
Antidiabetic treatment improves prognosis after radical resection in hepatocellular carcinoma patients with diabetes mellitus: a retrospective cohort study from 2000 to 2013
Introduction
Hepatocellular carcinoma (HCC) remains a major challenge in the field of liver disease worldwide (1). Liver resection is the standard treatment for early-stage liver cancer, with a survival rate of 60–80% at 5 years (2,3). However, only 20–30% of patients present with a resectable HCC at diagnosis. Many HCC patients are complicated with chronic hepatitis and liver cirrhosis, with the latter associated with a higher risk of developing HCC (4,5). Cirrhosis has also been closely associated with diabetes mellitus (DM) due to the disorder of glucose metabolism in the impaired liver (5-7). Clinically, there is a substantial proportion of patients with HCC who also have DM (8-10). Indeed, many epidemiological studies have suggested that DM may also increase the risk of cancer, especially HCC (11,12). DM is also proved as a independent prognostic factor in HCC patients (13).
Studies have suggested that insulin use may increase the risk of HCC, worsen the outcome of cirrhosis or HCC patients, and diabetic patients treated with metformin demonstrated a reduced risk of HCC (11,14-19). Some studies found that metformin could prolong the survival of HCC patients and reduce the tumor recurrence after hepatectomy (13,20), but others failed (19,21-24). There is still not enough evidence to prove whether metformin had priority over insulin or other antidiabetic drugs on treating DM in HCC patients. Therefore, this investigation examined prognosis after liver resection in HCC patients with DM who were treated with metformin, insulin or other medications. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-478/rc).
Methods
Patients who were diagnosed with HCC and DM from January 2000 to December 2013 were enrolled in this study. Patients who accepted radical liver resection were divided into two groups depending on whether they received antidiabetic treatment. The definitive diagnosis of HCC and DM followed the criteria of the European Association for the Study of the Liver (25) and the American Diabetes Association, respectively (26). The inclusion criteria for the cohort were as follows: (I) patients aged between 18 and 80 years old; (II) patients with a pathological diagnosis of HCC; (III) patients had not received any previous treatment for cancer; (IV) patients accepted radical liver resection; and (V) patients showed Child-Pugh A–B liver function. The following exclusion criteria were applied: (I) any co-existing malignancies; (II) patients from abroad who were unable to be followed-up regularly; (III) severe coagulability; and (IV) concomitant severe heart, lung, or kidney diseases. Patients who satisified the inclusion and exclusion criteria were all included in the cohort to reduce selection bias. The cohort size was designed to be as large as possible since this was an exploratory study. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Eastern Hepatobiliary Surgery Hospital (No. EHBHKY2022-K-022). Individual consent for this retrospective analysis was waived.
Hepatectomy
Hepatectomy was performed under general anesthesia with a right subcostal incision. A surgical margin of at least 1 cm was preferred when resecting the tumor, if possible. When the tumor was adjacent to major vessels, a 1 cm surgical margin could not be achieved, and the tumors was resected with a margin that was as wide as possible to avoid tumor residue. For multiple tumors, either a single liver resection or multiple resection was performed according to the tumor location. Pringle’s maneuver was routinely performed using a clamp/unclamp cycle of 15 minutes/5 minutes. Liver transection was performed using an ultrasonic scalpel or the clamp crushing method.
Follow-up
At 4 weeks after partial hepatectomy, patients were assessed by three-phase computed tomography (CT) or magnetic resonance imaging (MRI), liver function tests, and alpha fetoprotein (AFP) levels to exclude tumor residue. Patients were then followed-up every month in the first year, and thereafter, every 3 months to observe the tumor recurrence and metastasis. At each follow-up visit, ultrasound of the liver, liver function tests, and AFP levels were routinely performed. Chest X-ray and three-phase CT/MRI were performed every 3–6 months. Patients were recommended to an endocrinologist for monitoring and DM treatment. Fasting and postprandial blood glucose were checked every month and glycosylated hemoglobin was examined every 3–6 months. For patients with detectable level of hepatitis B virus DNA, a nucleoside analogue was recommended for anti-viral treatment. The diagnosis of HCC recurrence was in accordance with the criteria of the European Association for the Study of the Liver. Patients with tumor recurrence were actively treated with liver resection, transplantation, TACE, RFA, etc., depending on the tumor, liver function, and general condition of the patient.
Statistical analysis
Overall survival (OS) and disease-free survival (DFS) were calculated from the date the patient received liver resection to the date when patients died, experienced tumor recurrence, or the last follow-up. The last follow-up of the study was on 10th January 2014 or the date when the patients died. Patients who were lost to follow-up were censored from the last follow-up.
All data were entered into a computer and analyzed by SPSS 22.0 statistic software. Data was presented as median (range) for quantitative variables and absolute frequencies for qualitative variables. A cutoff value of 20 ng/mL serum AFP was applied. Comparison of data between the two groups was performed using the t test or Mann-Whitney U test for continuous data and χ2 test for categorical data. Propensity score matching wes performed to reduce the bias in the baseline data between the antidiabetic treatment group and no antidiabetic treatment group. A 1:1 matched analysis was done between two groups with a caliper width 0.1.
The OS and DFS rates were calculated using the life-table method. Comparisons of OS and DFS between the two groups and the survival curves were constructed using the Kaplan-Merier method. Multivariate Cox proportional hazard regression analysis was used to estimate prognostic factors which influence the OS and DFS, and all variables with a P value <0.1 by univariate comparison were subjected to the multivariate analysis. All tests were 2-sided and P<0.05 was considered statistically significant.
Results
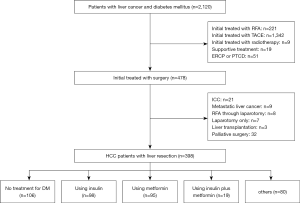
A total of 2,120 patients with liver cancer and DM were hospitalized in the Eastern Hepatobiliary Surgery Hospital from January 2000 to December 2013, of which, 478 patients received surgical treatment. Among the 478 patients, 398 patients were diagnosed with HCC and DM. Of the 398 patients with HCC and DM, 106 did not receive any treatment for diabetes and 292 received antidiabetic therapy, including 98 patients who received insulin alone, 95 who received metformin alone, 19 who received insulin plus metformin, and 80 who were given other drugs. The flowchart of the study is shown in Figure 1. The follow-up period ranged from 0 to 152 months, with a median of 42.5 months. The postoperative complication rate was 11.6% (46/398) and the 30-day perioperative mortality was 1.8% (7/398).
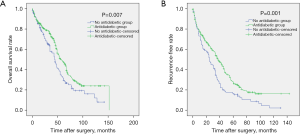
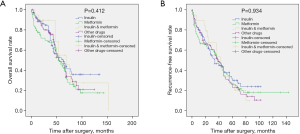
In the unmatched total cohort, the clinical data of the patients in the antidiabetic treatment group and the no treatment group are shown in Table 1. There was no significant difference between the 2 groups except for age, gender, alanine aminotransferase (ALT), aspartate aminotransferase (AST), serum fasting glucose, and tumor number. Patients in the antidiabetic treatment group were older (P<0.001), tended to be female (P=0.027), had lower ALT (P=0.001) and AST (P=0.045) levels, lower serum glucose levels (P=0.017), and more number of tumors (P=0.044). During the follow-up, 293 patients (73.6%) experienced tumor recurrence, including 201 patients in the antidiabetic treatment group and 92 patients in the no antidiabetic treatment group. A total of 231 patients (58.0%) died from disease progression during the course of this study. Survival analysis revealed that the 1-, 3-, 5-, and 7-year OS rates for the antidiabetic treatment group were 87.5%, 75.5%, 48.7%, and 29.1%, respectively, and for the no antidiabetic treatment group, the OS rates were 85.4%, 57.7%, 33.6%, and 19.1%, respectively (P=0.007, Figure 2A). The 1-, 3-, 5-, and 7-year recurrence-free survival (RFS) rates for the antidiabetic treatment group were 76.4%, 53.5%, 28.5%, and 17.5%, respectively and for the no antidiabetic treatment group, the RFS rates were 69.5%, 32.5%, 16.5%, and 10.7%, respectively (P=0.001, Figure 2B). Subgroup analysis demonstrated that there was no significant difference in OS (P=0.412, Figure 3A) and RFS (P=0.934, Figure 3B) among patients in the insulin group, metformin group, insulin plus metformin group, and other drugs group.
Table 1
| Parameter | Before propensity matching (n=398) | After propensity matching (n=200) | |||||
|---|---|---|---|---|---|---|---|
| Antidiabetic treatment group (n=292) | Control group (n=106) | P value | Antidiabetic treatment group (n=100) | Control group (n=100) | P value | ||
| Age (year) | 57.4±9.5 | 52.6±9.4 | <0.001 | 53.0±9.2 | 53.2±9.2 | 0.902 | |
| Gender (male/female) | 256/36 | 101/5 | 0.027 | 94/6 | 95/5 | 0.733 | |
| Platelet (×109/L) | 146.0±65.7 | 141.2±60.0 | 0.507 | 136.5±55.7 | 141.4±61.0 | 0.552 | |
| PT (s) | 12.3±1.1 | 12.5±1.3 | 0.097 | 12.3±1.1 | 12.5±1.2 | 0.224 | |
| ALT (IU/L) | 37.1 (9.5–450.8)# | 47.1 (15.0–359.4)# | 0.001‡ | 44.5 (9.5–450.8)# | 46.6 (15.0–359.4)# | 0.243‡ | |
| AST (IU/L) | 33.5 (11.1–533.9)# | 36.8 (12.5–248.0)# | 0.045‡ | 34.4 (11.1–533.9)# | 36.2 (12.5–209.6)# | 0.626‡ | |
| Albumin (g/L) | 41.7±4.1 | 42.2±3.7 | 0.262 | 41.8±4.1 | 42.3±3.7 | 0.331 | |
| TB (umol/L) | 14.3 (5.3–497.1)# | 15.8 (5.0–245.5)# | 0.094‡ | 15.1 (5.3–93.5)# | 15.8 (5.0–245.5)# | 0.471‡ | |
| AFP (≤20/>20 ng/mL) | 145/147 | 42/64 | 0.076 | 50/50 | 42/58 | 0.256 | |
| HBeAg (+/−) | 45/247 | 19/87 | 0.546 | 17/83 | 17/83 | 1 | |
| HBsAg (+/−) | 176/116 | 74/32 | 0.082 | 69/31 | 69/31 | 1 | |
| HCV (+/−) | 17/275 | 4/102 | 0.419 | 7/93 | 4/96 | 0.352 | |
| Serum glucose (mmol/L) | 8.2±3.1 | 9.1±3.5 | 0.017 | 8.6±3.3 | 9.0±3.6 | 0.42 | |
| Tumor size (cm) | 5.6±3.5 | 5.8±3.5 | 0.511 | 5.9±4.0 | 5.6±3.3 | 0.505 | |
| Tumor number (single/multiple) | 246/46 | 80/26 | 0.044 | 80/20 | 77/23 | 0.606 | |
| Vascular invasion (yes/no) | 20/272 | 7/99 | 0.931 | 6/94 | 5/95 | 0.756 | |
| Satellite tumors (yes/no) | 141/151 | 62/44 | 0.072 | 53/47 | 56/44 | 0.67 | |
| Edmondson classification (I–II/III–IV) | 62/230 | 17/89 | 0.251 | 21/79 | 17/83 | 0.471 | |
| BMI | 24.6±2.8 | 24.4±3.0 | 0.424 | 23.9±2.3 | 24.4±3.0 | 0.217 | |
| Liver cirrhosis (yes/no) | 151/141 | 61/45 | 0.302 | 61/39 | 55/45 | 0.39 | |
| Clavien-Dindo classification (0/I/II/IIIa/IIIa/IVa/IVb/V) | 262/4/4/9/2/5/1/5 | 89/6/3/5/0/1/0/2 | 0.184* | 88/4/3/1/2/1/0/1 | 87/5/3/2/0/1/0/2 | 0.831* | |
#, median (range); ‡, Mann-Whitney U test; *, 0 versus others. PT, prothrombin time; ALT, alanine aminotransferase; AST, asprine aminotransferase; TB, total bilirubin; AFP, alpha-fetoprotein; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; HCV, hepatitis C virus; BMI, body mass index.
Multivariate Cox proportional hazard regression analysis revealed that prothrombin time (PT) [hazard ratio (HR): 1.115, P=0.047], tumor size (HR: 1.059, P<0.001), tumor number (HR: 1.626, P=0.002), vascular invasion (HR: 2.074, P=0.003), satellite tumors (HR: 1.592, P=0.001), Edmondson classification (HR: 1.468, P=0.027), and antidiabetic treatment (HR: 0.722, P=0.014) were independent prognostic factors of RFS (Table 2). While tumor size (HR: 1.048, P=0.011), tumor number (HR: 1.779, P=0.001), vascular invasion (HR: 2.545, P<0.001), Edmondson classification (HR: 1.596, P=0.024), and antidiabetic treatment (HR: 0.713, P=0.021) were independent prognostic factors of OS (Table 3).
Table 2
| Variables | Univariate analysis, P value | Multivariate analysis | |
|---|---|---|---|
| Hazard ratio (95% CI) | P value | ||
| Age (year) | 0.023 | 0.993 (0.980–1.006) | 0.299 |
| Gender (male/female) | 0.184 | ||
| PT (s) | 0.010 | 1.115 (1.001–1.241) | 0.047 |
| ALT (IU/L) | 0.316 | ||
| AST (IU/L) | 0.373 | ||
| Albumin (g/L) | 0.120 | ||
| TB (mmol/L) | 0.077 | 1.003 (0.999–1.007) | 0.146 |
| AFP (≤20/>20 ng/mL) | <0.001 | 1.126 (0.866–1.465) | 0.376 |
| HBeAg (+/−) | 0.834 | ||
| HBsAg | 0.129 | ||
| BMI | 0.184 | ||
| Tumor size (cm) | <0.001 | 1.059 (1.027–1.091) | <0.001 |
| Tumor number (single/multiple) | <0.001 | 1.626 (1.197–2.210) | 0.002 |
| Vascular invasion (yes/no) | <0.001 | 2.074 (1.288–3.341) | 0.003 |
| Satellite tumors (yes/no) | <0.001 | 1.592 (1.221–2.077) | 0.001 |
| Edmondson classification (I–II/III–IV) | <0.001 | 1.468 (1.046–2.061) | 0.027 |
| Serum glucose | 0.157 | ||
| Platelet | 0.670 | ||
| HCV (+/−) | 0.539 | ||
| Antidiabetic treatment (no/yes) | 0.001 | 0.722 (0.557–0.937) | 0.014 |
| Liver cirrhosis (yes/no) | 0.009 | 0.971 (0.745–1.265) | 0.828 |
PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; AFP, alpha-fetoprotein; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; BMI, body mass index; HCV, hepatitis C virus.
Table 3
| Variables | Univariate analysis, P value | Multivariate analysis | |
|---|---|---|---|
| Hazard ratio (95% CI) | P value | ||
| Age (year) | 0.751 | ||
| Gender (male/female) | 0.635 | ||
| PT (s) | 0.037 | 1.057 (0.931–1.200) | 0.393 |
| ALT (IU/L) | 0.776 | ||
| AST (IU/L) | 0.110 | ||
| Albumin (g/L) | 0.094 | 0.986 (0.950–1.023) | 0.454 |
| TB (mmol/L) | 0.111 | ||
| AFP (≤ 20/>20 ng/mL) | <0.001 | 1.214 (0.907–1.625) | 0.193 |
| HBeAg (+/−) | 0.266 | ||
| HBsAg | 0.427 | ||
| BMI | 0.115 | ||
| Tumor size (cm) | <0.001 | 1.048 (1.011–1.086) | 0.011 |
| Tumor number (single/multiple) | <0.001 | 1.779 (1.263–2.505) | 0.001 |
| Vascular invasion (yes/no) | <0.001 | 2.545 (1.527–4.241) | <0.001 |
| Satellite tumors (yes/no) | <0.001 | 1.318 (0.979–1.773) | 0.069 |
| Edmondson classification (I–II/III–IV) | <0.001 | 1.596 (1.062–2.398) | 0.024 |
| Serum glucose | 0.727 | ||
| Platelet | 0.798 | ||
| HCV (+/−) | 0.708 | ||
| Antidiabetic treatment (no/yes) | 0.008 | 0.713 (0.535–0.951) | 0.021 |
| Liver cirrhosis (yes/no) | 0.006 | 1.141 (0.857–1.520) | 0.367 |
PT, prothrombin time; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TB, total bilirubin; AFP, alpha-fetoprotein; HBeAg, hepatitis B e antigen; HBsAg, hepatitis B surface antigen; BMI, body mass index; HCV, hepatitis C virus.
After propensity score-matching, a cohort including antidiabetic treatment group(n=100) and no antidiabetic treatment group (n=100) was established. There was no significant difference on baseline data between the 2 groups (Table 1). Survival analysis revealed that the 1-, 3-, 5-, and 7-year OS rates for the antidiabetic treatment group were 82.7%, 65.6%, 46.4%, and 33.6%, respectively, and for the no antidiabetic treatment group, the OS rates were 80.1%, 58.7%, 29.2%, and 20.1%, respectively (P=0.02). The 1-, 3-, 5-, and 7-year RFS rates for the antidiabetic treatment group were 66.1%, 48.1%, 23.8%, and 17.0%, respectively and for the no antidiabetic treatment group, the RFS rates were 61.6%, 23.2%, 16.2%, and 9.4%, respectively (P=0.02). Multivariate Cox proportional hazard regression analysis revealed that prothrombin time (PT) [hazard ratio (HR): 1.162, P=0.036], tumor size (HR: 1.098, P<0.001), tumor number (HR: 1.898, P=0.001), vascular invasion (HR: 3.209, P=0.001), satellite tumors (HR: 1.666, P=0.005) and antidiabetic treatment (HR: 0.669, P=0.015) were independent prognostic factors of RFS. While tumor size (HR: 1.096, P<0.001), tumor number (HR: 2.498, P<0.001), vascular invasion (HR: 6.284, P<0.001), liver cirrhosis (HR: 1.537, P=0.032), and antidiabetic treatment (HR: 0.563, P=0.002) were independent prognostic factors of OS.
Discussion
DM is considered one of the contributing factors in the oncogenesis of liver cancer (27). Several epidemiological studies have shown that the risk of liver cancer is higher in patients with DM compared to patients with no diabetes (28-30). Furthermore, many recent studies have found that DM can affect the prognosis of patients with HCC after hepatectomy (10,31,32). However, it remains to be elucidated whether treatment of diabetes can effectively improve the outcome of HCC patients with DM after liver resection. This study examined the association between treatment of diabetes and prognosis of HCC after liver resection, and investigated the prognostic effect of different antidiabetic drugs in HCC patients.
This study compared the RFS and OS of HCC patients receiving antidiabetic therapy and those with no antidiabetic treatment. The results showed that HCC patients with antidiabetic treatment obtained a better RFS and OS than those without antidiabetic treatment. Patients in the antidiabetic treatment group had lower fasting blood glucose, ALT, and AST levels, suggesting that patients who received antidiabetic treatment had a reduced inflammatory reaction when the blood glucose levels were controlled. Hyperglycemia has been shown to be positively associated with the risk of HCC (33), and it is also a significant risk factor in the prognosis of HCC patients after curative therapy (34). These studies indicated that better control of blood glucose levels may improve the prognosis of HCC and poor postoperative blood glucose control is an independent factor that negatively affects recurrence and survival. Thus, antidiabetic treatment may have a role in protecting the liver and improving the long-term survival of HCC patients by controlling blood glucose levels.
Several studies have reported that treatment with insulin is associated with poorer outcomes in patients with cirrhosis and an increased risk of HCC (14,19). While some studies have shown that metformin can inhibit the carcinogenesis of HCC and prolong the survival of HCC patients with DM (20,35,36), this remains controverisal (13,21). Subgroup analysis in our study failed to show that insulin nor metformin affects RFS or OS in HCC patients after hepatic resection. Several reasons may explain this observation. First, medication for DM patients is often combined and changeable, and therefore, patients cannot be precisely assigned to a metformin group or an insulin group. Second, most studies are retrospective with a limited sample size. Third, the control of blood glucose after antidiabetic medication is unknow in most studies, which may affect the prognosis of HCC.
There were some limitations to this study. First, it is a retrospective study which was conducted in a single institution and it may not be possible to generalize the results. Second, most patients in the study were HBV infected, only a few were HCV infected. It is therefore unclear whether these results apply to patients with other etiological factors. Third, there were differences in certain parameters between the antidiabetic treatment group and the no antidiabetic treatment group, and this may compromise the validity of the results. Future large-scale, multicentered, randomized, controlled trials are warranted to further validate these results.
In conclusion, antidiabetic treatment could provide a better outcome in terms of both RFS and OS after hepatic resection. There was no significant difference among the different types of antidiabetic therapies on the prognosis of HCC patients. Therefore, for HCC patients with DM, antidiabetic treatment should be recommended aggressively, regardless of which antidiabetic drugs are used.
Acknowledgments
Funding: The study was supported by Science Fund for Creative Research Groups, NSFC, China (Grant No. 81221061); National Natural Science Foundation of China (Grant No. 81471038, 81472285, and 31201025); Shanghai Health and Family Planning Commission Research Project Youth Project (Grant No. 20154Y0083) and Medical innovation research project from Shanghai Science and Technology Committee (Grant No. 20Y11905200).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-478/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-478/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-478/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by Ethics Committee of Eastern Hepatobiliary Surgery Hospital (No. EHBHKY2022-K-022). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin 2022;72:7-33. [Crossref] [PubMed]
- Zheng L, Zhang CH, Lin JY, et al. Comparative Effectiveness of Radiofrequency Ablation vs. Surgical Resection for Patients With Solitary Hepatocellular Carcinoma Smaller Than 5 cm. Front Oncol 2020;10:399. [Crossref] [PubMed]
- Ng KKC, Chok KSH, Chan ACY, et al. Randomized clinical trial of hepatic resection versus radiofrequency ablation for early-stage hepatocellular carcinoma. Br J Surg 2017;104:1775-84. [Crossref] [PubMed]
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med 2011;365:1118-27. [Crossref] [PubMed]
- Fattovich G, Stroffolini T, Zagni I, et al. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology 2004;127:S35-50. [Crossref] [PubMed]
- Wlazlo N, Beijers HJ, Schoon EJ, et al. High prevalence of diabetes mellitus in patients with liver cirrhosis. Diabet Med 2010;27:1308-11. [Crossref] [PubMed]
- Nielsen MF, Caumo A, Aagaard NK, et al. Contribution of defects in glucose uptake to carbohydrate intolerance in liver cirrhosis: assessment during physiological glucose and insulin concentrations. Am J Physiol Gastrointest Liver Physiol 2005;288:G1135-43. [Crossref] [PubMed]
- Poon RT, Fan ST, Wong J. Does diabetes mellitus influence the perioperative outcome or long term prognosis after resection of hepatocellular carcinoma? Am J Gastroenterol 2002;97:1480-8. [Crossref] [PubMed]
- Toyoda H, Kumada T, Nakano S, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma. Cancer 2001;91:957-63. [Crossref] [PubMed]
- Wang YY, Huang S, Zhong JH, et al. Impact of diabetes mellitus on the prognosis of patients with hepatocellular carcinoma after curative hepatectomy. PLoS One 2014;9:e113858. [Crossref] [PubMed]
- Kasmari AJ, Welch A, Liu G, et al. Independent of Cirrhosis, Hepatocellular Carcinoma Risk Is Increased with Diabetes and Metabolic Syndrome. Am J Med 2017;130:746.e1-7. [Crossref] [PubMed]
- He S, Wang J, Shen X, et al. Cancer and its predictors in Chinese adults with newly diagnosed diabetes and impaired glucose tolerance (IGT): a 30-year follow-up of the Da Qing IGT and Diabetes Study. Br J Cancer 2022; Epub ahead of print. [Crossref] [PubMed]
- Cho WR, Wang CC, Tsai MY, et al. Impact of metformin use on the recurrence of hepatocellular carcinoma after initial liver resection in diabetic patients. PLoS One 2021;16:e0247231. [Crossref] [PubMed]
- Liu J, Zhi Q, Liu Y, et al. Insulin promotes hepatocarcinoma tumorigenesis by up-regulating PKM2 expression. Exp Cell Res 2021;408:112872. [Crossref] [PubMed]
- Donadon V, Balbi M, Mas MD, et al. Metformin and reduced risk of hepatocellular carcinoma in diabetic patients with chronic liver disease. Liver Int 2010;30:750-8. [Crossref] [PubMed]
- Chen HP, Shieh JJ, Chang CC, et al. Metformin decreases hepatocellular carcinoma risk in a dose-dependent manner: population-based and in vitro studies. Gut 2013;62:606-15. [Crossref] [PubMed]
- Gupta SP, Mittal A, Sathian B, et al. Elevated serum insulin is an independent risk factor for hepatocellular carcinoma: a case control study from Nepal. Asian Pac J Cancer Prev 2013;14:7331-3. [Crossref] [PubMed]
- Chao LT, Wu CF, Sung FY, et al. Insulin, glucose and hepatocellular carcinoma risk in male hepatitis B carriers: results from 17-year follow-up of a population-based cohort. Carcinogenesis 2011;32:876-81. [Crossref] [PubMed]
- Yen FS, Lai JN, Wei JC, et al. Is insulin the preferred treatment in persons with type 2 diabetes and liver cirrhosis? BMC Gastroenterol 2021;21:263. [Crossref] [PubMed]
- Zhou J, Ke Y, Lei X, et al. Meta-analysis: The efficacy of metformin and other anti-hyperglycemic agents in prolonging the survival of hepatocellular carcinoma patients with type 2 diabetes. Ann Hepatol 2020;19:320-8. [Crossref] [PubMed]
- Chung YK, Hwang S, Song GW, et al. Absence of antitumor effects of metformin in sorafenib-treated patients with hepatocellular carcinoma recurrence after hepatic resection and liver transplantation. Ann Hepatobiliary Pancreat Surg 2018;22:297-304. [Crossref] [PubMed]
- Casadei Gardini A, Faloppi L, De Matteis S, et al. Metformin and insulin impact on clinical outcome in patients with advanced hepatocellular carcinoma receiving sorafenib: Validation study and biological rationale. Eur J Cancer 2017;86:106-14. [Crossref] [PubMed]
- Bhat M, Chaiteerakij R, Harmsen WS, et al. Metformin does not improve survival in patients with hepatocellular carcinoma. World J Gastroenterol 2014;20:15750-5. [Crossref] [PubMed]
- Amemiya H, Matsuda M, Saito R, et al. Impact of Insulin Treatment on Prognosis of non-B non-C Hepatocellular Carcinoma After Hepatectomy. Anticancer Res 2021;41:317-26. [Crossref] [PubMed]
- Bruix J, Sherman MPractice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology 2005;42:1208-36. [Crossref] [PubMed]
- American Diabetes Association. Standards of medical care in diabetes--2014. Diabetes Care 2014;37:S14-80. [Crossref] [PubMed]
- Mukherjee B, Bhattacharya S, Chakraborty S, et al. Is type 2 diabetes mellitus a predisposal cause for developing hepatocellular carcinoma? Curr Diabetes Rev 2015;11:64-70. [Crossref] [PubMed]
- Chen J, Han Y, Xu C, et al. Effect of type 2 diabetes mellitus on the risk for hepatocellular carcinoma in chronic liver diseases: a meta-analysis of cohort studies. Eur J Cancer Prev 2015;24:89-99. [Crossref] [PubMed]
- Zheng Z, Zhang C, Yan J, et al. Diabetes mellitus is associated with hepatocellular carcinoma: a retrospective case-control study in hepatitis endemic area. PLoS One 2013;8:e84776. [Crossref] [PubMed]
- Koh WP, Wang R, Jin A, et al. Diabetes mellitus and risk of hepatocellular carcinoma: findings from the Singapore Chinese Health Study. Br J Cancer 2013;108:1182-8. [Crossref] [PubMed]
- Wang YG, Wang P, Wang B, et al. Diabetes mellitus and poorer prognosis in hepatocellular carcinoma: a systematic review and meta-analysis. PLoS One 2014;9:e95485. [Crossref] [PubMed]
- Tsai MS, Lin CL, Chang SN, et al. Diabetes mellitus and increased postoperative risk of acute renal failure after hepatectomy for hepatocellular carcinoma: a nationwide population-based study. Ann Surg Oncol 2014;21:3810-6. [Crossref] [PubMed]
- Donadon V, Balbi M, Valent F, et al. Glycated hemoglobin and antidiabetic strategies as risk factors for hepatocellular carcinoma. World J Gastroenterol 2010;16:3025-32. [Crossref] [PubMed]
- Hosokawa T, Kurosaki M, Tsuchiya K, et al. Hyperglycemia is a significant prognostic factor of hepatocellular carcinoma after curative therapy. World J Gastroenterol 2013;19:249-57. [Crossref] [PubMed]
- Kang J, Li C, Gao X, et al. Metformin inhibits tumor growth and affects intestinal flora in diabetic tumor-bearing mice. Eur J Pharmacol 2021;912:174605. [Crossref] [PubMed]
- Schulte L, Scheiner B, Voigtländer T, et al. Treatment with metformin is associated with a prolonged survival in patients with hepatocellular carcinoma. Liver Int 2019;39:714-26. [Crossref] [PubMed]
(English Language Editor: J. Teoh)


