
Association between preoperative nutritional status, inflammation, and intestinal permeability in elderly patients undergoing gastrectomy: a prospective cohort study
Introduction
The incidence of gastric cancer ranks 5th among malignant tumors in the world, and the mortality rate ranks 3rd (1). Gastric cancer in elderly patients is often accompanied by malnutrition before surgery due to disorders in digestion and absorption, and different degrees of gastrointestinal obstruction (2-4). Tumor-derived cytokines, such as cytokines, such as interleukin (IL)-1, IL-6, and tumor necrosis factor, can cause anorexia and change liver metabolism by affecting hormone secretion (5,6), leading to the depletion of fat reserves, muscle atrophy, fatigue, and impaired activity. Additionally, surgical trauma stress and preoperative fasting can further exacerbate malnutrition. The incidence of malnutrition in patients with gastric cancer ranges from 19% to 60.2% (5,7), ranking it at the forefront of all tumors.
The intestinal epithelium is one of the most important determinants of intestinal permeability (8), and plays a key role as a selective barrier limiting the exposure of potentially harmful substances to the host (9). Intestinal permeability refers to the barrier function of the intestinal epithelium, manifested by its ability to control the passage of molecules through unmediated direct diffusion, independent of molecular concentration gradients or pressure, without the assistance of passive or active biochemical carrier systems (10). Defective gut barrier function can activate the chronic immune system, leading to local and systemic diseases, including celiac disease, colorectal cancer, inflammatory bowel disease, obesity, and diabetes (11-14). With age, the expression of epithelial tight junction proteins and microbial diversity decreases, the mucus layer thins, and the secretion of chronic pro-inflammatory factors increases, leading to impaired intestinal barrier function and intestinal permeability (15). However, the relationship of gut barrier function and inflammation remains to be clarified in elderly gastric cancer patients.
Impaired intestinal barrier function and altered gastrointestinal immune function have been observed in patients with obstructive jaundice and malnutrition and are associated with enhanced endotoxin exposure and an acute-phase response (16,17), leading to an increased risk of infectious complications. Malnutrition and inflammatory cytokines have been recognized as risk factors associated with postoperative complications and prolonged length of hospital stay (18-20). There is little evidence of any association between nutritional status and intestinal permeability in elderly gastric cancer patients, and the clinical significance of preoperative altered intestinal barrier function in the postoperative course is poorly understood. A previous study analyzed the relationship between preoperative intestinal barrier function and sepsis after radical upper gastrointestinal surgery and found that increased intestinal permeability before surgery did not predict a septic outcome (21). However, the impact of preoperative intestinal barrier function on postoperative recovery and complications is less clear, particularly in elderly patients.
This study sought to analyze whether there were any correlations between preoperative nutritional status, inflammatory cytokines, and intestinal permeability, and the effects of nutritional status and intestinal permeability on postoperative complications, length of hospital stay, and body weight loss in elderly patients with gastric cancer scheduled for surgery. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-367/rc).
Methods
This was a single-center prospective cohort study, which enrolled elderly patients scheduled for open radical gastrectomy at Zhongshan Hospital from April 2021 to September 2021. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the institutional Ethics Committee of Zhongshan Hospital (No. B2021-392), and all the patients signed the written informed consent form.
Patients
This study included gastric cancer patients eligible for open gastrectomy, aged 65 to 90 years, who had been classified as class I–II by the American Society of Anesthesiologists (ASA). Patients were excluded from the study if they had any diseases that interfered with intestinal permeability, such as renal failure, nephrotic syndrome, heart failure, diabetes, and thyroid disease. Patients with changes in surgical procedure or pathological diagnoses were dropped out.
Assessment of nutritional status
Patients’ nutritional status was assessed before surgery using the Mini Nutritional Assessment (MNA) and Nutrition Risk Screening-2002 (NRS2002). Additionally, the height and weight of the patients were recorded to calculate BMI, and the preoperative concentrations of free fatty acid (FFA), albumin, and prealbumin were recorded.
MNA
The MNA is an effective tool recommended by the European Society for Clinical Nutrition and Metabolism (ESPEN) that is used to evaluate the nutritional status of elderly patients (22). The standard questionnaire proposed by Guigoz et al. was used (23). It includes the following 4 parts: anthropometry, general status, dietary habits, and self-evaluation of health and nutritional status. Respondents can receive a total possible score of 30 points and were divided into the following 3 categories based on their score: well-nourished (≥24), mildly to moderately malnourished (17–23.5), or severely malnourished (<17). In this study, the categories were assessed as 1, 2, and 3 points according to the rating.
NRS2002
The NRS2002 is currently the most commonly used tool and was recommended by the ESPEN in 2002 and the Chinese Society for Parenteral and Enteral Nutrition in 2006 to assess the nutritional risk of hospitalized patients, including patient nutritional status [based on weight loss, body mass index (BMI), and general condition, or food intake] and disease severity (stress metabolism). Scores for each predictor range from 0 to 3, with an additional point added if the patient is aged over 70 years. A NRS score ≥3 is generally considered to indicate a nutritional risk.
Assessment of intestinal permeability
Intestinal permeability was assessed by D-lactate and intestinal fatty acid-binding protein (i-FABP). Blood samples were collected to detect the intestinal permeability and inflammatory cytokines. All the patients received the test preoperatively. Peripheral blood was drawn into an anticoagulant blood collection tube, centrifuged at 3,000 rpm for 10 min, collected in a cryopreservation tube, and stored at –80 ℃. D-lactate was detected using the colorimetric method (D-lactate Colorimetric Assay Kit, Sigma-Aldrich, Germany), and i-FABP was detected using the enzyme-linked immunoassay (ELISA) method (Human FABP2/I-FABP Immunoassay, R&D, USA) in accordance with the manufacturer’s instructions.
Assessment of inflammatory cytokines
Peripheral blood was drawn into an anticoagulant blood collection tube, and interleukin (IL)-6 and IL-10 were detected using the ELISA method (Human IL-6 ELISA Kit, Human IL-10 ELISA Kit, Immunoway, USA). The neutrophil and lymphocyte counts in the preoperative routine blood tests were recorded.
Assessment of postoperative recovery
Patients were followed throughout the study period and up to 30 d after surgery. Postoperative complications [as assessed by the Clavien-Dindo (CD) grading standard, under which a CD ≥ II indicates the occurrence of postoperative complications], time to first defecation, time to first liquid diet, and length of hospital stay (LOS) were recorded.
Statistical analyses
The analyses were performed using the Statistical Package for Social Sciences version 26.0 (SPSS, Inc., and IBM Company, Chicago, IL, USA). The continuous variables are presented as the mean (standard deviation) or median (interquartile range), and the categorical variables are described as frequencies and percentages. For the continuous variables, the t-test or the Mann-Whitney test was used for comparisons between groups. For the categorical variables, chi-square or Fisher’s exact tests were used for comparisons between groups. The Pearson correlation was used to evaluate the correlation of continuous variables with normal distribution, and the Spearman rank correlation method was used to evaluate correlations between the rank variables or variables that did not accord with a normal distribution. Using Canonical Correlation Analysis (CCA) to do the correlation between two groups of variables. Univariate and multivariate analysis were conducted using logistic regression. For all the analyses, all the P values were 2 sided, and values <0.05 were considered statistically significant.
Sample size calculation
This study estimated the sample size according to the correlation coefficient between the main research index i-FABP and MNA score. Set the test level α=0.05, power 1-β=0.10, R0=0.0, R1=0.3, using PASS software (version11, NCSS, USA) to calculate the required sample size of 112 cases, assuming a non-response rate of 20 %, the sample size is 134 cases.
Results
Characteristics of the enrolled patients
A total of 134 patients were included in the study, of whom 18 were not followed up to determine their postoperative recovery, body weight loss, and complications due to palliative surgery, abdominal exploration, and intraperitoneal hyperthermic chemotherapy. The patients had a median age of 69 years, and 81.3% of the patients were male. According to the MNA score, patients with mild to moderate malnutrition accounted for 50.7% of the cohort, and patients with severe malnutrition accounted for 32.1% of the cohort. The median NRS2002 score was 3 points, and 38.8% of the patients had scores >4. The laboratory indicators are shown in Table 1.
Table 1
| Items | Values |
|---|---|
| Age, years | 69 (66, 72) |
| Gender, n (%) | |
| Male | 109 (81.3) |
| Female | 25 (18.7) |
| BMI, kg/m2 | 22.1±2.5 |
| MNA, n (%) | |
| 1 | 23 (17.2) |
| 2 | 68 (50.7) |
| 3 | 43 (32.1) |
| NRS2002, median (IQR) | 3 (3, 4) |
| Laboratory tests | |
| L_C, ×109/L | 1.7 (1.3, 2.3) |
| N_C, ×109/L | 3.4 (2.8, 4.1) |
| Alb, g/L | 39.8±5.2 |
| PreAlb, mg/L | 204.7±51.8 |
| IL-6, pg/mL | 3.2 (2.175, 4.3) |
| IL-10, pg/mL | 0.375 (0.212, 0.625) |
| FFA, mmol/L | 0.75 (0.58, 0.98) |
| i-FABP, μg/L | 1.07 (0.67, 1.68) |
| D-Lac, μg/mL | 6.85±4.09 |
The data are shown as median (interquartile range) or mean ± standard deviation. BMI, body mass index; MNA, Mini Nutritional Assessment; NRS2002, Nutrition risk screening-2002; N_C, neutrophil count; L_C, lymphocyte count; Alb, albumin; preAlb, prealbumin; FFA, free fatty acid; i-FABP, intestinal fatty acid-binding protein; D-Lac, D-lactate.
Simple correlation analysis of intestinal permeability with nutritional, inflammatory, and recovery indicators
The results showed that the i-FABP was significantly negatively correlated with albumin (r=–0.409, P<0.001) and prealbumin (r=–0.397, P<0.001), and significantly positively correlated with the MNA (r=0.291, P=0.001), NRS2002 (r=0.284, P=0.001), and length of hospital stay (r=0.245, P=0.004) (see Table 2). D-lactate was significantly negatively correlated with BMI (r=–0.229, P=0.008), albumin (r=–0.426, P<0.001), and prealbumin (r=–0.358, P<0.001) levels, and significantly positively correlated with the NRS2002 (r=0.187, P=0.030), time to first defecation (r=0.264, P=0.002), and length of hospital stay (r=0.409, P<0.001). No correlation was found between i-FABP or D-lactate with age, FFA, IL-6, IL-10, body weight loss, time to fluid food, and postoperative complications (P>0.05).
Table 2
| Items | i-FABP | D-lac | |||
|---|---|---|---|---|---|
| Correlation coefficient (r) | P | Correlation coefficient (r) | P | ||
| Age | –0.104 | 0.231 | –0.124 | 0.152 | |
| BMI | –0.278 | 0.001 | –0.229 | 0.008 | |
| MNA | 0.291 | 0.001 | 0.164 | 0.059 | |
| NRS2002 | 0.284 | 0.001 | 0.187 | 0.030 | |
| Alb | –0.409 | <0.001 | –0.426 | <0.001 | |
| PreAlb | –0.397 | <0.001 | –0.358 | <0.001 | |
| FFA | 0.120 | 0.167 | –0.059 | 0.495 | |
| IL-6 | 0.121 | 0.163 | 0.025 | 0.777 | |
| IL-10 | –0.031 | 0.722 | –0.053 | 0.544 | |
| NLR | 0.249 | 0.004 | 0.229 | 0.008 | |
| CD ≥2 | 0.075 | 0.391 | –0.030 | 0.730 | |
| Body weight loss | 0.226 | 0.009 | 0.184 | 0.033 | |
| First defecation | 0.135 | 0.119 | 0.264 | 0.002 | |
| First fluid diet | 0.138 | 0.112 | 0.065 | 0.458 | |
| LOS | 0.245 | 0.004 | 0.409 | <0.001 | |
i-FABP, intestinal fatty acid-binding protein; D-Lac, D-lactic acid; BMI, body mass index; MNA, Mini Nutritional Assessment; NRS2002, Nutrition risk screening; Alb, albumin; preAlb, prealbumin; FFA, free fatty acid; NLR, neutrophil-to-lymphocyte ratio; CD, Clavien-Dindo classification; LOS, length of hospital stay.
CCA of intestinal permeability and nutritional parameters
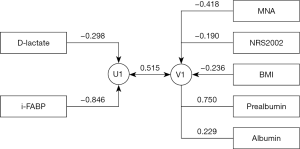
Define intestinal permeability parameters (D-lactate and i-FABP) as set U and nutritional parameters (MNA, NRS2002, BMI, albumin, and prealbumin) as set V for CCA. The canonical correlation coefficients were calculated, respectively, and Barlett χ2 hypothesis test was performed. The first pair of canonical correlation coefficients was statistically significant (r=0.515, eigenvalue =0.361, F=4.473, P<0.001). The first canonical correlation variable structure diagram was shown in Figure 1. D-lactate and i-FABP were negatively correlated with U1 (canonical correlation coefficients were −0.298 and −0.846). MNA, NRS2002, BMI were negatively correlated with V1 (canonical correlation coefficients were −0.418, −0.190, and −0.236), prealbumin and albumin were positively correlated with V1 (canonical correlation coefficients were 0.750 and 0.229).
Effect of nutritional status on postoperative recovery according to the MNA score
The patients were divided into the following three groups based on their preoperative MNA scores: well-nourished, mildly to moderately malnourished, and severely malnourished. The laboratory indicators, intestinal permeability, and postoperative recovery were compared. The results showed that there were statistically significant differences in the concentrations of BMI (P<0.001), prealbumin (P=0.005), FFA (P=0.015), i-FABP (P=0.001), and time to first defecation (P=0.048) among the three groups (see Table 3). Additionally, there was statistically significant difference in BMI (P=0.004) between the mildly to moderately malnourished group and the well-nourished group. The levels of BMI (P<0.001), prealbumin (P=0.002), and i-FABP (P=0.021) were significantly different between the well-nourished group and the severely malnourished group. The levels of BMI (P<0.001), FFA (P=0.011), i-FABP (P=0.001), and time to first flatus (P=0.041) were significantly different between the mildly to moderately malnourished group and the severely malnourished group. No significant difference was found in concentrations of albumin, IL-6, IL-10, lymphocyte and neutrophil count, D-lactate, time to fluid diet, body weight loss, LOS, and postoperative complications between groups (P>0.05).
Table 3
| Items | Well-nourished (n=33) | Mildly to moderately malnourished (n=58) | Severely malnourished (n=43) | F/χ2 | P |
|---|---|---|---|---|---|
| BMI, kg/m2 | 25.0 (22.1, 25.7) | 21.5 (21.0, 24.2)* | 19.8 (18.7, 20.8)*# | 60.994 | <0.001 |
| Age, years | 69 (68, 70) | 68 (65, 75) | 67 (66, 73) | 1.528 | 0.466 |
| Alb, g/L | 39.4±7.7 | 40.5±4.2 | 38.9±4.9 | 2.355 | 0.308 |
| PreAlb, mg/L | 228.1±66.9 | 208.7±46.1 | 188.9±45.7* | 10.448 | 0.005 |
| FFA, mmol/L | 0.85 (0.5, 1.02) | 0.70 (0.52, 0.92) | 0.84 (0.69, 1.02)# | 8.461 | 0.015 |
| IL-6, pg/mL | 2.4 (2.0, 3.5) | 3.2 (2.1, 3.8) | 3.3 (2.3, 6.7) | 5.031 | 0.081 |
| IL-10, pg/mL | 0.52 (0.28, 0.57) | 0.40 (0.21, 0.72) | 0.32 (0.21, 0.55) | 2.531 | 0.282 |
| L_C, ×109/L | 2.1 (1.5, 2.7) | 1.7 (1.3, 2.5) | 1.6 (1.3, 1.9) | 5.805 | 0.055 |
| N_C, ×109/L | 3.8 (2.8, 4.2) | 3.2 (2.8, 4.0) | 3.4 (2.8, 4.2) | 1.416 | 0.493 |
| i-FABP, µg/L | 0.84 (0.56, 1.49) | 1.03 (0.58, 1.29) | 1.54 (0.84, 2.30)*# | 14.563 | 0.001 |
| D-lac, µg/mL | 5.63±3.08 | 5.86±3.70 | 6.74±3.77 | 1.009 | 0.367 |
| First defecation, d | 6 (6, 7.25) | 6 (6, 7) | 7 (6, 8) # | 6.078 | 0.048 |
| First fluid diet, d | 6.5 (5.75, 11) | 7 (6, 9) | 8 (6, 9) | 0.274 | 0.872 |
| Body weight loss, % | 5.34±4.28 | 3.10±2.52 | 2.80±2.31 | 4.690 | 0.096 |
| LOS, d | 11 (8, 13) | 9 (8, 11.75) | 11 (8, 13) | 4.038 | 0.133 |
| PPC, n (%) | 3 (10.7) | 7 (13.2) | 4 (11.4) | 0.127 | 0.939 |
The data are shown as median (interquartile range) or mean ± standard deviation. *, in the pairwise comparison, compared to the well-nourished group, the difference was statistically significant; #, in the pairwise comparison, compared to the mildly to moderately malnourished group, the difference was statistically significant. BMI, body weight index; Alb, albumin; preAlb, prealbumin; FFA, free fatty acid; N_C, neutrophil count; L_C, lymphocyte count; i-FABP, intestinal fatty acid-binding protein; D-Lac, D-lactate; LOS, length of hospital stay; PPC, postoperative complications.
Univariate and multivariate analyses of postoperative complications
Postoperative complication was defined as Clavien-Dindo grades II-V. The overall morbidity rate was 12.1% (14 patients). The variables associated with complications in the univariate analysis are expressed in Table 4. In the univariate analysis, D-lactate (P=0.002) and i-FABP (P=0.032) were related to postoperative complications. In the multivariate analysis, D-lactate was independent risk factor of postoperative complication [odds ratio (OR) =1.354, 95% confidence interval (CI): 1.099–1.669, P=0.004].
Table 4
| Variables | N | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|---|
| χ2 | P | OR | 95% CI | P | |||
| Age | 116 | 2.469 | 0.096 | 0.936 | (0.832, 1.053) | 0.272 | |
| MNA | |||||||
| 1–2 | 81 | ||||||
| 3 | 35 | 0.019 | 0.889 | ||||
| NRS2002 | |||||||
| <4 | 73 | ||||||
| ≥4 | 43 | 0.493 | 0.483 | ||||
| BMI | 116 | 1.964 | 0.161 | ||||
| IL-6 | 116 | 0.435 | 0.509 | ||||
| IL-10 | 116 | 2.217 | 0.137 | ||||
| FFA | 116 | 0.769 | 0.380 | ||||
| D-Lac | 116 | 9.631 | 0.002 | 1.354 | (1.099, 1.669) | 0.004 | |
| i-FABP | 116 | 4.598 | 0.032 | 1.355 | (0.694, 2.648) | 0.374 | |
| Alb | 116 | 0.078 | 0.796 | ||||
| preAlb | 116 | 0.055 | 0.814 | ||||
| NLR | 116 | 0.081 | 0.775 | ||||
| Gender | |||||||
| Male | 98 | ||||||
| Female | 18 | 0.852 | 0.356 | ||||
| PNI | |||||||
| ≥48 | 68 | ||||||
| <48 | 48 | 3.444 | 0.063 | 2.480 | (0.609, 10.104) | 0.205 | |
MNA, Mini Nutritional Assessment; NRS2002, Nutrition risk screening; BMI, body weight index; FFA, free fatty acid; D-Lac, D-lactate; i-FABP, intestinal fatty acid-binding protein; Alb, albumin; preAlb, prealbumin; NLR, neutrophil-to-lymphocyte ratio; PNI, prognostic nutritional index.
Discussion
The protection of intestinal function plays a very important role in radical gastrointestinal tumor resection. The impairment of the intestinal barrier function can increase the risk of postoperative infectious complications, multiple organ failure, and mortality, and exacerbate local and systemic inflammatory stress responses in the intestine, influence the composition of the gut microbiome (24), leading to prolonged postoperative recovery (25). In this study, we found that 50.7% of elderly gastric cancer patients had preoperative mild to moderate malnutrition, and 32.1% had severe malnutrition. Additionally, the preoperative intestinal permeability indexes were significantly negatively correlated with preoperative albumin and prealbumin concentrations. There were significant positive correlations between the intestinal permeability indexes and the NRS2002, NLR, and hospital stay, but there were no significant correlations between the indexes and IL-6, IL-10, or postoperative complications.
There are various indicators for evaluating intestinal permeability, such as urinary excretion through oral probes (26), or assessing the levels of biomarkers, such as Zonulin, fatty acid-binding protein, citrulline, glucagon-like peptide-2, endotoxin, and D-lactate. FABP is a cytoplasmic protein of approximately 15 kDa that binds and transports fatty acids. In the absorption site of small intestinal villus, both hepatic (L-FABP) and intestinal (i-FABP) are expressed (27), while L-FABP is expressed in both the liver and kidney, and i-FABP is only expressed in the gut (28). When the intestinal epithelium is disrupted, i-FABP is released into the blood and can be detected in the plasma. Thus, i-FABP has been identified as a sensitive marker of intestinal ischemia, permeability, and barrier function (29). D-lactate is the product of fermentation and metabolism of various intestinal flora, but mammals do not have an enzyme system that degrades D-lactate, so D-lactate cannot be rapidly degraded. After the increase of intestinal permeability, D-lactate enters the blood through the damaged mucosa, which can lead to an increase in the concentration in the blood.
In this study, we found that preoperative intestinal permeability in elderly patients with gastric cancer was significantly correlated with nutritional indicators, such as albumin and prealbumin. Previous study has also provided evidence that malnutrition can increase intestinal permeability by altering the mucosal immune barrier and impairing intestinal barrier function (30). Other studies have shown that malnutrition is associated with atrophy of the intestinal villi, decreased intestinal weight (31), the opening of the tight junction, and abnormal mucin production (32).
Malnutrition is a common clinical symptom in elderly patients with gastric cancer, and the prevalence in this study was as high as 38.8% according to the NRS2002. In a study of malnutrition in German hospitals, 27.4% of patients were diagnosed with malnutrition according to the subjective global assessment method, and the prevalence of malnutrition among patients undergoing major abdominal surgery was 44% (33). A comprehensive assessment of nutritional status involves the following 5 areas: body mass index, a detailed nutritional history, the presence or absence of pathological weight loss, a decreased appetite and intake, and the severity of the underlying disease. This study examined 2 nutritional assessment scales, and a positive correlation was found between intestinal permeability and nutritional scores. Previous study has found a correlation between malnutrition and gastrointestinal permeability (34). However, there is no evidence that there is any causal relationship between the 2, and the 2 may occur at the same time and affect each other. Malnutrition may lead to intestinal mucosal atrophy and immunological abnormalities. Conversely, intestinal permeability may increase the likelihood of fluid and electrolyte loss, thereby exacerbating malnutrition.
In our study, no significant correlation was found between preoperative intestinal permeability and IL-6 or IL-10. In a rat model of open abdominal surgery, serum D-lactate and i-FABP were significantly decreased with the treatment of a beta-1 blocker, and necrosis factor-alpha and IL-6 were also decreased, indicating that the beta-1 blocker reduces systemic inflammatory responses and preserves the intestinal barrier function (35). However, there is still little evidence affirming the relationship between intestinal permeability and systemic inflammation in gastric cancer.
Previous study has evaluated the advantages of MNA in assessing malnutrition in elderly patients (22). A study of 157 cancer patients found that the sensitivity of MNA in diagnosing malnutrition was as high as 97% (36). However, MNA is less sensitive to dynamic changes in nutritional status during hospitalization and convalescence (37). Additionally, the tool has the following disadvantages: (I) the evaluation process takes a long time (15–20 min); (II) it is easy to over diagnose (i.e., the specificity is low) (38); and (III) it cannot assess patients with impaired cognitive function (39).
This study did not involve anthropometric indicators, such as grip strength, phase angle and other indicators, when evaluating nutritional status, and was a single-center study with a small sample size. Further research needs to be conducted on the long-term prognosis of patients in the future.
Conclusions
The incidence of preoperative malnutrition in elderly patients with gastric cancer is high, and preoperative intestinal permeability indicators (i.e., D-lactate and i-FABP) are significantly correlated with some nutritional indicators and postoperative recovery indicators. The preoperative concentration of D-lactate is an independent risk factor for postoperative complications, suggesting that altered gut barrier function before surgery could to some extent influence postoperative recovery in the elderly.
Acknowledgments
Funding: This work was sponsored by the Shanghai Municipal Key Clinical Specialty Unit (No. shslczdzk03603), the Clinical Research Project of the Zhongshan Hospital, Fudan University (Nos. 2021ZSQN47 and 2018ZSLC27), and the Clinical Research Plan of SHDC (No. SHDC2020CR3048B).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-367/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-367/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-367/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The protocol was approved by the institutional Ethics Committee of Zhongshan Hospital (No. B2021-392), and all the patients signed the written informed consent form.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin 2020;70:7-30. [Crossref] [PubMed]
- Xu R, Chen XD, Ding Z. Perioperative nutrition management for gastric cancer. Nutrition 2022;93:111492. [Crossref] [PubMed]
- Martín-Richard M, Carmona-Bayonas A, Custodio AB, et al. SEOM clinical guideline for the diagnosis and treatment of gastric cancer (GC) and gastroesophageal junction adenocarcinoma (GEJA) (2019). Clin Transl Oncol 2020;22:236-44. [Crossref] [PubMed]
- Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol 2019;14:26-38. [Crossref] [PubMed]
- Hébuterne X, Lemarié E, Michallet M, et al. Prevalence of malnutrition and current use of nutrition support in patients with cancer. JPEN J Parenter Enteral Nutr 2014;38:196-204. [Crossref] [PubMed]
- Seo SH, Kim SE, Kang YK, et al. Association of nutritional status-related indices and chemotherapy-induced adverse events in gastric cancer patients. BMC Cancer 2016;16:900. [Crossref] [PubMed]
- Fukuda Y, Yamamoto K, Hirao M, et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol 2015;22:S778-85. [Crossref] [PubMed]
- Kong SH, Lee HJ, Na JR, et al. Effect of perioperative oral nutritional supplementation in malnourished patients who undergo gastrectomy: A prospective randomized trial. Surgery 2018;164:1263-70. [Crossref] [PubMed]
- Ménard S, Cerf-Bensussan N, Heyman M. Multiple facets of intestinal permeability and epithelial handling of dietary antigens. Mucosal Immunol 2010;3:247-59. [Crossref] [PubMed]
- Travis S, Menzies I. Intestinal permeability: functional assessment and significance. Clin Sci (Lond) 1992;82:471-88. [Crossref] [PubMed]
- Fasano A, Not T, Wang W, et al. Zonulin, a newly discovered modulator of intestinal permeability, and its expression in coeliac disease. Lancet 2000;355:1518-9. [Crossref] [PubMed]
- He C, Yu T, Shi Y, et al. MicroRNA 301A Promotes Intestinal Inflammation and Colitis-Associated Cancer Development by Inhibiting BTG1. Gastroenterology 2017;152:1434-1448.e15. [Crossref] [PubMed]
- Suenaert P, Bulteel V, Lemmens L, et al. Anti-tumor necrosis factor treatment restores the gut barrier in Crohn's disease. Am J Gastroenterol 2002;97:2000-4. [Crossref] [PubMed]
- Grivennikov SI, Wang K, Mucida D, et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 2012;491:254-8. [Crossref] [PubMed]
- Branca JJV, Gulisano M, Nicoletti C. Intestinal epithelial barrier functions in ageing. Ageing Res Rev 2019;54:100938. [Crossref] [PubMed]
- Welsh FK, Farmery SM, MacLennan K, et al. Gut barrier function in malnourished patients. Gut 1998;42:396-401. [Crossref] [PubMed]
- Welsh FK, Ramsden CW, MacLennan K, et al. Increased intestinal permeability and altered mucosal immunity in cholestatic jaundice. Ann Surg 1998;227:205-12. [Crossref] [PubMed]
- Liu H, Jiao J, Zhu M, et al. Nutritional Status According to the Short-Form Mini Nutritional Assessment (MNA-SF) and Clinical Characteristics as Predictors of Length of Stay, Mortality, and Readmissions Among Older Inpatients in China: A National Study. Front Nutr 2022;9:815578. [Crossref] [PubMed]
- Nagata S, Maeda S, Nagamatsu S, et al. Prognostic Nutritional Index Considering Resection Range Is Useful for Predicting Postoperative Morbidity of Hepatectomy. J Gastrointest Surg 2021;25:2788-95. [Crossref] [PubMed]
- Dan Zeng CD, Tong YX, et al. Peripheral Lymphocyte Subsets Absolute Counts as Feasible Clinical Markers for Predicting Surgical Outcome in Gastric Cancer Patients After Laparoscopic D2 Gastrectomy: A Prospective Cohort Study. J Inflamm Res 2021;14:5633-46. [Crossref] [PubMed]
- Kanwar S, Windsor AC, Welsh F, Barclay GR, Guillou PJ, Reynolds JV. Lack of correlation between failure of gut barrier function and septic complications after major upper gastrointestinal surgery. Ann Surg 2000;231:88-95. [Crossref] [PubMed]
- Bozzetti F, Forbes A. The ESPEN clinical practice Guidelines on Parenteral Nutrition: present status and perspectives for future research. Clin Nutr 2009;28:359-64. [Crossref] [PubMed]
- Guigoz Y, Vellas B, Garry PJ, et al. Mini Nutritional Assessment: a practical assessment tool for grading the nutritional state of elderly patients. Facts Res Gerontology 1994;2:31-6.
- Maksimaityte V, Bausys A, Kryzauskas M, et al. Gastrectomy impact on the gut microbiome in patients with gastric cancer: A comprehensive review. World J Gastrointest Surg 2021;13:678-88. [Crossref] [PubMed]
- Kondrup J, Allison SP, Elia M, et al. ESPEN guidelines for nutrition screening 2002. Clin Nutr 2003;22:415-21. [Crossref] [PubMed]
- Virizuela JA, Camblor-Álvarez M, Luengo-Pérez LM, et al. Nutritional support and parenteral nutrition in cancer patients: an expert consensus report. Clin Transl Oncol 2018;20:619-29. [Crossref] [PubMed]
- Gajda AM, Storch J. Enterocyte fatty acid-binding proteins (FABPs): different functions of liver and intestinal FABPs in the intestine. Prostaglandins Leukot Essent Fatty Acids 2015;93:9-16. [Crossref] [PubMed]
- Storch J, Corsico B. The emerging functions and mechanisms of mammalian fatty acid-binding proteins. Annu Rev Nutr 2008;28:73-95. [Crossref] [PubMed]
- Schoultz I, Keita ÅV. The Intestinal Barrier and Current Techniques for the Assessment of Gut Permeability. Cells 2020;9:1909. [Crossref] [PubMed]
- van der Hulst RR, von Meyenfeldt MF, van Kreel BK, et al. Gut permeability, intestinal morphology, and nutritional depletion. Nutrition 1998;14:1-6. [Crossref] [PubMed]
- Steiner M, Bourges HR, Freedman LS, et al. Effect of starvation on the tissue composition of the small intestine in the rat. Am J Physiol 1968;215:75-7. [Crossref] [PubMed]
- Sherman P, Forstner J, Roomi N, et al. Mucin depletion in the intestine of malnourished rats. Am J Physiol 1985;248:G418-23. [PubMed]
- Pirlich M, Schütz T, Norman K, et al. The German hospital malnutrition study. Clin Nutr 2006;25:563-72. [Crossref] [PubMed]
- Norman K, Pirlich M, Schulzke JD, et al. Increased intestinal permeability in malnourished patients with liver cirrhosis. Eur J Clin Nutr 2012;66:1116-9. [Crossref] [PubMed]
- Tan S, Zhou F, Zhang Z, et al. Beta-1 blocker reduces inflammation and preserves intestinal barrier function after open abdominal surgery. Surgery 2021;169:885-93. [Crossref] [PubMed]
- Read JA, Crockett N, Volker DH, et al. Nutritional assessment in cancer: comparing the Mini-Nutritional Assessment (MNA) with the scored Patient-Generated Subjective Global Assessment (PGSGA). Nutr Cancer 2005;53:51-6. [Crossref] [PubMed]
- Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr 2002;56:779-85. [Crossref] [PubMed]
- Cereda E. Mini nutritional assessment. Curr Opin Clin Nutr Metab Care 2012;15:29-41. [Crossref] [PubMed]
- Kaiser R, Winning K, Uter W, et al. Comparison of two different approaches for the application of the mini nutritional assessment in nursing homes: resident interviews versus assessment by nursing staff. J Nutr Health Aging 2009;13:863-9. [Crossref] [PubMed]
(English Language Editor: L. Huleatt)


