0.012 | 0.728 (0.590–0.899) | 0.003 | |
| Other | 0.946 (0.710–1.260) | 0.704 | 0.772 (0.576–1.034) | 0.082 | |
| Unknown | 0.132 (0.033–0.536) | 0.005 | 0.460 (0.113–1.881) | 0.280 | |
| Tumor size, cm | <0.001 | 0.180 | |||
| <3 | Ref | Ref | |||
| ≥3 | 2.594 (2.227–3.022) | <0.001 | 1.169 (0.990–1.381) | 0.066 | |
| Unknown | 2.082 (1.770–2.448) | <0.001 | 1.119 (0.941–1.331) | 0.202 | |
| Marital status | <0.001 | 0.491 | |||
| Single | Ref | Ref | |||
| Married | 1.519 (1.267–1.821) | <0.001 | 0.916 (0.757–1.108) | 0.364 | |
| Other | 1.749 (1.424–2.149) | <0.001 | 0.987 (0.796–1.224) | 0.907 | |
| Histologic type | <0.001 | ||||
| Colonic-type adenocarcinoma | Ref | Ref | |||
| Mucinous neoplasm | 0.842 (0.720–0.985) | 0.032 | 0.690 (0.580–0.821) | <0.001 | |
| Neuroendocrine neoplasm | 0.282 (0.178–0.449) | <0.001 | 0.968 (0.595–1.573) | 0.894 | |
| SRCC | 1.976 (1.599–2.443) | <0.001 | 1.109 (0.883–1.393) | 0.372 | |
| Malignant carcinoid | 0.335 (0.282–0.398) | <0.001 | 0.657 (0.536–0.806) | <0.001 | |
| Grade | <0.001 | ||||
| I | Ref | Ref | |||
| II | 2.440 (2.017–2.952) | <0.001 | 1.794 (1.471–2.187) | <0.001 | |
| III | 5.379 (4.408–6.564) | <0.001 | 2.905 (2.318–3.640) | <0.001 | |
| IV | 5.839 (4.082–8.350) | <0.001 | 3.128 (2.159–4.533) | <0.001 | |
| Unknown | 1.720 (1.404–2.107) | <0.001 | 2.136 (1.721–2.652) | <0.001 | |
| Stage | <0.001 | ||||
| Distant | Ref | Ref | |||
| Localized | 0.172 (0.146–0.202) | <0.001 | 0.236 (0.194–0.287) | <0.001 | |
| Regional | 0.390 (0.338–0.451) | <0.001 | 0.425 (0.362–0.499) | <0.001 | |
| Chemotherapy | |||||
| No/unknown | Ref | Ref | |||
| Yes | 2.577 (2.274–2.920) | <0.001 | 1.220 (1.050–1.417) | 0.009 | |
OS, overall survival; HR, hazard ratio; 95% CI, 95% confidence interval; SRCC, signet ring cell carcinoma.
Table 4
| Variable | Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | ||
| Age at diagnosis, years | <0.001 | <0.001 | |||
| <35 | Ref | Ref | |||
| 35–69 | 4.700 (3.094–7.141) | <0.001 | 1.477 (0.952–2.291) | <0.001 | |
| >69 | 9.994 (6.498–15.370) | <0.001 | 3.058 (1.931–4.845) | <0.001 | |
| Sex | |||||
| Female | Ref | NI | |||
| Male | 1.003 (0.867–1.161) | 0.966 | |||
| Race | 0.002 | 0.025 | |||
| Black | Ref | Ref | |||
| White | 0.770 (0.606–0.979) | 0.033 | 0.682 (0.532–0.874) | 0.002 | |
| Other | 1.004 (0.723–1.394) | 0.981 | 0.702 (0.502–0.982) | 0.039 | |
| Unknown | 0.089 (0.012–0.637) | 0.016 | 0.452 (0.064–3.392) | 0.452 | |
| Tumor size, cm | 0.180 | ||||
| <3 | Ref | Ref | |||
| ≥3 | 3.482 (2.891–4.194) | <0.001 | 1.209 (0.991–1.474) | 0.061 | |
| Unknown | 2.766 (2.267–3.375) | <0.001 | 1.179 (0.955–1.454) | 0.126 | |
| Marital status | 0.902 | ||||
| Single | Ref | Ref | |||
| Married | 1.714 (1.382–2.126) | <0.001 | 0.971 (0.774–1.218) | 0.799 | |
| Other | 1.773 (1.383–2.273) | <0.001 | 1.010 (0.779–1.309) | 0.939 | |
| Histologic type | <0.001 | <0.001 | |||
| Colonic-type adenocarcinoma | Ref | Ref | |||
| Mucinous neoplasm | 0.864 (0.724–1.032) | 0.107 | 0.684 (0.563–0.830) | <0.001 | |
| Neuroendocrine neoplasm | 0.179 (0.095–0.338) | <0.001 | 0705 (0.365–1.363) | 0.299 | |
| SRCC | 2.184 (1.732–2.755) | <0.001 | 1.094 (0.854–1.403) | 0.477 | |
| Malignant carcinoid | 0.224 (0.180–0.280) | <0.001 | 0.519 (0.402–0.669) | <0.001 | |
| Grade | <0.001 | ||||
| I | Ref | Ref | |||
| II | 3.181 (2.517–4.020) | <0.001 | 2.213 (1.738–2.818) | <0.001 | |
| III | 7.850 (6.182–9.969) | <0.001 | 3.774 (2.888–4.932) | <0.001 | |
| IV | 8.770 (5.938–12.951) | <0.001 | 3.982 (2.654–5.976) | <0.001 | |
| Unknown | 1.871 (1.444–2.42) | <0.001 | 2.514 (1.912–3.305) | <0.001 | |
| Stage | <0.001 | ||||
| Distant | Ref | Ref | |||
| Localized | 0.078 (0.062–0.099) | <0.001 | 0.132 (0.101–0.172) | <0.001 | |
| Regional | 0.325 (0.276–0.383) | <0.001 | 0.391 (0.327–0.468) | <0.001 | |
| Chemotherapy | |||||
| No/unknown | Ref | Ref | |||
| Yes | 3.912 (3.371–4.541) | <0.001 | 1.321 (1.111–1.570) | 0.002 | |
CSS, cancer-specific survival; HR, hazard ratio; 95% CI, 95% confidence interval; SRCC, signet ring cell carcinoma.
Discussion
Primary appendiceal cancer often presents with symptoms similar to acute appendicitis, and postoperative pathology is the gold-standard test for its diagnostic confirmation (16-18). However, the prognosis of patients with primary appendiceal cancer after surgery and whether chemotherapy is required in these patients are unknown. Furthermore, as a rare tumor, primary appendiceal cancer can be more accurately assessed using a large database than by conducting a single-cohort study. Therefore, we aimed to identify factors that influence the postoperative prognosis of patients with primary appendiceal cancer using the SEER database.
One earlier study that used data from 2000 to 2009 reported that the incidence of primary appendiceal cancer was increasing annually, with the most common histologic type being mucinous adenocarcinoma (1). The present work similarly found that the incidence of primary appendiceal cancer continued to increase in the period under study, with the overall age-adjusted incidence rate per 100,000 population steadily increasing from 0.58 to 1.63 between 2004 and 2015. However, unlike the earlier study, we found that malignant carcinoid was the most common histologic type. Research has shown that the incidence of primary appendiceal cancer is higher in older people than in younger people (19). Consistent with this, our study found that patients aged 69 and over had the highest risk of death among all the age groups in the analysis. We also found that the risk of death was higher for Black patients than for White patients. In a previous study, McCusker et al. reported that the OS rate was significantly worse in patients with SRCC than in patients with other tumor types (15). Lowly differentiated adenocarcinoma and SRCC histopathology are negative prognostic indicators (20-23). In line with this, in our analysis, patients with low-grade tumors (grade 1 or 2), who accounted for 80.3% of patients whose histological grade was known, had a favorable prognosis.
In our study, the death risk was highest in patients with SRCC (HR =1.976; 95% CI: 1.599–2.443). Among patients who undergo colon cancer surgery, those with SRCC have a roughly 50% increased risk of death compared to those with classical adenocarcinoma (24).
For surgically resectable appendiceal cancer, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy are widely accepted treatments (25). In terms of 5-year recurrence-free survival and 5-year OS, laparoscopic surgery is comparable to open surgery and can be considered as a treatment option for primary appendiceal cancer (26). Appendectomy alone offers a survival advantage over colectomy for patients with carcinoid tumors (27). Anti-vascular endothelial growth factor (anti-VEGF) agents should be seriously considered in the treatment of individuals with higher-grade appendiceal tumors who are not potential candidates for curative surgical resection (28). Previous studies have shown that chemotherapy benefits patients with appendiceal cancer who have high-grade or poorly differentiated tumors (29,30). However, there was no significant benefit from chemotherapy in analysis of all the patients included in this study. Furthermore, chemotherapy did not have a prolonged survival effect for patients with distant metastases. This observation was likely due to patients with “no chemotherapy” and “unknown” chemotherapy status being grouped together in the SEER database, which might have led to some deviation.
The inherent limitations of using data obtained from the SEER database were also limitations of this study. The SEER database does not provide detailed chemotherapy information such as drugs, dose, and duration; therefore, it is unclear whether the chemotherapy received by patients was given before, during, or after their surgery. Moreover, information regarding underlying diseases, genomics, postoperative complications and chemotherapy side effects is not available from the database.
Many clinical trials related to immunotherapy and targeted therapy in gastric and colorectal cancers have been conducted, and significant progress has been made in these areas. For example, the phase III ToGA (Trastuzumab for Gastric Cancer) trial found that the median OS of patients with HER2-positive locally advanced, recurrent, or metastatic gastric or gastroesophageal junction adenocarcinoma is improved by the addition of trastuzumab to first-line chemotherapy (31). At the 2020 ASCO Gastrointestinal Cancers Symposium, Boku et al. reported that the 3-year OS rate was higher in the nivolumab group than in the placebo group (5.6% vs. 1.9%) (32). As a result, nivolumab was approved in Japan as a third-line treatment for gastric cancer. As early as 2004, the United States Food and Drug Administration approved cetuximab, a monoclonal antibody targeted to epidermal growth factor receptor (EGFR), and bevacizumab, a humanized IgG monoclonal antibody targeted to VEGF-A, for the treatment of metastatic colorectal cancer (33,34). However, at present, the efficacy of these targeted agents against appendiceal tumors is unknown, and there is a lack of evidence from large clinical trials. Therefore, large-scale clinical trials for appendiceal cancer are required immediately.
Conclusions
Although primary appendiceal cancer is rare, an increasing trend in the incidence of appendiceal cancer occurred from 2004 to 2015. Age, race, tumor size, marital status, histologic type, grade, stage, and treatment with chemotherapy were found to significantly affect the postoperative prognosis of patients with primary appendiceal cancer. However, chemotherapy failed to prolong survival after surgery for patients with distant metastases. Although this was only a retrospective study and our findings are not conclusive, our results still provide valuable insight into the diagnosis of primary appendiceal cancer. In the immediate future, large clinical trials of chemotherapy and targeted therapy for appendiceal cancer are urgently needed.
Acknowledgments
Funding: This study was supported by the National Natural Science Foundation of China (Grant No. 82002479).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-454/rc
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-454/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Marmor S, Portschy PR, Tuttle TM, et al. The rise in appendiceal cancer incidence: 2000-2009. J Gastrointest Surg 2015;19:743-50. [Crossref] [PubMed]
- Singh H, Koomson AS, Decker KM, et al. Continued increasing incidence of malignant appendiceal tumors in Canada and the United States: A population-based study. Cancer 2020;126:2206-16. [Crossref] [PubMed]
- Roncati L, Gasparri P, Gallo G, et al. Appendix Tumor Microenvironment. Adv Exp Med Biol 2020;1226:87-95. [Crossref] [PubMed]
- Yao JC, Hassan M, Phan A, et al. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol 2008;26:3063-72. [Crossref] [PubMed]
- Wu YC, Wen L, Dou WD, et al. Clinicopathological analysis and surgical strategy of primary appendiceal neoplasms. Zhonghua Wei Chang Wai Ke Za Zhi 2021;24:1065-72. [PubMed]
- Connor SJ, Hanna GB, Frizelle FA. Appendiceal tumors: retrospective clinicopathologic analysis of appendiceal tumors from 7,970 appendectomies. Dis Colon Rectum 1998;41:75-80. [Crossref] [PubMed]
- Gehrig PA, Boggess JF, Ollila DW, et al. Appendix cancer mimicking ovarian cancer. Int J Gynecol Cancer 2002;12:768-72. [Crossref] [PubMed]
- Whitfield CG, Amin SN, Garner JP. Surgical management of primary appendiceal malignancy. Colorectal Dis 2012;14:1507-11. [Crossref] [PubMed]
- Izzo P, De Santis A, Pugliese F, et al. Cancers of the appendix. Case report and literatures review. Ann Ital Chir 2017;6. [PubMed]
- Wu H, Chintagumpala M, Hicks J, et al. Neuroendocrine Tumor of the Appendix in Children. J Pediatr Hematol Oncol 2017;39:97-102. [Crossref] [PubMed]
- Villanueva Saenz E, Perez-Aguirre J, Belmonte MC, et al. Appendix adenocarcinoma associated with ulcerative colitis: a case report and literature review. Tech Coloproctol 2006;10:54-6. [Crossref] [PubMed]
- Freeman HJ. Duplicated appendix complicated by appendiceal cancer. World J Gastroenterol 2011;17:135-6. [Crossref] [PubMed]
- Li X, Zhou J, Dong M, et al. Management and prognosis of low-grade appendiceal mucinous neoplasms: A clinicopathologic analysis of 50 cases. Eur J Surg Oncol 2018;44:1640-5. [Crossref] [PubMed]
- Zhang W, Tan C, Xu M, et al. Primary appendiceal mucinous neoplasm: Gynecological manifestations, management, and prognosis. Gynecol Oncol 2020;156:357-62. [Crossref] [PubMed]
- McCusker ME, Cote TR, Clegg LX, et al. Primary malignant neoplasms of the appendix: a population-based study from the surveillance, epidemiology and end-results program, 1973-1998. Cancer 2002;94:3307-12. [Crossref] [PubMed]
- Talan DA. Cancer of the appendix and nonoperative treatment of appendicitis shared decision making. J Surg Oncol 2019;120:1060-1. [Crossref] [PubMed]
- Seawell J, Sciarretta JD, Pahlkotter M, et al. The Understated Malignancy Potential of Nonoperative Acute Appendicitis. Am Surg 2019;85:712-6. [Crossref] [PubMed]
- Mallinen J, Rautio T, Gronroos J, et al. Risk of Appendiceal Neoplasm in Periappendicular Abscess in Patients Treated With Interval Appendectomy vs Follow-up With Magnetic Resonance Imaging: 1-Year Outcomes of the Peri-Appendicitis Acuta Randomized Clinical Trial. JAMA Surg 2019;154:200-7. [Crossref] [PubMed]
- Skendelas JP, Alemany VS, Au V, et al. Appendiceal adenocarcinoma found by surgery for acute appendicitis is associated with older age. BMC Surg 2021;21:228. [Crossref] [PubMed]
- Glehen O, Mohamed F, Sugarbaker PH. Incomplete cytoreduction in 174 patients with peritoneal carcinomatosis from appendiceal malignancy. Ann Surg 2004;240:278-85. [Crossref] [PubMed]
- Shapiro JF, Chase JL, Wolff RA, et al. Modern systemic chemotherapy in surgically unresectable neoplasms of appendiceal origin: a single-institution experience. Cancer 2010;116:316-22. [Crossref] [PubMed]
- Lieu CH, Lambert LA, Wolff RA, et al. Systemic chemotherapy and surgical cytoreduction for poorly differentiated and signet ring cell adenocarcinomas of the appendix. Ann Oncol 2012;23:652-8. [Crossref] [PubMed]
- Stewart JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol 2006;13:624-34. [Crossref] [PubMed]
- Bagante F, Spolverato G, Beal E, et al. Impact of histological subtype on the prognosis of patients undergoing surgery for colon cancer. J Surg Oncol 2018;117:1355-63. [Crossref] [PubMed]
- Milovanov V, Sardi A, Ledakis P, et al. Systemic chemotherapy (SC) before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) in patients with peritoneal mucinous carcinomatosis of appendiceal origin (PMCA). Eur J Surg Oncol 2015;41:707-12. [Crossref] [PubMed]
- Inoue A, Murata K, Komori T, et al. Open versus laparoscopic surgery for primary appendiceal tumors: a large multicenter retrospective propensity score-matched cohort study in Japan. Surg Endosc 2021;35:5515-23. [Crossref] [PubMed]
- Guzman C, Boddhula S, Panneerselvam N, et al. Appendiceal Carcinoid Tumors: Is There a Survival Advantage to Colectomy over Appendectomy? J Gastrointest Surg 2020;24:1149-57. [Crossref] [PubMed]
- Choe JH, Overman MJ, Fournier KF, et al. Improved Survival with Anti-VEGF Therapy in the Treatment of Unresectable Appendiceal Epithelial Neoplasms. Ann Surg Oncol 2015;22:2578-84. [Crossref] [PubMed]
- Kolla BC, Petersen A, Chengappa M, et al. Impact of adjuvant chemotherapy on outcomes in appendiceal cancer. Cancer Med 2020;9:3400-6. [Crossref] [PubMed]
- Wang G, Li Q, Chen W. Chemotherapy in the treatment of different histological types of appendiceal cancers: a SEER based study. BMC Cancer 2021;21:778. [Crossref] [PubMed]
- Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 2010;376:687-97. [Crossref] [PubMed]
- Boku N, Satoh T, Ryu MH, et al. Nivolumab in previously treated advanced gastric cancer (ATTRACTION-2): 3-year update and outcome of treatment beyond progression with nivolumab. Gastric Cancer 2021;24:946-58. [Crossref] [PubMed]
- Cunningham D, Humblet Y, Siena S, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med 2004;351:337-45. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)




