
Decreased expression of claudin-18.2 in alpha-fetoprotein-producing gastric cancer compared to conventional gastric cancer
Introduction
Alpha-fetoprotein (AFP), first identified in the 1950s, is an albuminoid superfamily protein that is synthesized in the fetus primarily by the liver, yolk sac, and tissues of gastrointestinal origin (1,2). Elevated AFP expression in adults is often associated with hepatocellular carcinoma (HCC) or with yolk sac tumor, and it has been used as an important diagnostic tool for the detection of these tumors, as well as an early predictor of tumor relapse or metastasis. However, some diseases other than HCC and YST are also associated with high serum levels of AFP.
AFP-producing gastric cancer (AFPGC), an emerging unique subtype of gastric cancer (GC), has been gaining increased attention in recent years due to its distinct clinicopathological features and significantly worse outcomes. AFPGC is defined as “hepatoid adenocarcinoma and related entities” by the World Health Organization (3). Since the first report of AFPGC by Bourreille et al. in 1970 (4), other cases have been reported sporadically, and the specifics of this disease have gradually been clarified (5-8). AFPGC patients were previously reported to show a high incidence of liver metastasis and have significantly shorter survival than AFP-negative patients (6,7). Generally, AFPGC can be divided into 4 histological subtypes according to its morphological and immunohistochemical features: common adenocarcinoma type (COM), hepatoid type (HPT), enteroblastic type (ENT), and yolk sac tumor type (YST). However, AFPGC often appears as a mixture of more than 1 histological type (5). The elevation of serum AFP levels or immunostaining of AFP in tumor cells, as well as the immunoreactivity of hepatocytic or oncofetal proteins in tumor cells, such as glypican 3 (GPC-3), hepatocyte paraffin antigen 1 (HepPar-1), arginase-1 (Arg-1), and spalt like transcription factor 4 (SALL4), can help to reach a diagnosis. Other molecules, including claudin-6 (CLDN-6), cluster of differentiation 24 (CD24), cyclin-dependent kinase inhibitor 1 (p21), and MYC proto-oncogene (c-myc), have also been reported to play a role in AFPGC (9,10).
Claudins are a family of at least 27 transmembrane proteins that play a crucial role in the formation of tight junctions. Aberrant claudin expression has been implicated in cancer progression (11). Different expression of claudins may have prognostic value in colon cancer (CLDN-1) (12), breast cancer (CLDN-6) (13), prostate cancer (CLDN-8) (14), and HCC and thyroid cancer (CLDN-10) (15,16). The isoform 2 of the tight junction molecule claudin-18.2 (CLDN18.2), first described by Niimi et al. (17), is a highly specific tight junction component that is confined to differentiated and stem gastric epithelial cells, where it controls the paracellular permeability to Na+ and H+ ions (18). As the dominant isoform that exclusively present in normal gastric tissue, gastric adenocarcinomas, and their metastases, CLDN18.2 has become an ideal candidate for monoclonal antibody binding with the capability of reduced off-target effects (19). In the FAST study, the addition of zolbetuximab, a novel chimeric monoclonal immunoglobulin G1 (IgG1) antibody highly specific for CLDN18.2, significantly improved the prognosis of advanced gastric/gastro-oesophageal junction and oesophageal adenocarcinoma patients (20). However, the expression of CLDN18.2 in AFPGC and possibility as a therapeutic target for AFPGC has not been elucidated.
In this study, we retrospectively collected 98 AFPGC cases and analyzed the clinicopathological characteristics and patient survival. We also investigated CLDN18.2 expression in AFPGC, focusing on its association with the clinicopathological and immunohistochemical parameters of this unusual GC subtype. We present the following article in accordance with the REMARK reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-462/rc).
Methods
Patient selection
A total of 98 patients with primary AFPGC were retrospectively recruited from the routine and consultation files of Fudan University Shanghai Cancer Center from 2010 to 2021. The diagnosis of AFPGC was reached by the combination of characteristic morphology (hepatoid or enteroblastic differentiation) and immunostaining [AFP (serum and IHC), GPC3, Arg-1 and SALL4] as previously described (5). Another 356 patients with stage-matched conventional GC (cGC) were enrolled as a control group from 2010 to 2012. The control group has the similar background of age, gender, and location. All available slides for each case were reviewed independently by at least two expert pathologists to confirm the diagnosis.
Clinicopathological features [including age, gender, overall survival (OS) time and status, tumor features, and serum AFP level] from all patients were obtained from medical records, pathological reports, and personal interviews. All tumors were staged according to the TNM classification system of the American Joint Committee on Cancer (8th version, 2018).
The patients were followed up every 3–6 months after diagnosis until 17 December, 2021. OS was defined as the length of time between surgery/biopsy and death of the patient or the last follow-up date. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Clinical Research Ethics Committee of the Fudan University Shanghai Cancer Center (No. 050432-4-2108*). Signed informed consent was obtained from all patients for the use of their tissues in this study.
Serum assays for AFP
For 71 hospitalized patients, blood samples were obtained from all patients within 1 week before the surgery. The serum AFP levels were measured as previously described (21). The cutoff value for serum AFP was 10.00 ng/mL.
Immunohistochemistry (IHC)
IHC staining was carried out on freshly cut 3–5 µm-thick paraffin sections using a Ventana automation system as previously described (Ventana Medical Systems, Tucson, AZ, USA) (21).
The antibodies used were as follows: GPC-3 (clone 1G12, prediluted; Maixin, Fuzhou, China), SALL-4 (clone 6E3, 1:100; Biocare, Pacheco, CA, USA), Arg-1 (monoclonal, 1:6,000; Sigma-Aldrich, St. Louis, MO, USA), HepPar-1 (clone OCH1E5, 1:100, Maixin), ERBB2 (HER2; Clone 4B5, prediluted; Ventana), and CLDN18.2 (monoclonal; 43-14A, prediluted; Ventana).
Microsatellite instability was assessed using IHC staining for mismatch repair (MMR) proteins, including MLH1 (clone M1, prediluted; Ventana), PMS2 (clone A16-4, prediluted; Ventana), MSH2 (clone G2-19-1129, prediluted; Ventana), and MSH6 (clone SP93, prediluted; Ventana). Loss of expression of any of the MMR proteins was considered to have occurred when there was no corresponding IHC staining in the nuclei in tumor cells, whereas adjacent normal colonic mucosa and/or stromal cells had nuclear staining by contrast.
Scoring CLDN18.2 staining
The expression of CLDN18.2 was evaluated using a H-score system according to 2 components: the intensity of staining and the percentage of tumor cells stained at different intensities. The intensity of the staining was classified as 0 (no stained cells), 1+ (weak membrane expression), 2+ (moderate membrane expression), and 3+ (strong membrane expression). The H-score was calculated by adding the multiplication of the different staining intensities in 4 gradations with each percentage of positive cells (22).
CLDN18.2 positivity was defined as moderate (2+) or strong (3+) CLDN18.2 staining in at least 40% of the tumor cells, while the remainder were defined as CLDN18.2 negative tumors (20).
Statistical analysis
All analyses were performed using SPSS 22.0 (IBM Corp., Armonk, NY, USA). GraphPad Prism 7.0 software (GraphPad Software Inc., San Diego, CA, USA) was used for scientific graphing. A Student’s t-test or a one-way analysis of variance (ANOVA) was applied to evaluate the differences of CLDN18.2 expression between two or multiple groups. Comparisons between serum AFP level and clinicopathological features (including age, gender, position, tumor size, differentiation, and stages), as well as the positive rate of CLDN18.2 were determined by chi-square test or Fisher’s exact test, as appropriate. Survival of AFPGC and cGC was calculated using the Kaplan-Meier method, with P values generated by the log-rank test to compare the cumulative incidences among different groups. A two-sided P values less than 0.05 were considered significant.
Results
Clinicopathological characteristics
The clinicopathological characteristics of the 98 AFPGC patients are summarized in Table 1. The cohort included 88 surgical excisions and 10 endoscopic biopsies. There were 78 males (79.6%) and 20 females (20.4%; male to female ratio =3.9:1). The median age of the patient cohort was 65 years, ranging from 22 to 82 years. The majority of the patients (59/71, 83.1%) showed elevated serum AFP level ranging from 24.14 to ≥3,630 ng/mL (median, 292 ng/mL; 97.78±1,308.39 ng/mL).
Table 1
| Features | N |
|---|---|
| Age (years), median [range] | 65 [22–82] |
| <60 | 33 |
| ≥60 | 65 |
| Gender | |
| Male | 78 |
| Female | 20 |
| Position | |
| Upper | 29 |
| Middle | 24 |
| Low | 36 |
| Tumor size (cm) | |
| <5 | 41 |
| ≥5 | 31 |
| Differentiation | |
| Moderate | 8 |
| Poor | 80 |
| T stage | |
| 1 | 7 |
| 2 | 23 |
| 3 | 33 |
| 4 | 25 |
| N stage | |
| 0 | 19 |
| 1 | 31 |
| 2 | 25 |
| 3 | 13 |
| Distant metastasis | |
| 0 | 57 |
| 1# | 18 |
| 2## | 4 |
| TNM stage | |
| I | 18 |
| II | 34 |
| III | 26 |
| IV | 11 |
| AFP-serum | |
| <10 | 12 |
| ≥10 | 59 |
#, synchronous distant metastases; ##, metachronous distant metastases. AFPGC, alpha-fetoprotein-producing gastric cancer; TNM, tumor node metastasis; AFP, alpha-fetoprotein.
The tumors were relatively evenly distributed in the stomach, with 36 (40.4%) occurring in the antrum, 24 (27.0%) in the body, and 29 (32.6%) in the fundus and cardia. Among all the 88 surgical specimens, 8 cases (9.1%) showed moderate differentiation, 80 cases (90.9%) showed poor differentiation, and none of the cases showed good differentiation. The invasion depth of 7 tumors (8.0%) was limited to the mucosa or submucosa (T1), while 81 cases (92.0%) invaded deeper than the submucosa (T2–T4). Lymph node metastasis was observed in 69 patients (78.4%). Synchronous distant metastases were observed in 18 patients, including 16 liver metastases and 2 multi-organ metastases, while another 4 patients developed metachronous metastases during the follow-up.
The clinicopathological features of the stage-matched control group are summarized in Table S1.
Pathological findings
The tumor morphology was classified into 4 patterns according to the description of Kinjo et al. (5). The COM, HPT, and ENT types were commonly seen in most of the cases (Figure 1A-1C). Transition between different subtypes were frequently seen. We didn’t find any tumors showing a typical appearance of the YST type, although 1 of the consultation cases was formerly misdiagnosed as a yolk sac tumor.
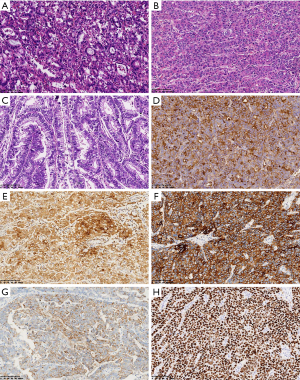
For IHC staining, nearly all the cases showed positive staining of at least 1 hepatocytic marker. Sixty-three of 74 cases (85.1%) showed variable GPC-3 expression, HepPar-1 was positive in 44 of 69 cases (63.8%), Arg-1 was positive in 22 of 60 cases (36.7%), focal AFP immunoreactivity was found in 46 of 52 cases (88.4%), and the ENT marker SALL4 was positive in 59 of 66 cases (89.4%; Figure 1D-1H). Immunoreactivity of at least 1 of the neuroendocrine markers (chromogranin A, CD56, and synaptophysin) was found in 19 of 50 cases (38%).
Microsatellite stability (MSS) status was detected in 52 patients, and all patients were confirmed as MSS by IHC. Human epidermal growth factor receptor 2 (HER2) was found to be positive (3+) in only 3 out of 81 patients (3.7%) and equivocal (2+) in 10 patients (12.3%), which was lower than previously reported for AFPGC.
Follow-up
Survival data of 85 patients were available for analysis. The final follow-up date was 17 Dec, 2021. As of the final follow-up, 19 patients had experienced disease-related death. The follow-up period of the AFPGC patients ranged from 1 to 129 months, and the median follow-up period was 16 (23.55±24.97) months. By dividing these patients into two groups according to the median value of preoperative serum AFP levels, we found that patients with a preoperative serum AFP level of ≥292 ng/mL showed significantly worse OS than the AFP-lower group (P=0.046; Figure 2A).
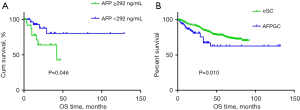
When compared with the stage-matched control group, the AFPGC patients exhibited a relatively poorer prognosis than the cGC patients, as was previously described (P=0.010; Figure 2B).
Notably, AFPGC patients with higher AFP levels were very prone to developing lymph node metastasis (P=0.024) and always showed a later TNM stage, which resulted in a poorer prognosis (P=0.020). However, no significant correlation was found between serum AFP level and patient age, gender, tumor position, tumor size, differentiation, invasion depth, or distant metastasis (Table 2).
Table 2
| Parameters | Serum AFP level, n | P value | |
|---|---|---|---|
| <292 | ≥292 | ||
| Age (years) | 0.864 | ||
| <60 | 11 | 12 | |
| ≥60 | 24 | 24 | |
| Gender | 0.387 | ||
| Male | 30 | 28 | |
| Female | 5 | 8 | |
| Position | 0.278 | ||
| Upper | 12 | 12 | |
| Middle | 13 | 8 | |
| Low | 10 | 16 | |
| Tumor size (cm) | 0.323 | ||
| <5 | 20 | 16 | |
| ≥5 | 13 | 17 | |
| Differentiation | 0.632 | ||
| Moderate | 4 | 3 | |
| Poorly | 30 | 33 | |
| T stage | 0.210 | ||
| 1 | 5 | 1 | |
| 2 | 10 | 7 | |
| 3 | 12 | 15 | |
| 4 | 6 | 10 | |
| N stage | 0.024* | ||
| 0 | 11 | 2 | |
| 1 | 12 | 12 | |
| 2 | 8 | 13 | |
| 3 | 2 | 6 | |
| Distant metastasis | 0.550 | ||
| 0 | 29 | 27 | |
| 1 | 6 | 8 | |
| TNM stage | 0.020* | ||
| I | 12 | 2 | |
| II | 10 | 14 | |
| III | 6 | 12 | |
| IV | 5 | 5 | |
*, P<0.05. AFP, alpha-fetoprotein; TNM, tumor node metastasis.
CLDN18.2 expression in AFPGC
CLDN18.2 staining was studied using whole tissue sections. A total of 51 AFPGC and 365 cGC samples were analyzed for CLDN18.2 expression. The overall expression rate of CLDN18.2 in AFPGC was low: 13 AFPGC tissues were completely devoid of any CLDN18.2 expression (25.5%). Of the positive AFPGC tissues, 27 cases showed a maximum of CLDN18.2 IHC 2+ or 3+ in no more than 30% of tumor cells (52.9%) (Figure S1). Only 11 cases (21.6%) were defined as CLDN18.2 positive according to the criteria described by Sahin et al. (20), which was much lower than the rate observed in cGC (38.5%; Figure 3A) (χ2=5.515; P=0.019). Among all clinicopathological parameters, tumor differentiation was the only factor that was significantly related to CLDN18.2 positivity (Table 3). There was no significant correlation between CLDN18.2 expression and the OS of the patients (Figure S2).
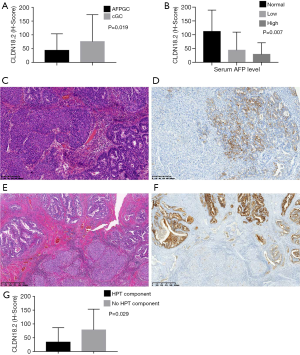
Table 3
| Parameters | CLDN18.2, n | P value | |
|---|---|---|---|
| Negative | Positive | ||
| Age (years), median [range] | 65 [36–82] | 0.964 | |
| <60 | 12 | 3 | |
| ≥60 | 29 | 7 | |
| Gender | 0.128 | ||
| Male | 33 | 10 | |
| Female | 8 | 0 | |
| Position | 0.767 | ||
| Upper | 14 | 4 | |
| Middle | 13 | 2 | |
| Low | 14 | 4 | |
| Tumor size (cm) | 0.942 | ||
| <5 | 20 | 5 | |
| ≥5 | 19 | 5 | |
| Differentiation | 0.046* | ||
| Moderate | 3 | 3 | |
| Poorly | 38 | 7 | |
| T stage | 0.142 | ||
| 1 | 2 | 2 | |
| 2 | 9 | 2 | |
| 3 | 21 | 2 | |
| 4 | 8 | 4 | |
| N stage | 0.705 | ||
| 0 | 8 | 2 | |
| 1 | 13 | 5 | |
| 2 | 15 | 2 | |
| 3 | 4 | 1 | |
| Metastasis | 0.581 | ||
| 0 | 34 | 9 | |
| 1 | 7 | 1 | |
| TNM stage | 0.785 | ||
| I | 7 | 3 | |
| II | 15 | 4 | |
| III | 12 | 2 | |
| IV | 6 | 1 | |
*, P<0.05. CLDN18.2, claudin-18.2; TNM, tumor node metastasis.
We divided the patients into three groups according to their serum AFP level (AFP normal, AFP low, and AFP high). Interestingly, we found that the expression of CLDN18.2 was significantly lower in patients with higher serum AFP levels (P=0.007; Figure 3B), suggesting that CLDN18.2 expression might be negatively correlated with the patient’s preoperative serum AFP level. We also found that CLDN18.2 tended to be expressed at a higher level in the HPT and COM areas than in HPT areas (Figure 3C-3F). The expression level of CLDN18.2 was significantly lower in AFPGC tissues with HPT components than those without (P=0.029; Figure 3G).
Discussion
The incidence rate of AFPGC has been reported to be 0.3–2% or 2.6–5.4% in different countries (23-25). Due to its unique clinicopathological features and significantly worse outcomes, AFPGC is attracting increasing attention. In this study, we analyzed 98 cases of AFPGC. We found some similar clinical and pathological features as previously described, such as (I) elevated serum AFP levels; (II) poor differentiation in most cases; (III) advanced TNM stage at first diagnosis; (IV) high likelihood of developing synchronous and metachronous distant metastasis; and (V) significantly worse prognosis, especially in patients with higher AFP levels. These findings suggest that AFPGC might have more aggressive biological behavior than cGC and should be recognized and managed accordingly.
Histologically, AFPGC is divisible into four main morphological subtypes: COM, ENT, HPT, and YST. Mixtures of different subtypes are frequently observed in clinical practice. It has been reported that many cases of AFPGC develop as COM or ENT in the mucosa, which differentiate into ENT and HPT during the process of tumor invasion and proliferation, acquiring AFP production ability (5). The YST type is rarely reported. In our case series, 1 consultation patient had been previously misdiagnosed with a YST that turned out to be an HPT-type AFPGC. The tumor showed a solid growth pattern composed of large polygonal eosinophilic hepatocyte-like neoplastic cells, which was histologically similar to a solid pattern yolk sac tumor. However, the existence of the intraepithelial neoplasia lesion in the peritumoral area and immunostaining of pan-cytokeratin and hepatocytic markers suggested that the tumor was more likely to be a primary AFPGC.
Clues pointing to the possibility of AFPGC include the following: (I) a HPT or ENT appearance of the tumor; (II) elevated preoperative serum AFP level; and (III) immunoreactivity of at least 1 hepatocytic or ENT marker. As an oncofetal glycoprotein rarely expressed in normal tissues, significant AFP synthesis signals a certain degree of retrodifferentiation (reversal of ontogeny) and a potential stemness (26,27), which may result in more aggressive behavior and lead to a poor prognosis. Serum AFP level is not only as a diagnostic and prognostic marker for AFPGC but can also be used as an indicator for therapeutic effect evaluation as well as a monitor of tumor progression. In our study, a majority of the patients showed elevated serum AFP levels prior to tumor resection and decreased remarkable after radical surgeries. Patients with higher serum AFP levels exhibited significantly worse clinical outcomes. A higher serum AFP level (≥292 ng/mL) was correlated with more lymph node metastases and a later TNM stage, and the postoperative elevation of serum AFP strongly indicated residual tumor, metastasis, or relapse. These results were consistent with other investigations.
We found neuroendocrine marker positivity in 38% of the patients. The combination of the strong reactivity of neuroendocrine markers, a high marker of proliferation Ki67 (Ki67) rate, and solid and organoid growth patterns could easily lead to the misdiagnosis of neuroendocrine tumors. It is also a difficult challenge for pathologists to distinguish AFPGC (particularly the HPT type) from HCC, especially when the tumors are first found outside the stomach. Correct categorization of the tumors should be made based on morphology, immunocytochemistry, and adequate history-taking. Immunoreactivity of hepatocyte-specific markers such as GPC-3, HepPar-1, and Arg-1 could be helpful for discriminating AFPGC from neuroendocrine tumors, while positive expression of caudal type homeobox 2 (CDX2) and SALL4, as well as a previous history of gastric tumor, would warrant the diagnosis of metastatic AFPGC. Moreover, abnormal expression of CLDN18.2, an emerging novel marker uniquely expressed in gastric epithelial cells and more likely to be found in GC (28), may also be helpful for the diagnosis of a small proportion of patients and the screening of potential patients who would benefit from the target therapy.
As a highly selective gastric lineage antigen, CLDN18.2 staining was observed at different intensities in 74% of the AFPGC cases in our study. But only 20% of these cases were defined as positive, which was much lower than the rate observed in cGC. CLDN18.2 positivity was significantly correlated with the differentiation of AFPGC. Tumors with elevated serum AFP levels showed significantly lower CLDN18.2 expression, which suggested that CLDN18.2 might be negatively correlated with a patient’s serum AFP levels. We also found that AFPGCs with an HPT component showed significantly less CLDN18.2 expression than those without, in accordance with the tendency that tumor areas with ENT and COM morphology show more CLDN18.2 expression than those with HPT morphology. The reason for this may be that the tumor cells lost gastric epithelial differentiation during the retro-differentiation progression, since the HPT type is regarded as a more invasive subtype compared to the COM and ENT types (5). More studies should be carried out to confirm this phenomenon.
There are no established treatment guidelines for AFPGC. Treatment of AFPGC usually follows that of cGC, with radical surgery regarded as the first-line approach, followed by adjuvant chemotherapy or targeted therapy if needed [such as S-1-based chemotherapies (29) or apatinib combined with other drugs (30)]. Trastuzumab has been confirmed as an additional useful therapeutic standard option for patients with HER2-positive advanced GCs and in aggressive variants of adenocarcinomas such as HER2-positive AFPGCs (31). Previous studies have reported that HER2 is overexpressed in 21.8–42.86% of AFPGCs (8,31,32). However, in our case, the amplification rate of HER2 was much lower. Through IHC staining, HER2 was found to be positive (3+) in only 3 cases and equivocal (2+) in 10 cases; the remainder of the cases were all HER2-negative (67, 83.75%). The fact that HER2 was not always routinely detected in AFPGC could have led to underestimated positive rates. Intratumoral heterogeneity of the tumor might also be a possible cause for this result.
As a highly selective gastric-lineage marker expressed in differentiated cells of the gastric mucosa, CLDN18.2 has become an emerging therapeutic target for GC (20,33). Zolbetuximab (IMAB362) is a novel chimeric IgG1 experimental antibody that binds CLDN18.2 on the tumor cell surface and induces immune effectors that activate antibody-dependent cell-mediated cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) (11). In the FAST study, the addition of zolbetuximab to epirubicin, oxaliplatin, and capecitabine (EOX) significantly improved progression-free survival and OS for patients with advanced CLDN18.2-positive gastric and gastroesophageal adenocarcinoma (20). However, in the patients with AFPGC, CLDN18.2 positivity (>40% of tumor cells with 2+ or 3+ staining intensity) was found in only 20% of the cases, which was significantly lower than the rate in the cGC cases. Thus, the enhanced efficacy from zolbetuximab might be limited to this small proportion of patients, adding more challenges to the treatment of AFPGCs.
Conclusions
AFPGC is a subtype of GC with distinct clinicopathological features and more aggressive biological behavior. This study found that CLDN18.2 expression was significantly decreased in AFPGC and was negatively correlated with the preoperative serum AFP level of the patients. The negative correlation between AFP and CLDN18.2 could be explained by retrodifferentiation of AFPGC. Decreased CLDN18.2 expression make it even more difficult to manage this malignancy. Therefore, special treatment strategies might be needed for this unique tumor type.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation of China (Nos. 81972249, 82172702, and 81902430), the Shanghai Clinical Science and Technology Innovation Project of Municipal Hospital (No. SHDC12020102), the Clinical Research Project of Shanghai Shenkang Hospital Development Center (No. SHDC2020CR4068), the Natural Science Foundation of Shanghai (Nos. 21ZR1414900 and 22ZR1413000), the Shanghai Science and Technology Development Fund (No. 19MC1911000), the Shanghai Municipal Key Clinical Specialty (No. shslczdzk01301), and the Shanghai ‘Rising Stars of Medical Talents’ Youth Development Program Youth Medical Talents-Specialist Program [No. SHWSRS(2020)087].
Footnote
Reporting Checklist: The authors have completed the REMARK reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-462/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-462/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-462/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Clinical Research Ethics Committee of the Fudan University Shanghai Cancer Center (No. 050432-4-2108*). Signed informed consent was obtained from all patients for the use of their tissues in this study.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Bergstrand CG, Czar B. Demonstration of a new protein fraction in serum from the human fetus. Scand J Clin Lab Invest 1956;8:174. [Crossref] [PubMed]
- Sun W, Liu Y, Shou D, et al. AFP (alpha fetoprotein): who are you in gastrology? Cancer Lett 2015;357:43-6. [Crossref] [PubMed]
- WHO Classification Tumours Editorial Board. editor. WHO classification of tumours: digestive system tumours. 5th ed. Lyon: IARC Press, 2019.
- Bourreille J, Metayer P, Sauger F, et al. Existence of alpha feto protein during gastric-origin secondary cancer of the liver. Presse Med (1893) 1970;78:1277-8. [PubMed]
- Kinjo T, Taniguchi H, Kushima R, et al. Histologic and immunohistochemical analyses of α-fetoprotein--producing cancer of the stomach. Am J Surg Pathol 2012;36:56-65. [Crossref] [PubMed]
- Murakami T, Yao T, Mitomi H, et al. Clinicopathologic and immunohistochemical characteristics of gastric adenocarcinoma with enteroblastic differentiation: a study of 29 cases. Gastric Cancer 2016;19:498-507. [Crossref] [PubMed]
- Wang Y, Sun L, Li Z, et al. Hepatoid adenocarcinoma of the stomach: a unique subgroup with distinct clinicopathological and molecular features. Gastric Cancer 2019;22:1183-92. [Crossref] [PubMed]
- He F, Fu Y, Sun Q, et al. Integrated clinicopathological and immunohistochemical analysis of gastric adenocarcinoma with hepatoid differentiation: an exploration of histogenesis, molecular characteristics, and prognostic markers. Hum Pathol 2021;115:37-46. [Crossref] [PubMed]
- Ushiku T, Shinozaki-Ushiku A, Maeda D, et al. Distinct expression pattern of claudin-6, a primitive phenotypic tight junction molecule, in germ cell tumours and visceral carcinomas. Histopathology 2012;61:1043-56. [Crossref] [PubMed]
- Liu X, Yu H, Cai H, et al. Expression of CD24, p21, p53, and c-myc in alpha-fetoprotein-producing gastric cancer: Correlation with clinicopathologic characteristics and survival. J Surg Oncol 2014;109:859-64. [Crossref] [PubMed]
- Singh P, Toom S, Huang Y. Anti-claudin 18.2 antibody as new targeted therapy for advanced gastric cancer. J Hematol Oncol 2017;10:105. [Crossref] [PubMed]
- Ouban A. Claudin-1 role in colon cancer: An update and a review. Histol Histopathol 2018;33:1013-9. [PubMed]
- Yang M, Li Y, Shen X, et al. CLDN6 promotes chemoresistance through GSTP1 in human breast cancer. J Exp Clin Cancer Res 2017;36:157. [Crossref] [PubMed]
- Ashikari D, Takayama KI, Obinata D, et al. CLDN8, an androgen-regulated gene, promotes prostate cancer cell proliferation and migration. Cancer Sci 2017;108:1386-93. [Crossref] [PubMed]
- Huang GW, Ding X, Chen SL, et al. Expression of claudin 10 protein in hepatocellular carcinoma: impact on survival. J Cancer Res Clin Oncol 2011;137:1213-8. [Crossref] [PubMed]
- Xiang Z, Zhong C, Chang A, et al. Immune-related key gene CLDN10 correlates with lymph node metastasis but predicts favorable prognosis in papillary thyroid carcinoma. Aging (Albany NY) 2020;12:2825-39. [Crossref] [PubMed]
- Niimi T, Nagashima K, Ward JM, et al. claudin-18, a novel downstream target gene for the T/EBP/NKX2.1 homeodomain transcription factor, encodes lung- and stomach-specific isoforms through alternative splicing. Mol Cell Biol 2001;21:7380-90. [Crossref] [PubMed]
- Pellino A, Brignola S, Riello E, et al. Association of CLDN18 Protein Expression with Clinicopathological Features and Prognosis in Advanced Gastric and Gastroesophageal Junction Adenocarcinomas. J Pers Med 2021;11:1095. [Crossref] [PubMed]
- Athauda A, Chau I. Claudin 18.2-a FAST-moving target in gastric cancer? Ann Oncol 2021;32:584-6. [Crossref] [PubMed]
- Sahin U, Türeci Ö, Manikhas G, et al. FAST: a randomised phase II study of zolbetuximab (IMAB362) plus EOX versus EOX alone for first-line treatment of advanced CLDN18.2-positive gastric and gastro-oesophageal adenocarcinoma. Ann Oncol 2021;32:609-19. [Crossref] [PubMed]
- Ren F, Weng W, Zhang Q, et al. Clinicopathological features and prognosis of AFP-producing colorectal cancer: a single-center analysis of 20 cases. Cancer Manag Res 2019;11:4557-67. [Crossref] [PubMed]
- Specht E, Kaemmerer D, Sänger J, et al. Comparison of immunoreactive score, HER2/neu score and H score for the immunohistochemical evaluation of somatostatin receptors in bronchopulmonary neuroendocrine neoplasms. Histopathology 2015;67:368-77. [Crossref] [PubMed]
- Chang YC, Nagasue N, Abe S, et al. Comparison between the clinicopathologic features of AFP-positive and AFP-negative gastric cancers. Am J Gastroenterol 1992;87:321-5. [PubMed]
- Ushiku T, Uozaki H, Shinozaki A, et al. Glypican 3-expressing gastric carcinoma: distinct subgroup unifying hepatoid, clear-cell, and alpha-fetoprotein-producing gastric carcinomas. Cancer Sci 2009;100:626-32. [Crossref] [PubMed]
- Yamazawa S, Ushiku T, Shinozaki-Ushiku A, et al. Gastric Cancer With Primitive Enterocyte Phenotype: An Aggressive Subgroup of Intestinal-type Adenocarcinoma. Am J Surg Pathol 2017;41:989-97. [Crossref] [PubMed]
- Matsunou H, Konishi F, Jalal RE, et al. Alpha-fetoprotein-producing gastric carcinoma with enteroblastic differentiation. Cancer 1994;73:534-40. [Crossref] [PubMed]
- Kuhlmann WD, Peschke P. Hepatic progenitor cells, stem cells, and AFP expression in models of liver injury. Int J Exp Pathol 2006;87:343-59. [Crossref] [PubMed]
- Li WT, Jeng YM, Yang CY. Claudin-18 as a Marker for Identifying the Stomach and Pancreatobiliary Tract as the Primary Sites of Metastatic Adenocarcinoma. Am J Surg Pathol 2020;44:1643-8. [Crossref] [PubMed]
- Harada M, Tsujimoto H, Ichikura T, et al. A case of a long-term survival achieved by surgical treatment and chemotherapy for late recurrence of AFP-producing gastric cancer. Surg Case Rep 2019;5:106. [Crossref] [PubMed]
- Li N, Bai C, Zhang R, et al. Efficacy and safety of apatinib for the treatment of AFP-producing gastric cancer. Transl Oncol 2021;14:101004. [Crossref] [PubMed]
- Giuffrè G, Ieni A, Barresi V, et al. HER2 status in unusual histological variants of gastric adenocarcinomas. J Clin Pathol 2012;65:237-41. [Crossref] [PubMed]
- Fujimoto M, Matsuzaki I, Nishino M, et al. HER2 is frequently overexpressed in hepatoid adenocarcinoma and gastric carcinoma with enteroblastic differentiation: a comparison of 35 cases to 334 gastric carcinomas of other histological types. J Clin Pathol 2018;71:600-7. [Crossref] [PubMed]
- Sahin U, Schuler M, Richly H, et al. A phase I dose-escalation study of IMAB362 (Zolbetuximab) in patients with advanced gastric and gastro-oesophageal junction cancer. Eur J Cancer 2018;100:17-26. [Crossref] [PubMed]


