
The evolving treatment paradigm of locally advanced rectal cancer: a narrative review
Introduction
Over the past century, the management of locally advanced rectal cancer (LARC) (i.e., clinical tumor stage T3-T4, or any T with involved regional lymph nodes) has improved dramatically thanks to the enormous advancements in surgical techniques as well as the incorporation of radiotherapy and chemotherapy in the multimodal treatment paradigm. Indeed, local recurrences (LR) and overall survival (OS) rates have significantly improved after the introduction of preoperative treatment and total mesorectal excision (TME). However, there is still relatively a high rate of distant recurrences of nearly 30% which lead eventually to death (1). A decade ago, management of LARC constituted mainly of neoadjuvant radiotherapy/chemoradiotherapy followed by surgery and optional adjuvant chemotherapy. Recently, the introduction of total neoadjuvant therapy (TNT) (i.e., delivering full dose radiotherapy and systemic chemotherapy prior to surgery) showed promising results (2,3). Furthermore, some reports suggest that pursuing a watch-and-wait (WW) strategy offers a non-invasive therapeutic alternative for patients who achieve clinical complete response (cCR) after neoadjuvant treatment with non-inferior long-term outcomes and with less morbidity (4-6).
Here we discuss the evolution of LARC management with all its aspects. In particular, we present the different perioperative approaches highlighting the different radiotherapy regimens applied in this setting, the role of chemotherapy and its timing, and the very-appealing, not yet widely adopted, organ preservation (OP) approach.
Our objective is to propose a treatment algorithm, based on available evidence, for patient selections aiming to deliver the best treatment, oncologically, with maximal preservation of quality of life and avoiding unnecessary side effects. We present the following article in accordance with the Narrative Review reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-13/rc).
Methods
We conducted a literature review through Google Scholar and PubMed (Table 1). We included only phase 2/3 trials that examined various perioperative strategies in LARC. With regards to articles that discussed OP, the selection criteria were expanded thus observational and retrospective studies were also eligible.
Table 1
| Items | Specification |
|---|---|
| Date of search (specified to date, month and year) | August 1st, 2021 |
| Databases and other sources searched | PubMed, Google scholar |
| Search terms used (including MeSH and free text search terms and filters) | “locally advanced rectal cancer”, “perioperative therapy in rectal cancer”, “neoadjuvant therapy”, “total neoadjuvant treatment”, “organ preservation”, “interval to surgery”, “short course radiotherapy”, “chemoradiation in rectal cancer” |
| Timeframe | Trials fully published until the date of search (August 1st, 2021) |
| Inclusion and exclusion criteria (study type, language restrictions etc.) | Phase 2/3 trials with mature results published in English. Articles that discussed organ preservation could also be observational and retrospective studies |
| Selection process (who conducted the selection, whether it was conducted independently, how consensus was obtained, etc.) | Search was conducted by MA and AH. All papers were assessed by all authors for eligibility. There were no cases of disagreement between authors |
| Any additional considerations, if applicable | N/A |
Role of radiotherapy
Historically, LR rates after surgical resection alone of LARC were unacceptably high. Indeed, in the pre-TME era, up to 30% of patients suffered from LR, which is frequently associated with significant morbidities. From a surgical perspective, treatment of such relapses would involve pelvic exenteration, which also entails high morbidity and mortality rates. Therefore, there was an urgent need to try to tackle this by offering adjuvant radiotherapy in order to optimize local control (LC) and sterilize the surgical bed.
The most widely practiced contemporary radiotherapy regimens are the short radiotherapy course (SCRT) which is comprised of 25 Gy given in 5 fractions and the long chemoradiotherapy course (LCCRT) which is comprised of 45–50 Gy given in 25 fractions with concurrent fluoropyrimidine (5-fluorouracil or capcetabine). Various large-scale trials have explored and demonstrated the added benefit of radiotherapy in the treatment paradigm of LARC (Table 2).
Table 2
| Study | Study arms | Patients # | Median follow-up | Primary end-point | Findings |
|---|---|---|---|---|---|
| GITSG 7175 (7) | Arm A: no adjuvant therapy; arm B: adjuvant radiation; arm C: adjuvant chemotherapy 5-FU + semustine; arm D: adjuvant radiotherapy and chemotherapy (combined) | 227 | 80 months | OS | Combined modality significantly improved time to recurrence, DFS and OS compared to no adjuvant therapy |
| NSABP R-02 (8) | Arm A: adjuvant chemotherapy 5-FU+semustine+vincristine or 5-FU + LV; arm B: adjuvant chemotherapy and radiotherapy | 694 | 93 months | DFS, OS | Radiotherapy did not affect OS and DFS. Radiotherapy significantly reduced 5-year LR (13% vs. 8%) |
| Swedish Rectal Cancer Trial (9) | Arm A: non-TME surgery only; arm B: neoadjuvant SCRT followed by surgery | 1,168 | 13 years | LR, OS | Pre-operative SCRT significantly reduced LR (26% vs. 9%) and improved OS by 29% |
| Dutch Trial (10) | Arm A: TME surgery only; arm B: neoadjuvant SCRT followed by TME | 1,861 | 12 years | LR, OS | SCRT significantly reduced 10-year LR (11% vs. 5%) in all subgroups. No significant difference in OS was observed though patients with stage III and negative CRM had improved OS associated with SCRT |
| German trial CAO/ARO/AIO-94 (11) | Arm A: neoadjuvant LCCRT with concurrent 5-FU and adjuvant 5-FU; arm B: adjuvant LCCRT with concurrent 5-FU and adjuvant 5-FU | 823 | 11 years | OS | No differences in OS, DFS or DM were noted. Arm A had significantly less 10-year LR (7.1% vs. 10.1%), as well as less acute and chronic G3/4 toxicity |
| MRC CR07 (12) | Arm A: neoadjuvant SCRT; arm B: selective adjuvant LCCRT with concurrent 5-FU restricted to CRM+ | 1,350 | 4 years | LR | Neoadjuvant therapy significantly reduced 3-year LR (4.4% vs. 10.6%) and improved 3-year DFS (77.5% vs. 71.5%.). No OS difference |
LARC, locally advanced rectal cancer; OS, overall survival; DFS, disease free survival; LR, local recurrence; SCRT, short-course radiotherapy; LCCRT, long-course chemoradiotherapy; 5-FU, 5-florouracil; LV, leucovorin; CRM, circumferential resection margin; DM, distant metastasis.
The potential role of radiotherapy was initially explored in the postoperative setting. Various randomized controlled trials (RCTs), such as the GITSG 7175 (7) and the NSABP R-02 (8), demonstrated the added benefit of adjuvant radiotherapy in addition to chemotherapy by improving LC rates.
The pivotal Swedish Rectal Cancer Trial (9) was among the first studies that demonstrated the feasibility and efficacy of preoperative radiotherapy. In this trial, patients were randomized between upfront surgery versus preoperative SCRT followed by surgery after 1 week. After median follow-up of 13 years, LR and OS were significantly improved in recipients of preoperative radiotherapy (9% vs. 26% and 38% vs. 30% respectively). One major limitation of the Swedish trial is that the surgical techniques used then are now considered inadequate and outdated. TME is now considered the standard of care in the surgical management of LARC due to better LC compared to blunt dissection (13). Therefore, after the results of the Swedish trials were published, many questioned the value of preoperative radiotherapy in the TME era, as this higher-quality technique was not utilized in the Swedish trial. To address this issue, the Dutch trial (11,14) tested the combination of SCRT with TME versus TME alone. The advantage of radiotherapy was preserved in terms of LR (2-year LR rates were 2.4% vs. 8.2%). However, and conversely to the Swedish trial, no OS differences were observed between the two groups.
Preoperative radiotherapy yields several potential advantages over postoperative radiotherapy. First, it may lead to downstaging thus potentially enabling a less morbid and sphincter-preserving surgery. Second, it is believed to be more tolerable as patients may be in worse condition after surgery. Third, treatment may be less toxic as radiation fields encompass a clinical target volume, which actually contain macroscopic disease, or a segment of diseased bowel destined to be sacrificed in surgery. This is in contrast to the clinical target volume in the postoperative setting, which may contain bowel loops that fills the operation field thus increasing radiation dose to healthy bowels with a subsequent increase in toxicity. Finally, it is believed that radiation is more potent in the preoperative setting as tumor vasculature and oxygenation are intact. Perhaps the most contemporary study that properly evaluated both strategies was the one conducted by Sauer and colleagues. In this phase 3 study (11) patients with resectable LARC were randomized between preoperative and postoperative LCCRT. The findings of this trial did not reveal OS or DFS differences between the two arms. However, less LR (6% vs. 13%) and grade 3–4 toxicity (27% vs. 40%) were observed among patients in the preoperative group. The MRC CR07 (12) sought to determine whether a risk-adapted approach could be implemented hence offering radiotherapy only to patients with high-risk features based on pathological assessment. In this study, 1,350 patients were randomized to either preoperative SCRT or selective postoperative LCCRT for patients with involved circumferential resection margin (CRM). Preoperative radiotherapy reduced the 3 years’ absolute risk of LR by 6.2% and improved 3-years DFS by 6.2% as well. Interestingly, the proportion of patients who had positive CRM was very similar between groups yet a significant difference in LR rates was observed (4% vs. 11%). This illustrates the prognostic significance of positive CRM in patients who proceed to upfront surgery, with these individuals being at higher risk of developing LR despite postoperative chemoradiation.
Radiosensitizers
Fluoropyrimidines are considered the main radiosensitizers in rectal cancer. Originally, 5-FU was administered as bolus infusion before and after radiation therapy (15). Later on, O’Connell et al. (16) showed that concurrent continuous 5-FU infusion is superior to bolus 5-FU in terms of relapse rates and OS. The FFCD 9203 (17) evaluated the added value of fluoropyrimidine-based chemotherapy given concomitantly with preoperative radiotherapy. This study demonstrated improved LR and pCR rates associated with chemoradiation compared with radiotherapy alone (8.1% vs. 16.5% and 11.4% vs. 3.6% respectively) with no difference in sphincter preservation rates. The EORTC 22921 (18) trial, which aimed to explore the benefit of chemotherapy and its timing (given either preoperatively, postoperatively, or both) compared with preoperative radiotherapy alone, also showed that chemotherapy, irrespective of its timing, further improved LC without major impact on OS or DFS. The NSABP-R04 (19) compared capecitabine with infusional 5-FU and demonstrated non-inferior sphincter-preservation, downstaging, pCR and toxicity rates.
The addition of oxaliplatin was also explored but the results were generally disappointing. In the ACCORD 12/0405-PRODIGE 2 trial (20), patients who received CAPOX (capecitabine plus oxaliplatin) suffered more from preoperative grade 3–4 toxicities without improvement in sphincter preservation or pCR rates. Similar conclusions were drawn from the STAR-01, NSABP-R04 and PETACC-6 trials (19,21,22). The controversial German CAO/ARO/AIO-04 trial (23) showed improvement in pCR rates and 3-year DFS associated with the addition of oxaliplatin, without increased toxicity. Nonetheless, this study had serious structural variabilities other than oxaliplatin incorporation, thus its results must be carefully interpreted.
Concurrent irinotecan showed promising activity in phase I/II trials (24,25), but two phase III trials (26,27) showed higher toxicity rates without significant added benefits.
Monoclonal antibodies, such as cetuximab, pantimumab and bevacizumab (28-32), which proved to be effective the metastatic setting, have failed to yield positive results when combined with radiotherapy perioperatively. Table 3 summarizes selected trials, which aimed to explore the benefit of concurrent chemoradiation.
Table 3
| Study | Study arms | Patients # | Median follow-up | Primary end-point | Findings |
|---|---|---|---|---|---|
| J. O’Connell trial (16) | Arm A: adjuvant chemotherapy (5-FU + semustine) and LCCRT with concurrent CVI 5-FU; arm B: adjuvant chemotherapy (5-FU) and LCCRT with concurrent CVI 5-FU; arm C: adjuvant chemotherapy (5-FU + semustine) and LCCRT with concurrent bolus 5-FU; arm D: adjuvant chemotherapy (5-FU) and LCCRT with concurrent bolus 5-FU | 660 | 46 months | OS | CVI significantly improved time to relapse and OS over bolus 5-FU. Systemic 5-FU alone is equivalent to doublet 5-FU + semustine |
| FFCD 9203 (17) | Arm A: neoadjuvant LCRT 45 Gy; arm B: neoadjuvant LCCRT 45 Gy given concurrently with 5-FU + LV | 733 | 81 months | OS | LCCRT significantly improved pCR rates (3.6% vs. 11.4%), reduced 5-year LR rates (16.5% vs. 8.1%), with more grade3/4 acute toxicity (2.7% vs. 14.6%). No OS or sphincter preservation differences |
| EORTC 22921 (18) | Arm A: neoadjuvant LCRT 45 Gy; arm B: neoadjuvant LCCRT 45 Gy with concurrent 5-FU + LV; arm C: same as arm A + adjuvant chemotherapy 5FU + LV; arm D: same as arm B + adjuvant chemotherapy 5FU + LV | 1,011 | 10.4 years | OS | Addition of chemotherapy whether in neoadjuvant or adjuvant settings nearly halved LR rates, though having no impact on OS, DFS, and DM rates. Notably, adjuvant chemotherapy does not affect DFS or OS regardless of the type of neoadjuvant therapy given |
| NSABP-R04 (19) | Arm A: CVI 5-FU; arm B: capecitabine; arm C: CVI 5-FU + oxaliplatin; arm D: capecitabine + oxaliplatin | 1,608 | 5 years | LRC | Capecitabine can be a good replacement for CVI 5-FU yielding similar LRC, DFS, OS and toxicity. The addition of oxaliplatin increased toxicity without improving outcomes |
| ACCORD 12/0405-PRODIGE (20) | Arm A: neoadjuvant LCCRT 45 Gy with concurrent capecitabine; arm B: neoadjuvant LCCRT 50 Gy with concurrent capecitabine + oxaliplatin | 598 | 5 years | pCR | No difference in pCR, DFS, OS, LRC. Arm B had increased acute G3/4 toxicity 11% vs. 25% |
| STAR-01 (21) | Arm A: neoadjuvant LCCRT 50.4 Gy with concurrent CVI 5-FU; arm B: neoadjuvant LCCRT 50.4 Gy with concurrent CVI 5-FU + oxaliplatin | 739 | 8.8 years | OS | Addition of oxaliplatin did not improve OS, pCR or EFS, rather increased G3/4 acute toxicity (8% vs. 24%) |
| PETACC-6 (22) | Arm A: neoadjuvant LCCRT 45–50.4 Gy with concurrent capecitabine and adjuvant capecitabine; arm B: similar to arm A with the addition of oxaliplatin to capeciatbine in both neoadjuvant and adjuvant treatments | 1,094 | 68 months | DFS | No improvement in outcomes. Similar 5-year DFS and OS around 71% and 80% respectively. Greater G3/4 adverse events in arm B |
| CAO/ARO/AIO-04 (23) | Arm A: neoadjuvant LCCRT 50.4 Gy with concurrent CVI 5-FU and Adjuvant 5-FU; arm B: neoadjuvant LCCRT 50.4 Gy with concurrent CVI 5-FU + oxaliplatin and adjuvant 5-FU + oxaliplatin | 1,236 | 50 months | DFS | Adding oxaliplatin significantly improved pCR and 3-year DFS (71.2% vs. 75.9%) with similar acute and late toxicity profiles. Comment: 5-FU chemotherapy protocols were substantially different between arms in both the neo-adjuvant and adjuvant settings as well as number of adjuvant cycles and addition of Oxaliplatin to adjuvant treatment in arm B |
OS, overall survival; DFS, disease free survival; LRC, locoregional control; pCR, pathologic complete response; EFS, event free survival; LCCRT, long-course chemoradiotherapy; LCRT, long-course radiotherapy; 5-FU, 5-florouracil; LV, leucovorin; CRM, circumferential resection margin; DM, distant metastasis; CVI, continuous venous infusion.
SCRT vs. LCRT
Althought neoadjuvant radiotherapy was firmly established as a standard of care based on several high-quality RCT (9,11,14), controversy remains regarding the optimal radiotherapy regimen.
Apparently, SCRT is more cost-effective and significantly shorter, making it particularly attractive to both patients and physicians alike. However, there have been concerns regarding suboptimal tumor downstaging and pathological response hence decreasing the likelihood of achieving sphincter-preserving surgery.
The pivotal polish I colorectal study (33) sought to determine whether the higher downstaging rate associated with LCCRT would be eventually translated to a higher rate of sphincter-preserving surgeries. The trial randomized patients with locally advanced disease (cT3–4) to either LCCRT followed by surgery after 4–6 weeks or SCRT followed by surgery within 1 week. With over 300 patients enrolled, and after a median follow-up of 48 months, no significant differences in OS, DFS and LC were detected between both groups and the rates of sphincter-preserving surgeries were not significantly different. Moreover, SCRT was not associated with more late toxicities compared to LCCRT. Therefore, based on the results of Polish I trial, it appears that SCRT is a valid alternative to LCCRT. However, there are several potential confounding factors in the Polish I trial that should be considered. First, more patients in the SCRT went on to receive adjuvant chemotherapy compared to those after LCCRT and this may potentially affect the DM and OS rates. Second, a large subset of patient (nearly 40%) in the SCRT had actually pT1–2 disease and given that surgery was performed in a relatively short interval after the end of SCRT, it is unlikely that this finding is fully explained by the downstaging effect of SCRT and this may actually reflect imprecisions in clinical staging. Thus, it could be that the SCRT group harbored more patients with more favorable prognosis.
The T-TROG 01.04 (34) randomized patients with cT3N0-2 disease to SCRT with early surgery and six months of adjuvant 5-FU vs. LCCRT with concurrent 5-FU and 4 months of adjuvant 5-FU. Again, LCCRT resulted in higher pCR rates (15% vs. 1%) and pathologic downstaging (45% vs. 28%) but no differences in CRM+, sphincter preservation, late toxicity, or long term oncological outcomes were detected. Concerningly, SCRT trended towards higher LR rates (7.5% vs. 4.4%), raising questions whether it could be due to a higher proportion of patients with low-lying tumor (less than 5 cm from anal verge) in that group (30% vs. 19%). These concerns are further intensified by a subgroup analysis of low-lying tumors which showed a trend towards poorer LC after SCRT in this setting. As it was demonstrated in the Polish II (35,36) study, the pCR rates after SCRT could be augmented by administering 3 cycles of FOLFOX following SCRT and before surgery.
To conclude, both preoperative SCRT and LCCRT are valid regimens to be used with available data to support oncological benefit of both regimens. The main advantage of the SCRT is its short duration which could be critical in places where access to radiotherapy units is not trivial and capacity is limited. Further, in the era of COVID-19 pandemic and when hospital visits should be minimized, the SCRT could be very appealing. Nonetheless, it should be acknowledged that all the above-mentioned trials had a heterogenous patient population and that a subset of patients had “good” tumours in the first place. This could underestimate the potential benefit of downstaging achieved in the LCCRT compared with SCRT. In addition, one should keep in mind the alarming signal regarding the suboptimal response of low-lying tumours to SCRT. Table 4 highlights various important factors that should be taken into consideration when choosing the preoperative radiotherapy regimen. We would suggest implementing LCCRT for cT4 tumour, Node-positive disease, threatened mesorectal fascia, and low-lying tumours. In these situations, we believe that it is imperative to choose the regimen with the higher chances of achieving downstaging. In other scenarios (mainly middle-height cT3N0 tumours) SCRT is certainly a good, less expensive, and less time-consuming alternative.
Table 4
| Indications | SCRT | LCRT |
|---|---|---|
| T4 | √ | |
| Node positive | √ | |
| Threatened MRF | √ | |
| Presence of oligometastasis | √ | |
| Low lying tumors | √ | |
| Organ preservation | √ | |
| None of the above | √ |
SCRT, short-course radiotherapy; LCCRT, long-course chemoradiotherapy; MRF, mesorectal fascia.
Interval to surgery
Findings from several RCTs suggest that prolonging the interval between radiation to surgery has a desirable impact on pCR, downstaging, and probably sphincter preservation calling for further investigation.
In the French Lyon R90-01 (37) trial, over 200 patients were treated (between the years 1991 and 1995) with preoperative radiation constituted of 39 Gy in 13 fractions followed by surgery either 2 or 6–8 weeks later. Longer interval to surgery substantially improved overall response rate (71% vs. 53%) as well as pathological response. Notably, postoperative complications rate and profile were similar between both arms. In 1998, the Stockholm III trial (38) was launched with the aim to determine the optimal radiotherapy fractionation and timing to surgery. In this RCT, patients with resectable tumour were randomized between SCRT followed by surgery within 1 week, SCRT followed by surgery after 4–8 weeks, or long-course radiotherapy followed by surgery within 4–8 weeks. After long-term follow up, no major differences in oncological outcomes were observed between the three groups. However, in a pooled, post-hoc analysis of patients who were treated with SCRT (immediate vs. delayed surgery), postoperative complications were less encountered in the SCRT with delayed surgery compared with SCRT and immediate surgery. The most commonly observed relapse pattern in Stockholm III was distant-only relapse. Although no major difference was observed in the DM rate between the three groups, many speculated that a prolonged interval to surgery could have had a negative impact, as systemic-dose chemotherapy was not delivered in a timely manner. In addition, only a small subset of patients in Stockholm III had actually received adjuvant chemotherapy. These findings motivated the investigators to call for consideration of integrating chemotherapy in the preoperative course during the waiting period for surgery thus potentially improving DM rates as well as pathological response even further. With this regard, Garcia-Aguilar et al. (39) reported incremental rising in pCR rates with longer interval to surgery filled with increasing cycles of FOLFOX following LCCRT. This has led to an impressive pCR rate of nearly 40% after 6 cycles of FOLFOX during 20 weeks’ interval to surgery, without increasing surgical difficulty, post-operative morbidity and mortality.
The phase III GRECCAR-6 study (40) primarily aimed to assess the effect of the waiting interval after preoperative LCCRT on the pCR rates. Patients with LARC were randomly assigned to either 7-weeks or 11-weeks period after LCCRT and before surgery took place. On pathological examination, there was no significant difference between the shorter and the longer waiting intervals. However, the 11-weeks group was associated with increased overall morbidity and a trend toward poorer perineal healing. Further, the quality of the mesorectal specimen, which strongly reflects the quality of surgery and oncological outcomes (13,41), was inferior in the 11-weeks compared to the 7 weeks-period. Based on these findings, a longer waiting period exceeding 7–8 weeks does not increase the pCR with a possible detrimental effect in terms of perioperative morbidity and quality of surgery.
In summary, current evidence suggests that a waiting period of 7–8 weeks after either SCRT or LCRRT is feasible with a possible benefit in terms of downsizing and increased likelihood of pCR. If there is no intention to deliver systemic dose chemotherapy preoperatively, we advise against longer waiting periods based on the GRECCAR-6 findings.
Adjuvant chemotherapy
In the pre-multimodal treatment era, adjuvant chemotherapy had positive impact on DFS and OS (42). Currently, with preoperative therapy being standard of care, the role of adjuvant chemotherapy has become controversial. Up to date, there is no robust evidence that demonstrated a meaningful benefit of adjuvant chemotherapy in completely resected LARC following preoperative (chemo)radiotherapy (43-45).
A Cochrane meta-analysis (46) which aimed to provide an answer to this question pooled around 9,200 patients with rectal cancer from 21 RCTs which compared surgery alone versus adjuvant chemotherapy. This meta-analysis showed a significant risk reduction in death and disease recurrence associated with adjuvant chemotherapy. However, this meta-analysis suffered from major limitations. First, most of the included trials did not mandate the delivery of neither pre nor postoperative radiotherapy, and second, a large subset of them were conducted in the pre-TME era. Therefore, the relevance of these findings in the contemporary management of LARC is questionable.
Perhaps the EORTC 22921 (18) trial is the most relevant study that evaluated the role of adjuvant chemotherapy in the multimodal treatment context. Essentially, this trial enrolled over 1,000 patients with cT3/T4 disease, and randomized them to either preoperative radiotherapy or chemoradiotherapy followed by adjuvant chemotherapy or surveillance. After a median follow-up of 10.4 years, no significant improvement in OS or DFS was associated with adjuvant chemotherapy regardless of the allocated preoperative treatment group. There were less LRs when chemotherapy was integrated in the perioperative treatment. Consequently, the authors concluded that no benefit attributed to adjuvant chemotherapy could be established. Having said that, there are several key issues in this trial that should be acknowledged. First, adherence to adjuvant chemotherapy was poor as the majority of patients who were allocated to adjuvant chemotherapy either did not complete or did not start at all the planned treatment course. Moreover, besides having locally advanced disease (pT3/4), most patients did not have bad prognostic factors such as pathologically involved nodes or positive margins. This means that a potential benefit of adjuvant chemotherapy might have been “diluted” by the majority of the trial participants who did not harbor a significant risk of distant relapse.
In summary, there is no compelling evidence that adjuvant chemotherapy leads to improved outcomes in the setting LARC treated with preoperative (chemo)radiotherapy. Plausible explanations include underpowered trials which recruited heterogenous groups of patients hence were unable to detect small yet significant improvements, low compliance to adjuvant chemotherapy after surgery, and the relatively late targeting of micrometastasis owing to time spent in preoperative treatment and postoperative recovery. The recommendation of using adjuvant chemotherapy is mainly extrapolated from the metastatic setting as well as from the adjuvant setting in colon cancer.
Total neoadjuvant approach
Unlike loco-regional recurrences (LRR), which are significantly mitigated by preoperative radiotherapy, distant relapse rates have not been profoundly improved by preoperative radiotherapy. Indeed, in the preoperative radiotherapy plus TME era, the relapse pattern has dramatically shifted with distant relapses now accounting for the majority of treatment failures (25–30%). As mentioned before, various trials failed to demonstrate significant effectiveness of adjuvant chemotherapy. Therefore, there was a growing interest in delivering both (chemo)radiotherapy and systemic-doses of chemotherapy preoperatively [i.e., total neoadjuvant treatment (TNT)].
TNT yields several advantages. First, it allows the delivery of systemic-dose of chemotherapy in a timely manner. Second, it could increase the proportion of patients who achieve pCR. Third, it may be more tolerable as patients may be in a worse state after surgery and possible colostomy. Lastly, it could enhance chemotherapy effect thanks to intact vasculature before surgery.
Several groups have explored the delivery of chemotherapy before (induction) or after (consolidation) neoadjuvant radiotherapy or LCCRT. Of note, some groups have also speculated that consolidation chemotherapy may have a greater impact on tumor response by the added value of prolonging the interval between radiotherapy and surgery.
The Spanish GCR-3 trial (47) was one of the first encouraging studies which showed the promise of TNT strategy. In this phase 2 trial, patients were randomized between 4 cycles of induction CAPOX followed by LCCRT and surgery versus LCCRT followed by surgery and adjuvant CAPOX. Although no differences in OS or DFS were detected, the TNT strategy was associated with higher compliance and more favorable acute toxicity profile.
Recently, two hallmark phase 3 RCTs have demonstrated the feasibility and efficacy of TNT. In RAPIDO trial (2), 912 patients were randomized between a TNT approach, which consisted of SCRT followed by six cycles of CAPOX or nine cycles of FOLFOX4 followed by TME, versus standard-of-care approach, which consisted of LCCRT followed by TME and optional adjuvant chemotherapy. Eligible patients in RAPIDO had high-risk features based on pelvic MRI, namely having either T4 disease, extramural vascular invasion, cN2, involved mesorectal fascia, or enlarged lateral lymph nodes. The primary endpoint of the study was disease related-treatment failure (DRTF). After median follow of nearly five years, DRTF was significantly improved in the TNT arm and this was attributed to significant reduction in DM (30% relative risk reduction that corresponds to 7% absolute risk reduction). Furthermore, the proportion of patients who achieved pCR in the TNT arm was significantly higher (28% vs. 14%). Interestingly, there was no significant difference in LRR rates. Sensitivity analysis within the standard-of-care group did not show any impact of adjuvant chemotherapy on the probability of DRTF. In terms of tolerability, 85% of patients in the TNT group completed the allocated treatment and more than 90% of them were able to undergo surgery with curative intent. Hence, the RAPIDO-derived TNT strategy appears to be effective in reducing DM rates with an acceptable toxicity profile. Of note, out of the 187 patients who were intended to have adjuvant chemotherapy in the standard-of-care arm, only 118 (63%) completed the allocated treatment, thus illustrating again the low compliance/poor tolerability of adjuvant chemotherapy. The second study was conducted by the French PRODIGE intergroup. In PRODIGE-23 (3), 461 patients were randomized between TNT and standard-of-care regimens. Here, TNT consisted of 6 cycles of FOLFIRINOX followed by LCCRT followed by surgery, whereas the standard-of-care arm included LCCRT followed by surgery. Both groups received adjuvant chemotherapy. The duration of adjuvant chemotherapy was group-dependent, with the TNT group receiving therapy for 3 months whereas standard-of-care group receiving it for 6 months. Unlike RAPIDO, PRODIGE-23 was more permissive in terms of eligibility criteria with patients having stage II disease being also eligible. Yet, only a minority of the study population (7%) had stage II disease. After median follow-up of about 4 years, 3-year DFS was significantly improved in the TNT group. As in RAPIDO, the difference was mainly attributable to the reduction in DM with findings mirroring those in RAPIDO. Moreover, similarly to RAPIDO, pCR rate was significantly higher in the TNT group (28% vs. 12%) yet no significant differences in LRR or spinchter-preserving surgeries were detected. TNT was tolerable and the majority of patients managed to complete the allocated therapy.
Both studies’ findings were concordant and showed significant improvement in DFS mainly due to improvement in DM rates. However, no differences in OS were observed in both studies yet data are still immature. Patients with metastatic disease tend to respond to systemic therapy initially and for a durable period, hence a reliable OS analysis would need longer follow-up to elucidate the true effect of reducing the incidence of metastatic relapse. It is of interest to note that LRR was not reduced by utilizing the TNT approach despite the higher pCR rates. A plausible explanation might be that there is a subset of non-responders who may actually progress during the prolonged period of neoadjuvant therapy hence tempering a LRR benefit driven by those who achieve pCR. Of note, the proportion of patients who had pT4 disease was somewhat higher in TNT arm compared to the standard-of-care arm in RAPIDO thus enforcing the hypothesis that some refractory tumors may actually progress during the prolonged interval to surgery. Therefore, predictive biomarkers before embarking on TNT approach would aid in proper selection of patients eligible for this strategy.
Furthermore, many oncologists feel that it is unreasonable to offer TNT for all newcomers with LARC as it might lead to overtreatment. Biomarkers in this context can shed more light on the patient’s risk of developing metastatic disease or alternatively their chances of responding to chemotherapy (e.g., microsatellite instability status known to correlate with poor response to fluoropyrimidine-based chemotherapy) thus mapping patients who could be spared such an intensified regimen of therapy.
Apart from patient selection, there are additional unanswered questions that need to be addressed in order to optimize the TNT strategy. First, what is the optimal regimen and duration of systemic chemotherapy given in TNT? Most phase 2 trials and RAPIDO used oxaliplatin-based regimens whereas in PRODIGE-23, FOLFIRINOX was used based on the improved response to this regimen in the metastatic setting. Cautiously saying, based on the comparable efficacy figures in RAPIDO/other phase 2 trials and PRODIGE-23, irinotecan does not seem to offer a significant additional benefit. Moreover, the addition of irinotecan might increase toxicity due to more gastrointestinal and hematological adverse events. The duration of neoadjuvant chemotherapy is also variable between studies and is a matter of debate. The Polish II (35) study failed to demonstrate the superiority of preoperative SCRT followed by 3 cycles of FOLFOX over LCCRT. The TNT approach used in Polish II was similar to RAPIDO with a key difference in the number of chemotherapy cycles used preoperatively (3 cycles of FOLFOX in Polish II vs. 9 cycles of FOLFOX or 6 cycles of CAPOX in RAPIDO). Garcia-Aguilar et al. (39) showed that the pCR rate increased with escalated consolidation cycles of FOLFOX after LCCRT with 38% of patients achieving pCR after 6 cycles of chemotherapy. In multivariate analysis, patients who received 6 cycles of chemotherapy were significantly more likely to have pCR compared with patients who received LCCRT only. Thus, based on the current evidence, it seems that 6 to 9 cycles of neoadjuvant FOLFOX (or its equivalence) are appropriate. Second, what is the optimal sequencing that should be used in TNT (consolidation vs. induction chemotherapy)? Induction chemotherapy offers the advantage of earlier targeting of micrometastasis. However, chemotherapy may actually provide survival advantage to chemotherapy-resistant clones, hence reducing the chances of eradicating such clones with radiotherapy. Additionally, tumors that are inherently unresponsive to chemotherapy may progress during the induction period. Thus, it seems sensible to address local disease first to prevent local progression, with subsequent consolidation chemotherapy. Furthermore, delivering consolidation chemotherapy provides an added value of interval prolongation between surgery and radiotherapy which may further improve pathological response. The CAO/ARO/AIO-12 (48) trial aimed to select the more promising TNT sequencing by applying pick-the-winner design. In that study, patients who were treated with consolidation chemotherapy exhibited higher compliance and less grade 3–4 toxicity during LCCRT. On the other hand, more patients in the induction arm managed to complete all the allocated chemotherapy cycles compared to those in the consolidation arm. More importantly, the pCR rate was higher in the consolidation arm reaching the pre-defined rate (25%) required to demonstrate superiority over standard chemoradiation while the induction arm failed to fulfill this criterion. Third, how long surgery could be deferred from the end of radiotherapy without compromising surgical quality? As previously mentioned, a longer waiting interval between radiotherapy and surgery was associated with more pelvic fibrosis, higher postoperative morbidity and lower quality of TME, thus raising concerns regarding the feasibility of surgery after TNT as the interval between the end of radiotherapy and surgery could be more than 12 weeks. Nevertheless, trials assessing TNT strategy did not rise any alarming signals regarding quality of surgery. Pelvic fibrosis was reported to be more prominent in the TNT groups yet no significant differences in R0 resections, types of operation, or quality of TME were observed. Finally, what is the optimal radiotherapy regimen that should be used? It seems that both SCRT and LCCRT-based TNT approaches yield encouraging results. These findings are in line with previous (33,34) reports that demonstrated the comparable efficacy and toxicity of both regimens. Based on current data, it seems that the choice between the two protocols relies mainly on institutional experience and preference.
OP
Although surgical intervention has always been the mainstay of the multi-modal therapy in LARC, it is not without significant morbidity and quality of life compromise. In particular, colostomy, which is often needed, can have great psychological and physiological impacts on patients, as many find it too difficult to come to terms with living with a stoma, even temporarily. Moreover, mortality rate after surgery is estimated to be around 1–2% particularly among older individuals and patients with comorbidities (49). Hence, OP is a desirable goal as both patients and their physicians seek cure with a minimal toll on quality of life and emotional well-being.
Several studies (50,51) indicate that pCR is associated with very good oncological outcomes including high control rates (both locally and systematically) and OS. Therefore, it is speculated that in this subset of patients who achieve pCR, surgery could be safely avoided. Recently, OP for LARC with watch-and-wait strategy has gained wider recognition, being proposed for selected patients achieving cCR after neoadjuvant treatment (4,52).
Habr-Gama et al. (53) sought to compare the outcomes of patients with LARC who achieved cCR following LCRRT and were managed nonoperatively with subsequent observation, with those who went on to have surgery after LCRRT and were proved to have pCR. Five-year OS and DFS were 100% and 92% in the non-surgical group compared to 88% and 83% in the resected group. Notably, none of the patients developed pelvic recurrence and no difference in distant recurrences was detected. Similarly, Beard and colleagues (54) reported their findings related to 31 LARC patients undergoing non-operative management following LCCRT, showing favorable outcomes with 3-year LC, DFS and OS of 77.4%, 74.4% and 93.4% respectively. Nodal stage was the only variable predictive of relapse. In addition, most LR were salvageable by surgery, albeit the outcomes of those were ultimately poor with over half developing DM.
In a meta-analysis (6) including over 860 patients treated with LCCRT, following cCR, the 2-year local regrowth was 15.7%, with no difference in DM, DFS or OS between operative and non-operative management. Patients who went on to have surgery and proved to have a pCR in surgical specimen, had similar rates of regrowth and OS compared to those with cCR who were managed non-surgically but with better DFS (probably due to discordance between pCR and cCR status). The international watch and wait database (IWWD) (5,55), which is the largest registry that aims to collect and report the outcomes of patients with LARC who had neoadjuvant therapy and did not undergo surgery, reported the outcomes of 880 patients who had cCR after neoadjuvant therapy. Nearly half of the included patients had T3 disease and involved nodes. After a median follow-up of 3.3 years, the 2-year local regrowth was 25.2%, of which 97% recurred in the bowel wall. Around 5% of patients had pelvic relapse which involved both intra-luminal and pelvic nodes regrowth, yet only 3% of patients suffered from isolated pelvic nodal relapse. Most regrowths occurred relatively early within the first 24 months. In addition, most documented LRs were amenable to salvage surgery as 78% of evaluable patients had surgery with curative intent. Long-term oncological outcomes showed DM rate of 8%, 5-year OS and DFS of 85% and 94% respectively. Noteworthy, staging modalities at baseline and reassessment were not ideal as only 71% had MRI scan at reassessment. Of note, delayed local regrowth after 7 years occurred, therefore long-term, close surveillance is mandated.
The ongoing organ preservation in rectal adenocarcinoma (OPRA) trial (56) is a multicenter phase 2 trial which aim to prospectively evaluate OP strategy by randomizing patients to either consolidation or induction chemotherapy combined with LCCRT. All patients were staged with MRI before embarking on TNT. After 8–12 weeks from treatment completion, patients underwent comprehensive clinical assessment including digital rectal examination, flexible sigmoidoscopy and MRI. Those who were deemed to have cCR or near cCR were offered watchful waiting. The preliminary results of over 300 patients, showed similar 3-year DFS and DM free survival, but consolidation chemotherapy had significantly better OP rates compared with induction chemotherapy (58% vs. 43%).
Despite encouraging reports, there is still no consensus about patient selection criteria for OP. The current evidence supporting the role of OP still suffers from major limitations. First, there is large heterogenicity both in treatments and patient characteristics across different studies. Second, most of the reports did not mandate either a staging MRI or a comprehensive clinical assessment after neoadjuvant therapy which should include at least 3 modalities (digital rectal examination, endoscopy and repeated MRI). Third, there is a concern that a discordance between cCR and pCR may lead to undertreatment of some patients hence missing the window of opportunity for cure (57-60). Finally, most of these studies were reported after relatively short follow-up periods. In conclusion, despite optimistic and promising data, OP should not yet be regarded as standard in daily clinical practice. Currently, it may be contemplated when patients are deemed to be too frail to have surgery or for patients who are adamant not to undergo surgery. For the latter group, and providing that patient did achieve cCR, a comprehensive and open discussion between patient and physician is warranted before pursuing such an approach with the emphasize of the lack of firmly established evidence to date supporting this strategy and the importance of patient compliance with an intensive surveillance schedule.
Summary
The current treatment paradigm of LARC is a very a good example of how multimodal and multidisciplinary management can improve outcomes of patients with cancer (Figure 1). LARC harbors meaningful risks of both local and distant relapses. Preoperative radiotherapy has undoubtedly proven to be effective in reducing chances of loco-regional relapses. Despite the lack of convincing evidence that preoperative radiotherapy facilitate more sphincter-preserving surgeries, the fact that it can reduce the incidence of pelvic recurrences that may have required pelvic exenteration is a great achievement. Adjuvant chemotherapy has failed to demonstrate clear benefit in terms of DFS and OS with a possible explanation of low compliance after TME and late targeting of microscopic disease. The earlier introduction of systemic-dose chemotherapy in the TNT approach reduces DM rates and may possibly be proven to improve survival with longer follow-up.
As treatment intensity escalates, so does the need for more reliable risk-stratification biomarkers in order to better select patients for such treatment and avoid overtreatment (Figure 2). For a start, it is important to bear in mind that safety data from RAPIDO (2) and PRODIGE-23 (3) stems from a study population that had a good performance status of 0–1 (with 80% of patients who had performance status 0) and with the majority of patients being younger than 65 years of age. Additionally, most patients in RAPIDO and PRODIGE-23 had stage III disease, hence questioning the applicability and the necessity of TNT for patients with stage II. Based on current data, we feel that patients with cT3N0 will be overtreated with TNT while those with cT4N0 might benefit from such therapy and a patient-physician open discussion should steer the decision making process. Molecular biomarkers will be of great value should they be discovered.
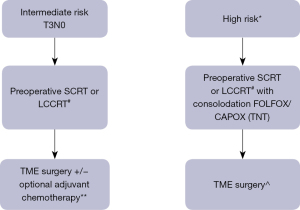
Finally, pursuing an OP strategy in LARC should be offered to carefully selected patients or in the context of clinical trials or in very highly selected. We believe that conducting a RCT to evaluate OP versus standard-of-care treatment will be extremely challenging. It is likely that patients who will opt to take part in such a trial will have an inherent preference to a certain approach (most probably OP as it is the experimental arm) and non-compliance to the allocated therapy could be expected to be high with major rates of protocol deviations.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the Narrative Review reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-13/rc
Peer Review File: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-13/prf
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-13/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Gollins S, Sebag-Montefiore D. Neoadjuvant Treatment Strategies for Locally Advanced Rectal Cancer. Clin Oncol (R Coll Radiol) 2016;28:146-51. [Crossref] [PubMed]
- Bahadoer RR, Dijkstra EA, van Etten B, et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) versus preoperative chemoradiotherapy, TME, and optional adjuvant chemotherapy in locally advanced rectal cancer (RAPIDO): a randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:29-42. [Crossref] [PubMed]
- Conroy T, Bosset JF, Etienne PL, et al. Neoadjuvant chemotherapy with FOLFIRINOX and preoperative chemoradiotherapy for patients with locally advanced rectal cancer (UNICANCER-PRODIGE 23): a multicentre, randomised, open-label, phase 3 trial. Lancet Oncol 2021;22:702-15. [Crossref] [PubMed]
- Habr-Gama A, São Julião GP, Vailati BB, et al. Organ Preservation in cT2N0 Rectal Cancer After Neoadjuvant Chemoradiation Therapy: The Impact of Radiation Therapy Dose-escalation and Consolidation Chemotherapy. Ann Surg 2019;269:102-7. [Crossref] [PubMed]
- van der Valk MJM, Hilling DE, Bastiaannet E, et al. Long-term outcomes of clinical complete responders after neoadjuvant treatment for rectal cancer in the International Watch & Wait Database (IWWD): an international multicentre registry study. Lancet 2018;391:2537-45. [Crossref] [PubMed]
- Dossa F, Chesney TR, Acuna SA, et al. A watch-and-wait approach for locally advanced rectal cancer after a clinical complete response following neoadjuvant chemoradiation: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol 2017;2:501-13. [Crossref] [PubMed]
- Thomas PR, Lindblad AS. Adjuvant postoperative radiotherapy and chemotherapy in rectal carcinoma: a review of the Gastrointestinal Tumor Study Group experience. Radiother Oncol 1988;13:245-52. [Crossref] [PubMed]
- Wolmark N, Wieand HS, Hyams DM, et al. Randomized trial of postoperative adjuvant chemotherapy with or without radiotherapy for carcinoma of the rectum: National Surgical Adjuvant Breast and Bowel Project Protocol R-02. J Natl Cancer Inst 2000;92:388-96. [Crossref] [PubMed]
- Swedish Rectal Cancer Trial. Improved survival with preoperative radiotherapy in resectable rectal cancer. N Engl J Med 1997;336:980-7. [Crossref] [PubMed]
- van Gijn W, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial. Lancet Oncol 2011;12:575-82. [Crossref] [PubMed]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Sebag-Montefiore D, Stephens RJ, Steele R, et al. Preoperative radiotherapy versus selective postoperative chemoradiotherapy in patients with rectal cancer (MRC CR07 and NCIC-CTG C016): a multicentre, randomised trial. Lancet 2009;373:811-20. [Crossref] [PubMed]
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993;341:457-60. [Crossref] [PubMed]
- Kapiteijn E, Marijnen CA, Nagtegaal ID, et al. Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer. N Engl J Med 2001;345:638-46. [Crossref] [PubMed]
- Krook JE, Moertel CG, Gunderson LL, et al. Effective surgical adjuvant therapy for high-risk rectal carcinoma. N Engl J Med 1991;324:709-15. [Crossref] [PubMed]
- O’Connell MJ, Martenson JA, Wieand HS, et al. Improving adjuvant therapy for rectal cancer by combining protracted-infusion fluorouracil with radiation therapy after curative surgery. N Engl J Med 1994;331:502-7. [Crossref] [PubMed]
- Gérard JP, Conroy T, Bonnetain F, et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3-4 rectal cancers: results of FFCD 9203. J Clin Oncol 2006;24:4620-5. [Crossref] [PubMed]
- Bosset JF, Calais G, Mineur L, et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol 2014;15:184-90. [Crossref] [PubMed]
- Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. JNCI J Natl Cancer Inst 2015;107:248. [Crossref] [PubMed]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical outcome of the ACCORD 12/0405 PRODIGE 2 randomized trial in rectal cancer. J Clin Oncol 2012;30:4558-65. [Crossref] [PubMed]
- Aschele C, Cionini L, Lonardi S, et al. Primary tumor response to preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer: pathologic results of the STAR-01 randomized phase III trial. J Clin Oncol 2011;29:2773-80. [Crossref] [PubMed]
- Schmoll HJ, Stein A, Van Cutsem E, et al. Pre- and Postoperative Capecitabine Without or With Oxaliplatin in Locally Advanced Rectal Cancer: PETACC 6 Trial by EORTC GITCG and ROG, AIO, AGITG, BGDO, and FFCD. J Clin Oncol 2021;39:17-29. [Crossref] [PubMed]
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979-89. [Crossref] [PubMed]
- Gollins S, Sun Myint A, Haylock B, et al. Preoperative chemoradiotherapy using concurrent capecitabine and irinotecan in magnetic resonance imaging-defined locally advanced rectal cancer: impact on long-term clinical outcomes. J Clin Oncol 2011;29:1042-9. [Crossref] [PubMed]
- Willeke F, Horisberger K, Kraus-Tiefenbacher U, et al. A phase II study of capecitabine and irinotecan in combination with concurrent pelvic radiotherapy (CapIri-RT) as neoadjuvant treatment of locally advanced rectal cancer. Br J Cancer 2007;96:912-7. [Crossref] [PubMed]
- Kalofonos HP, Bamias A, Koutras A, et al. A randomised phase III trial of adjuvant radio-chemotherapy comparing Irinotecan, 5FU and Leucovorin to 5FU and Leucovorin in patients with rectal cancer: a Hellenic Cooperative Oncology Group Study. Eur J Cancer 2008;44:1693-700. [Crossref] [PubMed]
- Sebag-Montefiore D, Adams R, Gollins S, et al. ARISTOTLE: A phase III trial comparing concurrent capecitabine with capecitabine and irinotecan (Ir) chemoradiation as preoperative treatment for MRI-defined locally advanced rectal cancer (LARC). J Clin Oncol 2020;38:4101. [Crossref]
- Dewdney A, Cunningham D, Tabernero J, et al. Multicenter randomized phase II clinical trial comparing neoadjuvant oxaliplatin, capecitabine, and preoperative radiotherapy with or without cetuximab followed by total mesorectal excision in patients with high-risk rectal cancer (EXPERT-C). J Clin Oncol 2012;30:1620-7. [Crossref] [PubMed]
- Gollins S, West N, Sebag-Montefiore D, et al. Preoperative chemoradiation with capecitabine, irinotecan and cetuximab in rectal cancer: significance of pre-treatment and post-resection RAS mutations. Br J Cancer 2017;117:1286-94. [Crossref] [PubMed]
- Pinto C, Di Fabio F, Maiello E, et al. Phase II study of panitumumab, oxaliplatin, 5-fluorouracil, and concurrent radiotherapy as preoperative treatment in high-risk locally advanced rectal cancer patients (StarPan/STAR-02 Study). Ann Oncol 2011;22:2424-30. [Crossref] [PubMed]
- Nogué M, Salud A, Vicente P, et al. Addition of bevacizumab to XELOX induction therapy plus concomitant capecitabine-based chemoradiotherapy in magnetic resonance imaging-defined poor-prognosis locally advanced rectal cancer: the AVACROSS study. Oncologist 2011;16:614-20. [Crossref] [PubMed]
- Masi G, Vivaldi C, Fornaro L, et al. Total neoadjuvant approach with FOLFOXIRI plus bevacizumab followed by chemoradiotherapy plus bevacizumab in locally advanced rectal cancer: the TRUST trial. Eur J Cancer 2019;110:32-41. [Crossref] [PubMed]
- Bujko K, Nowacki MP, Nasierowska-Guttmejer A, et al. Long-term results of a randomized trial comparing preoperative short-course radiotherapy with preoperative conventionally fractionated chemoradiation for rectal cancer. Br J Surg 2006;93:1215-23. [Crossref] [PubMed]
- Ngan SY, Burmeister B, Fisher RJ, et al. Randomized trial of short-course radiotherapy versus long-course chemoradiation comparing rates of local recurrence in patients with T3 rectal cancer: Trans-Tasman Radiation Oncology Group trial 01.04. J Clin Oncol 2012;30:3827-33. [Crossref] [PubMed]
- Ciseł B, Pietrzak L, Michalski W, et al. Long-course preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: long-term results of the randomized Polish II study. Ann Oncol 2019;30:1298-303. [Crossref] [PubMed]
- Bujko K, Wyrwicz L, Rutkowski A, et al. Long-course oxaliplatin-based preoperative chemoradiation versus 5 × 5 Gy and consolidation chemotherapy for cT4 or fixed cT3 rectal cancer: results of a randomized phase III study. Ann Oncol 2016;27:834-42. [Crossref] [PubMed]
- Francois Y, Nemoz CJ, Baulieux J, et al. Influence of the interval between preoperative radiation therapy and surgery on downstaging and on the rate of sphincter-sparing surgery for rectal cancer: the Lyon R90-01 randomized trial. J Clin Oncol 1999;17:2396. [Crossref] [PubMed]
- Erlandsson J, Holm T, Pettersson D, et al. Optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer (Stockholm III): a multicentre, randomised, non-blinded, phase 3, non-inferiority trial. Lancet Oncol 2017;18:336-46. [Crossref] [PubMed]
- Garcia-Aguilar J, Chow OS, Smith DD, et al. Effect of adding mFOLFOX6 after neoadjuvant chemoradiation in locally advanced rectal cancer: a multicentre, phase 2 trial. Lancet Oncol 2015;16:957-66. [Crossref] [PubMed]
- Lefevre JH, Mineur L, Kotti S, et al. Effect of Interval (7 or 11 weeks) Between Neoadjuvant Radiochemotherapy and Surgery on Complete Pathologic Response in Rectal Cancer: A Multicenter, Randomized, Controlled Trial (GRECCAR-6). J Clin Oncol 2016;34:3773-80. [Crossref] [PubMed]
- Martling A, Cedermark B, Johansson H, et al. The surgeon as a prognostic factor after the introduction of total mesorectal excision in the treatment of rectal cancer. Br J Surg 2002;89:1008-13. [Crossref] [PubMed]
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21-9. [Crossref] [PubMed]
- Breugom AJ, van Gijn W, Muller EW, et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann Oncol 2015;26:696-701. [Crossref] [PubMed]
- Glynne-Jones R, Counsell N, Quirke P, et al. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann Oncol 2014;25:1356-62. [Crossref] [PubMed]
- Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200-7. [Crossref] [PubMed]
- Petersen SH, Harling H, Kirkeby LT, et al. Postoperative adjuvant chemotherapy in rectal cancer operated for cure. Cochrane Database Syst Rev 2012;CD004078. [Crossref] [PubMed]
- Fernandez-Martos C, Garcia-Albeniz X, Pericay C, et al. Chemoradiation, surgery and adjuvant chemotherapy versus induction chemotherapy followed by chemoradiation and surgery: long-term results of the Spanish GCR-3 phase II randomized trial†. Ann Oncol 2015;26:1722-8. [Crossref] [PubMed]
- Fokas E, Allgäuer M, Polat B, et al. Randomized phase II trial of chemoradiotherapy plus induction or consolidation chemotherapy as total neoadjuvant therapy for locally advanced rectal cancer: CAO/ArO/AIO-12. J Clin Oncol 2019;37:3212-22. [Crossref] [PubMed]
- Rutten HJ, den Dulk M, Lemmens VE, et al. Controversies of total mesorectal excision for rectal cancer in elderly patients. Lancet Oncol 2008;9:494-501. [Crossref] [PubMed]
- Burbach JP, den Harder AM, Intven M, et al. Impact of radiotherapy boost on pathological complete response in patients with locally advanced rectal cancer: a systematic review and meta-analysis. Radiother Oncol 2014;113:1-9. [Crossref] [PubMed]
- MacGregor TP, Maughan TS, Sharma RA. Pathological grading of regression following neoadjuvant chemoradiation therapy: the clinical need is now. J Clin Pathol 2012;65:867-71. [Crossref] [PubMed]
- Rupinski M, Szczepkowski M, Malinowska M, et al. Watch and wait policy after preoperative radiotherapy for rectal cancer; management of residual lesions that appear clinically benign. Eur J Surg Oncol 2016;42:288-96. [Crossref] [PubMed]
- Habr-Gama A, Perez RO, Nadalin W, et al. Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results. Ann Surg 2004;240:711-7; discussion 717-8. [Crossref] [PubMed]
- Beard BW, Rao AR, Schumacher A, et al. Watchful Waiting after Clinical Complete Response to Neoadjuvant Chemoradiation for Rectal Cancer. Int J Radiat Oncol 2019;105:E161. [Crossref]
- Fernandez LM, São Julião GP, Figueiredo NL, et al. Conditional recurrence-free survival of clinical complete responders managed by watch and wait after neoadjuvant chemoradiotherapy for rectal cancer in the International Watch & Wait Database: a retrospective, international, multicentre registry study. Lancet Oncol 2021;22:43-50. [Crossref] [PubMed]
- Garcia-Aguilar J, Patil S, Kim JK, et al. Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial. J Clin Oncol 2020;38:4008. [Crossref]
- Joye I, Deroose CM, Vandecaveye V, et al. The role of diffusion-weighted MRI and (18)F-FDG PET/CT in the prediction of pathologic complete response after radiochemotherapy for rectal cancer: a systematic review. Radiother Oncol 2014;113:158-65. [Crossref] [PubMed]
- de Jong EA, ten Berge JC, Dwarkasing RS, et al. The accuracy of MRI, endorectal ultrasonography, and computed tomography in predicting the response of locally advanced rectal cancer after preoperative therapy: A metaanalysis. Surgery 2016;159:688-99. [Crossref] [PubMed]
- Glynne-Jones R, Hughes R. Complete Response after Chemoradiotherapy in Rectal Cancer (Watch-and-Wait): Have we Cracked the Code? Clin Oncol (R Coll Radiol) 2016;28:152-60. [Crossref] [PubMed]
- Smith FM, Wiland H, Mace A, et al. Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy. Dis Colon Rectum 2014;57:311-5. [Crossref] [PubMed]


