
Efficacy and safety of sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a two-center study in China
Introduction
Hepatocellular carcinoma (HCC) is currently the 6th most common cancer and the 2nd leading cause of cancer-related deaths worldwide (1). The incidence of HCC around the globe follows the geographical distribution of the hepatitis B and C viruses, as infection with either virus is a significant risk factor of HCC (2-4). The management of HCC is multidisciplinary (3,5). However, patients with advanced HCC still have a dismal prognosis, with a 5-year overall survival (OS) of 31% for localized disease, 11% for regional disease, and 2% for metastatic disease (6).
Most patients with HCC are diagnosed at an advanced stage (7). Patients with advanced HCC who have progressed after 1st-line therapy have a poor prognosis. Sorafenib is a standard 1st-line systemic targeted therapy for advanced HCC (8). Unfortunately, patients who fail to respond to sorafenib have an OS of only 8 months without treatment (9-11).
Regorafenib can inhibit the activity of the protein kinases involved in angiogenesis, oncogenesis, metastasis, and tumor immunity (12-14). The molecular structures of regorafenib and sorafenib are very similar, but regorafenib has a special molecular target and a more potent pharmacological activity than sorafenib. In 2017, the RESORCE trial released encouraging results (15-17). According to the results of the RESORCE trial, regorafenib improved OS with a hazard ratio (HR) of 0.63 [95% confidence interval (CI): 0.50–0.79; 1-sided P<0.0001], and patients treated regorafenib had a median OS of 10.6 months (95% CI: 9.1–12.1 months), while those who received a placebo had a median OS of 7.8 months (95% CI: 6.3–8.8 months) (15). Significant improvements were also found in relation to progression-free survival (PFS), time-to-progression (TTP), the disease control rate, and overall tumor response (15). In recent years, there has been increasing evidence that the application of regorafenib in combination with other adjuvant therapies, such as transarterial chemoembolization (TACE) and immune checkpoint inhibitors, may benefit some patients (18-20).
Regorafenib is a standard 2nd-line treatment for patients with advanced HCC, and the sequential therapy of regorafenib after progression to sorafenib has been adopted as an evidence-based treatment (3,5,21). However, patients in Asia, especially those in China, display different clinical characteristics to patients in the West (22-26). Previous studies have shown that Regorafenib-related adverse events were more frequent in Asian populations than in non-Asian populations, including hand-foot skin reaction (27-29) and liver function adverse events (30-32). These variances are possibly due to racial differences affecting drug absorption or pharmacokinetics or improved management of regorafenib-related toxicities. Beyond that, it remains unclear which clinical features would benefit patients from sequential therapy. Thus, our study was designed to assess the efficacy and safety and analyzed the prognostic factors of sequential therapy with sorafenib and regorafenib among advanced HCC patients in China. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-397/rc).
Methods
Patients
This is a two-center, retrospective, cohort research study. Patients with advanced HCC receiving sequential therapy at two large hospitals (Tianjin Medical University Cancer Institute and Hospital and Tianjin First Central Hospital) in China from October 2018 to April 2020 were included in the study. To be eligible for inclusion in the study, patients had to meet the following inclusion criteria: (I) be aged ≥18 years; (II) have Barcelona Clinic Liver Cancer (BCLC) stage B or C (2); (III) have previously undergone but failed to respond to treatment with sorafenib (i.e., had received sorafenib for ≥20 days and had documented radiological progression or had stopped taking the drug because of unbearable adverse reactions); (IV) had liver function status Child-Pugh A; (V) had adequate bone marrow, liver, and renal function; and (VI) had an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0–1. Patients were excluded from the study if they met any of the following exclusion criteria are (I) had received other treatments between the withdrawal of sorafenib and the start of regorafenib or had received other treatments during regorafenib treatment; (II) had a history of other tumors except for HCC; and/or (III) had severe cardiovascular or respiratory disease or human immunodeficiency virus infection. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Ethics Committee of the Tianjin Medical University Cancer Institute and Hospital (No. bc2022135) and Tianjin First Central Hospital (No. 2022N256KY), with the requirement for informed consent waived. And this study complied with the Good Clinical Practice guidelines and applicable local laws. Any patient data that could identify individual patients were anonymized and de-identified before analysis.
Therapy
The patients were converted directly to regorafenib after failing to respond to sorafenib monotherapy. Based on previous trials, the starting dose was 160 mg of regorafenib once daily for 3 weeks, followed by 1 week of no treatment per cycle (15-17,33,34). A reduced starting dose of <160 mg/day regorafenib was allowed for some patients (15). The patients underwent evaluations every 4–6 weeks to determine the efficacy and safety of the treatment according to physiological, laboratory, and radiological results.
Data collection and definition
The following data were gathered from the patient charts: sex, age, history of hepatitis, history of chronic disease, unhealthy living habits, previous treatment (basic antiviral therapy and treatment before sorafenib), data on sorafenib treatment (tolerance and type of progression after sorafenib, enlargement of the original/new intrahepatic lesions, or new extrahepatic metastatic lesions), baseline data of regorafenib [ECOG-PS, Child-Pugh class, radiological evaluation, alpha-fetoprotein (AFP), and BCLC stage], data on regorafenib treatment [the start date of the regorafenib treatment, time of radiological progression according to the Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 and mRECIST, and adverse events of regorafenib treatment], and date of death or last follow-up. The patient data were anonymized and de-identified before the analysis.
Concentrations of alanine aminotransferase (ALT), aspartate aminotransferase (AST), bilirubin, and AFP were assessed before each treatment cycle. A radiological evaluation, using computed tomography or magnetic resonance imaging scans, was conducted. OS was defined as the time from the initiation of regorafenib to death from any cause. PFS was defined as the time from the initiation of regorafenib to the date of disease progression or death from any cause before progression. Semiannual follow-up was based on telephone questionnaire, with additional follow-up procedures when needed. Safety was assessed by the frequency of treatment-emergent adverse events. Adverse events were graded using National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.03.
Statistical analysis
All the statistical analyses were conducted using SPSS 23 (IBM, Armonk, NY, USA). The normally distributed continuous data were tested using the Kolmogorov-Smirnov test and are expressed as the mean ± standard deviation. The non-normally distributed continuous data are expressed as the median (range). Loss to follow-up and missing values were excluded from the study. The PFS of regorafenib treatment patients was estimated using Kaplan-Meier plots of medians with 95% CIs. A univariable analysis was conducted using the Kaplan-Meier method, and differences were evaluated using the log-rank test. The univariable Cox proportional-hazards model was fitted to each variable. Next, all variables with a two-sided P value <0.05 and other significant factors identified in previous studies were included in the multivariable analysis using a stepwise Cox hazard-regression model to evaluate their value as independent predictors of PFS and OS. P values <0.05 were considered statistically significant.
Results
Patient characteristics
A total of 55 patients with advanced HCC received sequential therapy from October 2018 to April 2020. However, 5 patients were lost to follow-up, and 7 were excluded for not receiving regular reviews or missing critical clinical information. Table 1 sets out the baseline characteristics of the 43 patients included in the study. Patients had a mean age of 60.4±9.1 years, and 39 (90.7%) patients were male. Of the patients, 35 (81.4%) had a history of hepatitis B, and 60% had received previous antiviral therapy. Of the patients, 14 (32.6%) patients had AFP levels ≥400 ng/mL before 2nd-line treatment. All patients had Child-Pugh class A cirrhosis and ECOG 0–1, and 60.5% and 39.5% of the patients were classified as BCLC stage B and C, respectively. All 43 patients were confirmed to show radiological progression during sorafenib therapy. Additionally, 5 (11.6%) patients had a macrovascular invasion. Further, 8 (18.6%) patients had extrahepatic metastasis, with the most common metastatic site being the lungs (n=8, 18.6%), followed by the bones (n=5, 11.6%). In relation to the patterns of progression during sorafenib treatment, 18 (41.9%), 11 (25.6%), 10 (23.3%), and 4 (9.3%) patients were classified as having growth of intrahepatic or extrahepatic lesions or both, a new extrahepatic lesion, a new intrahepatic lesion, or unknown, respectively. Almost all the patients underwent pre-treatment before regorafenib. During the sorafenib administration, 40 patients displayed a tolerance to sorafenib.
Table 1
| Variable | N=43 |
|---|---|
| Age, years, mean ± SD | 60.4±9.1 |
| Male, n (%) | 39 (90.7) |
| History of diabetes, n (%) | 12 (27.9) |
| History of hypertension, n (%) | 20 (46.5) |
| Smoking history, n (%) | 20 (46.5) |
| History of alcoholism, n (%) | 9 (20.9) |
| Etiology, n (%) | |
| Hepatitis B | 35 (81.4) |
| None | 8 (18.6) |
| Standard antiviral therapy, n (%) | 21/35 (60.0) |
| AFP (ng/mL), median (interquartile range) | 32.4 (4.4, 821.7) |
| AFP ≥400 ng/mL, n (%) | 14 (32.6) |
| Child-Pugh class A, n (%) | 43 (100.0) |
| ECOG-PS ≤1, n (%) | 43 (100.0) |
| ALT (U/L), median (interquartile range) | 31.0 (16.0, 53.5) |
| ALT >60 U/L, n (%) | 10 (23.3) |
| AST (U/L), median (interquartile range) | 37.0 (22.5, 65.0) |
| AST >60 U/L, n (%) | 11 (25.6) |
| ALB (g/L), median (interquartile range) | 40.3 (36.3, 45.1) |
| Barcelona Clinic Liver Cancer stage, n (%) | |
| B | 26 (60.5) |
| C | 17 (39.5) |
| Macrovascular invasion, n (%) | 5 (11.6) |
| Extrahepatic metastasis, n (%) | 8 (18.6) |
| Lung, n (%) | 8 (18.6) |
| Bone, n (%) | 5 (11.6) |
| Adrenal, n (%) | 2 (4.7) |
| Macrovascular invasion and/or extrahepatic disease, n (%) | 13 (30.2) |
| Pattern of progression on previous sorafenib treatment, n (%) | |
| Growth of intrahepatic or extrahepatic lesions, or both | 18 (41.9) |
| New extrahepatic lesion | 11 (25.6) |
| New intrahepatic lesion | 10 (23.3) |
| Unknown | 4 (9.3) |
| Pretreatment, n (%) | 35 (81.4) |
| Pre-resection, n (%) | 28 (65.1) |
| Pre-local ablation, n (%) | 6 (14.0) |
| Pre-TACE, n (%) | 31 (72.1) |
| Pre-liver transplantation, n (%) | 7 (16.3) |
| Pre-radiotherapy, n (%) | 4 (9.3) |
| Tolerance of sorafenib, tolerance, n (%) | 40 (93.0) |
HCC, hepatocellular carcinoma; AFP, alpha-fetoprotein; ECOG-PS, Eastern Cooperative Oncology Group Performance Status; ALT, alanine transaminase; AST, aspartate aminotransferase; ALB, albumin; TACE, transarterial chemoembolization.
Effectiveness of regorafenib
The starting dose of regorafenib was determined according to each patient’s basic condition (80–160 mg). With a median follow-up period of 8 months (95% CI: 11.3–11.4 months), the median PFS and OS (see Figure 1) were 11.0 months (95% CI: 5.8–16.2 months) and 17.0 months (95% CI: 12.8–21.2 months), respectively. We confirmed that 14 patients had radiological progression after regorafenib administration.

Factors associated with PFS
Among the variables, AFP, alanine transaminase (ALT), and aspartate aminotransferase (AST) before 2nd-line treatment were significant risk factors for PFS in the univariable analyses (see Table 2). The Kaplan-Meier curves of the PFS of HCC patients treated with regorafenib after sorafenib for the before 2nd-line treatment AFP <400 and ≥400 ng/mL groups showed significant differences (see Figure 2A). Additionally, significant differences were also found between normal (≤60 U/L) and abnormal (>60 U/L) ALT (P=0.003) and AST (P=0.009) before 2nd-line treatment (see Figure 2B,2C). The multivariable Cox proportional-hazards regression analysis showed that AFP (HR =0.225; 95% CI: 0.073–0.688; P=0.009), ALT (HR =0.195; 95% CI: 0.051–0.741; P=0.016), AST (HR =0.209; 95% CI: 0.063–0.697; P=0.011), and extrahepatic metastasis (HR =0.074; 95% CI: 0.009–0.608; P=0.015) before 2nd-line treatment were independently associated with PFS (see Table 2). No factors were found to be associated with OS (see Table 3).
Table 2
| Variable | Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 1.007 | 0.958–1.058 | 0.78 | ||||
| Female sex | 3.03 | 0.396–23.185 | 0.286 | ||||
| History of diabetes | 0.408 | 0.163–1.022 | 0.056 | ||||
| History of hypertension | 0.626 | 0.245–1.602 | 0.328 | ||||
| Smoking history | 0.566 | 0.229–1.402 | 0.219 | ||||
| History of alcoholism | 1.495 | 0.435–5.142 | 0.524 | ||||
| Hepatitis B | 0.971 | 0.281–3.359 | 0.963 | 1.158 | 0.273–4.923 | 0.842 | |
| Pretreatment | 0.424 | 0.098–1.838 | 0.251 | ||||
| Pre-resection | 1.235 | 0.484–3.152 | 0.658 | ||||
| Pre-local ablation | 1.394 | 0.322–6.047 | 0.657 | ||||
| Pre-TACE | 0.939 | 0.337–2.615 | 0.904 | ||||
| Pre-liver transplantation | 1.864 | 0.424–8.199 | 0.41 | ||||
| Pre-radiotherapy | 0.852 | 0.194–3.736 | 0.832 | ||||
| Tolerance of sorafenib | 1.452 | 0.333–6.338 | 0.62 | ||||
| AFP ≥400 vs. <400 ng/mL | 0.319 | 0.125–0.811 | 0.016* | 0.225 | 0.073–0.688 | 0.009* | |
| ALT >60 vs. ≤60 U/L | 0.27 | 0.105–0.694 | 0.007* | 0.195 | 0.051–0.741 | 0.016* | |
| AST >60 vs. ≤60 U/L | 0.323 | 0.129–0.809 | 0.016* | 0.209 | 0.063–0.697 | 0.011* | |
| Hypoproteinemia | 0.484 | 0.170–1.381 | 0.175 | 0.527 | 0.168–1.653 | 0.272 | |
| ALB before 2nd-line treatment | 0.958 | 0.894–1.027 | 0.23 | ||||
| Pattern of progression on previous sorafenib treatment | |||||||
| Growth of intrahepatic or extrahepatic lesions, or both | 1 | ||||||
| New extrahepatic lesion | 4.141 | 0.518–33.103 | 0.18 | ||||
| New intrahepatic lesion | 1.198 | 0.124–11.599 | 0.876 | ||||
| Unknown | 2.798 | 0.325–24.065 | 0.349 | ||||
| BCLC stage | |||||||
| Stage B | 1 | 1 | |||||
| Stage C | 0.517 | 0.209–1.580 | 0.154 | 1.53 | 0.276–8.492 | 0.627 | |
| Macrovascular invasion | 0.823 | 0.235–2.877 | 0.76 | 0.499 | 0.070–3.648 | 0.499 | |
| Extrahepatic metastasis | 0.377 | 0.133–1.070 | 0.067 | 0.074 | 0.009–0.608 | 0.015* | |
| Macrovascular invasion and/or extrahepatic disease | 0.465 | 0.185–1.170 | 0.104 | – | – | – | |
*, P values <0.05 were considered statistically significant. PFS, progression-free survival; HR, hazard ratio; CI, confidence interval; TACE, transarterial chemoembolization; AFP, α-fetoprotein; AST, aspartate transaminase; ALT, alanine transaminase; BCLC, Barcelona Clinic Liver Cancer.
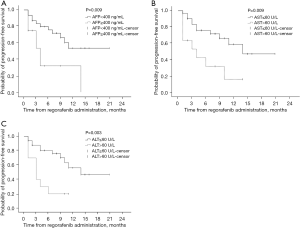
Table 3
| Variable | HR | 95% CI | P |
|---|---|---|---|
| Age | 1.011 | 0.936–1.092 | 0.781 |
| Female | 0.912 | 0.111–7.520 | 0.932 |
| History of diabetes | 4.048 | 0.501–32.732 | 0.19 |
| History of hypertension | 4.15 | 0.795–21.633 | 0.091 |
| Smoking history | 0.826 | 0.219–3.121 | 0.778 |
| History of alcoholism | 34.27 | 0.033–35,300.905 | 0.318 |
| Hepatitis B | 0.03 | 0.000–47.744 | 0.35 |
| Pretreatment | 0.665 | 0.081–5.443 | 0.704 |
| Pre-resection | 0.831 | 0.206–3.354 | 0.795 |
| Pre-local ablation | 0.738 | 0.141–3.847 | 0.718 |
| Pre-TACE | 1.09 | 0.269–4.423 | 0.904 |
| Pre-liver transplantation | 2.2 | 0.258–18.720 | 0.471 |
| Pre-radiotherapy | 0.708 | 0.087–5.760 | 0.747 |
| Tolerance of sorafenib | 1.908 | 0.233–15.606 | 0.547 |
| AFP ≥400 vs. <400 ng/mL | 0.43 | 0.112–1.645 | 0.217 |
| ALT >60 vs. ≤60 U/L | 0.387 | 0.086–1.736 | 0.215 |
| AST >60 vs. ≤60 U/L | 0.269 | 0.066–1.099 | 0.067 |
| ALB before 2nd-line treatment | 0.944 | 0.845–1.054 | 0.302 |
| Pattern of progression on previous sorafenib treatment | |||
| Growth of intrahepatic or extrahepatic lesions, or both | 1 | ||
| New extrahepatic lesion | 24,528.378 | 0–4.863E+147 | 0.952 |
| New intrahepatic lesion | 28,019.74338 | 0–5.5567E+147 | 0.951 |
| Unknown | 27,202.69259 | 0–5.3946E+147 | 0.952 |
| BCLC stage | |||
| Stage B | 1 | ||
| Stage C | 0.709 | 0.175–2.869 | 0.629 |
| Macrovascular invasion | 1.902 | 0.219–16.500 | 0.56 |
| Extrahepatic metastasis | 0.524 | 0.124–2.214 | 0.38 |
| Macrovascular invasion and/or extrahepatic disease | 0.795 | 0.186–3.397 | 0.757 |
OS, overall survival; HR, hazard ratio; CI, confidence interval; AFP, α-fetoprotein; AST, aspartate transaminase; ALT, alanine transaminase; ALB, albumin; BCLC, Barcelona Clinic Liver Cancer.
Safety and tolerability
The regorafenib-related adverse events are summarized in Table 4. A total of 31 (72.1%) patients experienced at least 1 treatment-related adverse event. Most of adverse events were able to be managed by dose modifications and appropriate supportive care. The most common toxicities were hand-foot skin reactions (n=21, 48.8%), diarrhea (n=14, 32.6%), and hypertension (n=6, 14%). The most common grade 3–4 toxicities were hypoalbuminemia (n=2, 4.7%), anemia (n=2, 4.7%), and thrombocytopenia (n=2, 4.7%). Most of the adverse reactions were < grade 3.
Table 4
| Variable | Any grade (N=43), n (%) | Grades 3–4 (N=43), n (%) |
|---|---|---|
| Any adverse event | 31 (72.1) | 3 (7.0) |
| Hand-foot skin reaction | 21 (48.8) | 0 |
| Diarrhea | 14 (32.6) | 0 |
| Elevated serum AST/ALT | 6 (14.0) | 1 (2.3) |
| Hypertension | 6 (14.0) | 0 |
| Hypoalbuminemia | 5 (11.6) | 2 (4.7) |
| Thrombocytopenia | 3 (7.0) | 2 (4.7) |
| Elevated serum blood bilirubin | 3 (7.0) | 1 (2.3) |
| Anemia | 2 (4.7) | 2 (4.7) |
| Gastrointestinal bleeding | 1 (2.3) | 1 (2.3) |
| Fatigue and decreased appetite | 1 (2.3) | 1 (2.3) |
| Alopecia | 1 (2.3) | 0 |
HCC, hepatocellular carcinoma; AST, aspartate transaminase; ALT, alanine transaminase.
A total of 19 patients discontinued regorafenib due to disease progression, adverse events, or death during the observation period. No patient stopped taking the drug permanently because of serious adverse events. There were 9 deaths during the observation period. Of the 9 deaths, 1 patient died from liver failure caused by regorafenib treatment, and 5 patients died from non-tumor–related causes.
Discussion
The efficacy of regorafenib was confirmed by the RESORCE trial in advanced HCC patients who showed progression after treatment with sorafenib (15-17). This study sought to verify the efficacy and safety of sorafenib sequential to regorafenib in the treatment of advanced HCC patients based on real-world data from China. The results strongly suggest that sequential treatment with sorafenib and regorafenib is well-tolerated and effective in patients with advanced HCC. Further, wine identified some pre-treatment clinical indicators that can be used to screen patients with a good prognosis.
Our results complement those of the RESORCE trial, which excluded many more patients with complex clinical conditions. Indeed, the RESORCE trial excluded patients intolerant to sorafenib due to adverse events and those who required a sorafenib dose reduction to <400 mg/d. Due to the impressive physique of the Chinese patients, such patients were not excluded in the present study. Our study’s median PFS and OS were comparable to those of the RESORCE trial and those in other reports from Japan and Korea (15,34,35), which further confirms the efficacy of sequential treatment in patients with advanced HCC even in real-world therapy settings.
In previous studies and this study, while palmar-plantar erythrodysesthesia, diarrhea, fatigue, decreased appetite, elevated AST, and hypertension were the most common adverse events, most of these events were < grade 3, and could be controlled by adjusting the dose and providing optimal supportive care (15,34,35). Further, in our cohort, no patient stopped taking the drug permanently because of severe adverse reactions.
Research on the mechanism of sequential therapy of the two targeted drugs is still underway. A previous in vitro experiment showed that samples treated with regorafenib and sorafenib differed in protein expression compared to those treated with a placebo (36). The pattern of protein upregulation by the two drugs was similar, indicating that the rapidly accelerated fibrosarcoma (RAF)/Mitogen-activated protein kinase kinase (MAPKK)/extracellular-signal-regulated kinases (ERK) pathway was activated, but sorafenib downregulated more proteins than regorafenib. Both regorafenib and sorafenib were effective in a mouse liver cancer model, but several cases showed better regorafenib activity, which may explain the significant efficacy of regorafenib in patients with sorafenib resistance (36).
The outstanding results of sequential therapy could play a very positive role in guiding clinical treatment (37). This study found no significant associations between pre-treatment, sorafenib tolerance, the pattern of progression on previous sorafenib treatment, BCLC stage, or macrovascular invasion before 2nd-line treatment and the PFS or OS of 2nd-line treatment. The RESORCE trial also found that regorafenib improved the outcomes of patients with HCC with a good liver function reserve (15). Regardless of the previous pattern of progression of sorafenib and regardless of the last dose of sorafenib, regorafenib produced a definite effect (17). A further report suggests that therapy with sequential sorafenib followed by regorafenib might result in an unprecedented median OS of 26 months (16). We verified these findings in our research.
The present study showed that AFP ≥400 ng/mL, ALT ≥60 IU/L, and AST ≥60 IU/L before 2nd-line treatment were associated with PFS in the univariable analyses. The multivariable Cox proportional-hazards regression analysis also showed that AFP ≥400 ng/mL, ALT ≥60 IU/L, and AST ≥60 IU/L before 2nd-line treatment were independently associated with PFS. It may be that patients with a good liver function reserve before 2nd-line treatment are more likely to benefit from the sequential treatment. Further, AFP might be used as a clinical indicator for screening people who are likely to benefit from sequential therapy.
Previous studies had found that patients with a Child-Pugh score of 5 before sorafenib treatment had a significantly better prognosis than patients with a score of 6 (15,38,39). This is because patients with a score of 5 can be switched early from TACE to sorafenib if TACE is not effective and they can then be switched from sorafenib to regorafenib if they are refractory to sorafenib (15,16,33). This may be an essential strategy for improving survival in the future. The long survival time of 26 months achieved by timely sequential therapy is almost comparable to trae4ditional TACE in the treatment of intermediate-stage HCC (33,40). A recent study of patients with recurrent HCC after liver transplantation demonstrated that sequential therapy with sorafenib and regorafenib significantly prolonged OS (28.8 months), and was an independent predictor of OS (41). In recent years, some studies have found that nivolumab, cabozantinib, or regorafenib produce excellent treatment effects after sorafenib treatment failure in advanced HCC patients, but the difference was not statistically significant (42,43). Now that the potential of sorafenib-regorafenib sequential therapy to significantly improve patient prognosis is more pronounced, it may be necessary to re-evaluate the appropriate time at which to start sorafenib. With the help of some efficient and commonly used clinical indicators, patients eligible for sorafenib therapy can receive it promptly and thus benefit from all currently available therapies.
Currently, it is not apparent which patients will benefit from sequential treatment. A previous study has shown that multiple proteins and micro ribonucleic acids might be predictive of OS in HCC patients treated with regorafenib, and the analysis of the association between baseline plasma levels of 266 proteins and responses to regorafenib treatment identified 5 biomarkers, including Angiopoietin-1 (ANG-1), cystatin B, Latency associated peptide transforming growth factor beta-1 (LAP TGF-b1), Lectin-like oxidized LDL receptor-1 (LOX-1), and Macrophage inflammatory protein-1a (MIP-1a) as possible predictors of OS, and 47 biomarkers, including the 5 predictive for OS, as possible predictors of TTP (44). A previous study highlighted the predictive role of AFP, the neutrophil-to-lymphocyte ratio, and extrahepatic spread in predicting the efficacy of sequential therapy (45). In our study, the Cox proportional-hazards regression analysis showed that AFP [hazard ratio (HR) =0.225; 95% CI: 0.073–0.688; P=0.009], ALT (HR =0.195; 95% CI: 0.051–0.741; P=0.016), AST (HR =0.209; 95% CI: 0.063–0.697; P=0.011), and presence of extrahepatic metastasis (HR =0.074; 95% CI: 0.009–0.608; P=0.015) before 2nd-line treatment were independently associated with PFS. Based on these clinical characteristics it may be possible to distinguish patients who would benefit from sequential therapy. However, these findings need to be validated.
The present study had some limitations. This study was only conducted at 2 hospitals, which led to a small sample size. Patients were informed of the possible adverse reactions before they took the drug. However, some patients still did not pay enough attention to drug-related adverse reactions, which led to a lack of monitoring of critical adverse reactions (such as proteinuria). There was no comparator group. In addition, the analyses were limited to the data available in the patient charts. Other studies have also proposed to predict the prognosis of sorafenib and regorafenib sequential therapy based on clinical indicators (46,47), but there is still no accepted method for predicting the potential population likely to benefit from sequential therapy.
In conclusion, sequential therapy with sorafenib and regorafenib is well-tolerated and effective in patients with advanced HCC. Additionally, patients with a good liver function reserve and a high level of AFP before 2nd-line treatment may benefit more from sequential treatment. This evidence supports the results of the clinical trials (15-17,35).
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Grant No. 82173317), the National Science and Technology Major Project of China (Grant No. 2018ZX10302205), and the Science and Technology Development Fund of Tianjin Education Commission for Higher Education (Grant No. 2017KJ199).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-397/rc
Data Sharing Statement: available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-397/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-397/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the ethics committee of Tianjin Medical University Cancer Institute and Hospital (No. bc2022135) and Tianjin First Central Hospital (No. 2022N256KY), with the requirement for informed consent waived. And this study complied with the Good Clinical Practice guidelines and applicable local laws. Any patient data that could identify individual patients were anonymized and de-identified before analysis.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021;71:209-49. [Crossref] [PubMed]
- Marrero JA, Kulik LM, Sirlin CB, et al. Diagnosis, Staging, and Management of Hepatocellular Carcinoma: 2018 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2018;68:723-50. [Crossref] [PubMed]
- Vogel A, Cervantes A, Chau I, et al. Hepatocellular carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018;29:iv238-iv55. [Crossref] [PubMed]
- Shengir M, Elgara M, Sebastiani G. Metabolic and cardiovascular complications after virological cure in hepatitis C: What awaits beyond. World J Gastroenterol 2021;27:1959-72. [Crossref] [PubMed]
- Burger JA, Barr PM, Robak T, et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 2020;34:787-98. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Global Burden of Disease Cancer Collaboration. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 2017;3:524-48. [Crossref] [PubMed]
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 2008;359:378-90. [Crossref] [PubMed]
- Llovet JM, Decaens T, Raoul JL, et al. Brivanib in patients with advanced hepatocellular carcinoma who were intolerant to sorafenib or for whom sorafenib failed: results from the randomized phase III BRISK-PS study. J Clin Oncol 2013;31:3509-16. [Crossref] [PubMed]
- Zhu AX, Kudo M, Assenat E, et al. Effect of everolimus on survival in advanced hepatocellular carcinoma after failure of sorafenib: the EVOLVE-1 randomized clinical trial. JAMA 2014;312:57-67. [Crossref] [PubMed]
- Zhu AX, Park JO, Ryoo BY, et al. Ramucirumab versus placebo as second-line treatment in patients with advanced hepatocellular carcinoma following first-line therapy with sorafenib (REACH): a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol 2015;16:859-70. [Crossref] [PubMed]
- Cerrito L, Ponziani FR, Garcovich M, et al. Regorafenib: a promising treatment for hepatocellular carcinoma. Expert Opin Pharmacother 2018;19:1941-8. [Crossref] [PubMed]
- Personeni N, Pressiani T, Santoro A, et al. Regorafenib in hepatocellular carcinoma: latest evidence and clinical implications. Drugs Context 2018;7:212533. [Crossref] [PubMed]
- Heo YA, Syed YY. Regorafenib: A Review in Hepatocellular Carcinoma. Drugs 2018;78:951-8. [Crossref] [PubMed]
- Bruix J, Qin S, Merle P, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017;389:56-66. [Crossref] [PubMed]
- Finn RS, Merle P, Granito A, et al. Outcomes with sorafenib (SOR) followed by regorafenib (REG) or placebo (PBO) for hepatocellular carcinoma (HCC): Results of the international, randomized phase 3 RESORCE trial. J Clin Oncol 2017;35. [Crossref]
- Worns MA, Merle P, Granito A, et al. Survival by pattern of tumor progression during prior sorafenib (SOR) treatment in patients with hepatocellular carcinoma (HCC) in the phase III RESORCE trial comparing second-line treatment with regorafenib (REG) or placebo. Oncology Research and Treatment 2017;40:161.
- Han Y, Cao G, Sun B, et al. Regorafenib combined with transarterial chemoembolization for unresectable hepatocellular carcinoma: a real-world study. BMC Gastroenterol 2021;21:393. [Crossref] [PubMed]
- Fondevila F, Méndez-Blanco C, Fernández-Palanca P, et al. Anti-tumoral activity of single and combined regorafenib treatments in preclinical models of liver and gastrointestinal cancers. Exp Mol Med 2019;51:1-15. [Crossref] [PubMed]
- Cheng AL, Hsu C, Chan SL, et al. Challenges of combination therapy with immune checkpoint inhibitors for hepatocellular carcinoma. J Hepatol 2020;72:307-19. [Crossref] [PubMed]
- Su GL, Altayar O, O'Shea R, et al. AGA Clinical Practice Guideline on Systemic Therapy for Hepatocellular Carcinoma. Gastroenterology 2022;162:920-34. [Crossref] [PubMed]
- Song TJ, Fong Y, Cho SJ, et al. Comparison of hepatocellular carcinoma in American and Asian patients by tissue array analysis. J Surg Oncol 2012;106:84-8. [Crossref] [PubMed]
- Choo SP, Tan WL, Goh BKP, et al. Comparison of hepatocellular carcinoma in Eastern versus Western populations. Cancer 2016;122:3430-46. [Crossref] [PubMed]
- Sun J, Wu J, Liu C, et al. Typing of biliary tumor thrombus influences the prognoses of patients with hepatocellular carcinoma. Cancer Biol Med 2021; [Epub ahead of print]. [Crossref] [PubMed]
- Liu D, Luo Y, Chen L, et al. Diagnostic value of 5 serum biomarkers for hepatocellular carcinoma with different epidemiological backgrounds: A large-scale, retrospective study. Cancer Biol Med 2021;18:256-70. [Crossref] [PubMed]
- Sun D, Li H, Cao M, et al. Cancer burden in China: trends, risk factors and prevention. Cancer Biol Med 2020;17:879-95. [Crossref] [PubMed]
- Ueda T, Uemura H, Tomita Y, et al. Efficacy and safety of axitinib versus sorafenib in metastatic renal cell carcinoma: subgroup analysis of Japanese patients from the global randomized Phase 3 AXIS trial. Jpn J Clin Oncol 2013;43:616-28. [Crossref] [PubMed]
- Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 2009;10:25-34. [Crossref] [PubMed]
- Zhou A. Management of sunitinib adverse events in renal cell carcinoma patients: the Asian experience. Asia Pac J Clin Oncol 2012;8:132-44. [Crossref] [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Li J, Qin S, Xu R, et al. Regorafenib plus best supportive care versus placebo plus best supportive care in Asian patients with previously treated metastatic colorectal cancer (CONCUR): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2015;16:619-29. [Crossref] [PubMed]
- Yoshino T, Komatsu Y, Yamada Y, et al. Randomized phase III trial of regorafenib in metastatic colorectal cancer: analysis of the CORRECT Japanese and non-Japanese subpopulations. Invest New Drugs 2015;33:740-50. [Crossref] [PubMed]
- Kudo M. Regorafenib as Second-Line Systemic Therapy May Change the Treatment Strategy and Management Paradigm for Hepatocellular Carcinoma. Liver Cancer 2016;5:235-44. [Crossref] [PubMed]
- Yoo C, Park JW, Kim YJ, et al. Multicenter retrospective analysis of the safety and efficacy of regorafenib after progression on sorafenib in Korean patients with hepatocellular carcinoma. Invest New Drugs 2019;37:567-72. [Crossref] [PubMed]
- Ogasawara S, Ooka Y, Itokawa N, et al. Sequential therapy with sorafenib and regorafenib for advanced hepatocellular carcinoma: a multicenter retrospective study in Japan. Invest New Drugs 2020;38:172-80. [Crossref] [PubMed]
- Kissel M, Berndt S, Fiebig L, et al. Antitumor effects of regorafenib and sorafenib in preclinical models of hepatocellular carcinoma. Oncotarget 2017;8:107096-108. [Crossref] [PubMed]
- An L, Liao H, Yuan K. Efficacy and Safety of Second-line Treatments in Patients with Advanced Hepatocellular Carcinoma after Sorafenib Failure: A Meta-analysis. J Clin Transl Hepatol 2021;9:868-77. [Crossref] [PubMed]
- Ogasawara S, Chiba T, Ooka Y, et al. Characteristics of patients with sorafenib-treated advanced hepatocellular carcinoma eligible for second-line treatment. Invest New Drugs 2018;36:332-9. [Crossref] [PubMed]
- Guan YS, He Q. Sorafenib: activity and clinical application in patients with hepatocellular carcinoma. Expert Opin Pharmacother 2011;12:303-13. [Crossref] [PubMed]
- Kudo M, Han G, Finn RS, et al. Brivanib as adjuvant therapy to transarterial chemoembolization in patients with hepatocellular carcinoma: A randomized phase III trial. Hepatology 2014;60:1697-707. [Crossref] [PubMed]
- Iavarone M, Invernizzi F, Ivanics T, et al. Regorafenib Efficacy After Sorafenib in Patients With Recurrent Hepatocellular Carcinoma After Liver Transplantation: A Retrospective Study. Liver Transpl 2021;27:1767-78. [Crossref] [PubMed]
- Kuo YH, Yen YH, Chen YY, et al. Nivolumab Versus Regorafenib in Patients With Hepatocellular Carcinoma After Sorafenib Failure. Front Oncol 2021;11:683341. [Crossref] [PubMed]
- Casadei-Gardini A, Rimassa L, Rimini M, et al. Regorafenib versus cabozantinb as second-line treatment after sorafenib for unresectable hepatocellular carcinoma: matching-adjusted indirect comparison analysis. J Cancer Res Clin Oncol 2021;147:3665-71. [Crossref] [PubMed]
- Teufel M, Seidel H, Köchert K, et al. Biomarkers Associated With Response to Regorafenib in Patients With Hepatocellular Carcinoma. Gastroenterology 2019;156:1731-41. [Crossref] [PubMed]
- Bang Y, Yoo C, Lonardi S, et al. Sequential Treatment of Sorafenib-Regorafenib Versus Sorafenib-Physician's Choice: A Propensity Score-Matched Analysis. Target Oncol 2021;16:401-10. [Crossref] [PubMed]
- Hong YM, Yoon KT, Cho M. Systemic immune-inflammation index predicts prognosis of sequential therapy with sorafenib and regorafenib in hepatocellular carcinoma. BMC Cancer 2021;21:569. [Crossref] [PubMed]
- Wang HW, Chuang PH, Su WP, et al. On-Treatment Albumin-Bilirubin Grade: Predictor of Response and Outcome of Sorafenib-Regorafenib Sequential Therapy in Patients with Unresectable Hepatocellular Carcinoma. Cancers (Basel) 2021;13:3758. [Crossref] [PubMed]


