
The bevacizumab plus oxaliplatin-based chemotherapy regimen is more suitable for metastatic colorectal cancer patients with a history of schistosomiasis: a clinical retrospective analysis
Introduction
Schistosomiasis is a snail-borne disease caused by parasitic blood-dwelling flukes that affects almost 250 million people globally. It is a public health problem in tropical and subtropical regions of Africa, Asia, the Caribbean and South America. There were about 12 million schistosomiasis patients in China in 1950s (1). Although great achievements have been attained in treating schistosomiasis, there were still 37,601 schistosomiasis patients in China by the end of 2017 (2). Kunshan city lies in the Yangtze river basin, and is a endemic area of schistosomiasis (1). Schistosomiasis often results in hepatic fibrosis, splenomegaly, and thrombocytopenia. The basic platelet counts of the schistosomiasis patients are low, and might be much lower during chemotherapy, which leads to increased risk of bleeding. If the platelet count does not meet the requirements for chemotherapy, the patient’s treatment will be delayed or even not be carried out as planned, which directly affects their prognosis. Therefore the impacts of treatment regimens on platelet counts are critically important for those patients.
Oxaliplatin or irinotecan in combination with 5-fluorouracil regimens are the backbones of systemic treatment for metastatic or recurrent colorectal cancer patients. However, thrombocytopenia is one of the commonest treatment-related adverse events of them. Angiogenesis is an attractive therapeutic target for patients with cancer, and it can be inhibited by bevacizumab (3). Bevacizumab (Avastin, Genentech, South San Francisco, CA) is a recombinant humanized immunoglobulin monoclonal antibody that binds with and inhibits the activity of human vascular endothelial growth factor-A (VEGF-A) (4). Bevacizumab enhances the effect of chemotherapy in colorectal cancer and was approved by the FDA as the first angiogenesis inhibitor to treat metastatic colorectal cancer (mCRC) (5). However, it has several adverse effects including hypertension, proteinuria, thromboembolic events, wound-healing complications, congestive heart failure, and gastrointestinal perforation (6-11). Bevacizumab plus oxaliplatin-based chemotherapy and bevacizumab plus irinotecan-based chemotherapy are equally effective. The impacts of the two regimens on platelet counts remain unknown as the results varied among different clinical trials. A study has reported that bevacizumab was associated with an increased risk of all grades of thrombocytopenia (12). It increased the incidence of grade 1 or 2 thrombocytopenia from irinotecan-based chemotherapy in patients with mCRC (13,14). However, researches also reported that bevacizumab can reduce the rate of thrombocytopenia from oxaliplatin-based chemotherapy because of its protective impact on oxaliplatin-induced hepatic sinusoidal injury (HSI) (15,16). For mCRC patients with a history of schistosomiasis, the basic platelet counts of whom are more likely to be low, choosing a regimen with less impact on platelet count is very important. Because of survival improvement of bevacizumab on chemotherapy and protective effect of bevacizumab on oxaliplatin-induced splenomegaly and thrombocytopenia, bevacizumab plus oxaliplatin-based chemotherapy is potentially more effective and safer. In order to find the answer, we conducted this retrospective analysis. We present the following article in accordance with the STROBE reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-207/rc).
Methods
Patients
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Affiliated Kunshan Hospital of Jiangsu University Institutional Review Board (No. 2022-03-006-K01). Individual consent for this retrospective analysis was waived. Bevacizumab has been widely used in China since September 1, 2017, before which it was not covered by insurance. We retrospectively reviewed the medical records of all patients with mCRC who received oxaliplatin-based chemotherapy or irinotecan-based chemotherapy from September 1, 2017, to June 30, 2019, in the Affiliated Kunshan Hospital of Jiangsu University. Patients were included if they were mCRC patients with a treatment history for schistosomiasis and if they received oxaliplatin-based chemotherapy or irinotecan-based chemotherapy as a first-line treatment for no less than 4 cycles. The exclusion criteria were then applied to the 195 identified cases as follows: receiving a regimen containing cetuximab, lack of available imaging, absence of spleen on imaging, prior liver resection, presence of known hepatitis or cirrhosis, lack of information on platelet counts. The final population analyzed consisted of 153 patients.
Data collection
The baseline data collected consisted of patient demographics, body mass index (BMI, a person’s weight in kilograms divided by the square of height in meters), and disease characteristics. From the first cycle until the completion of chemotherapy, data were collected on numbers of cycles delivered, spleen sizes, and platelet counts. Contrast-enhanced computed tomography (CT) studies were performed with a multidetector row-64 CT scanner (Light-Speed, GE Healthcare) with a collimation of 5 mm. Spleen volume was calculated as vertical diameter × transverse diameter × posterior diameter × 0.445+29 (cm3). Thrombocytopenia was defined as a platelet count of less than 100,000/mm3. The changes in spleen volumes and platelet counts were determined by comparisons with the baseline pretreatment values. The distribution of each categorical variable was summarized by its frequencies and percentages. The distribution of each continuous variable was summarized in term of its medians and ranges. Many researches have demonstrated that bevacizumab enhances the effect of chemotherapy in colorectal cancer. Bevacizumab plus oxaliplatin-based chemotherapy and bevacizumab plus irinotecan-based chemotherapy are equally effective. And the main purpose of this study is to find a safer treatment regimen for mCRC patients with a history of schistosomiasis, whose platelet counts are more likely to be low. So we didn’t consider survival and progression outcomes.
Statistical analysis
The primary end points were the comparisons of the 6-month cumulative incidence rates of splenic enlargement of 10% or greater and thrombocytopenia between bevacizumab and non-bevacizumab treatment cohorts.
Comparison studies between groups were carried out with the Fisher exact test and Wilcoxon rank-sum test. Time-to-event distributions were estimated by the Kaplan-Meier curves, and variable comparisons between treatment groups were made using the Log-rank test. The Cox proportional hazards regression model was used to characterize associations between patient characteristics and the incidences of splenomegaly and thrombocytopenia. All statistical analyses were performed with Stata 14.0 (StataCorp., College Station, TX). All statistical tests were two-sided, and P<0.05 was considered statistically significant.
Results
The baseline characteristics
The final population analyzed consisted of 153 patients, including 73 bevacizumab-treated patients and 80 non-bevacizumab-treated patients. Baseline characteristics for the two groups, including age, sex, tumor site, BMI, chemotherapy cycles, spleen size, and platelet count, were similar (Table 1). The median age of patients was 63 [51–79] years old for the bevacizumab cohort, and 58 [53–89] years old for non-bevacizumab cohort (P=0.12). Sex (P=0.50) and tumor sites (P=0.33) of patients were equally distributed in the two groups. BMI was calculated as weight (kg)/(height (m))2, and the median values were 21.1 (15.5–29.2) for the bevacizumab group and 21.5 (15.9–33.8) for the non-bevacizumab group (P=0.64). No significant differences were found in chemotherapy cycles {6 [6–24] vs. 6 [6–16]; P=0.68}, baseline spleen sizes [152.2 (74.1–529.6) vs. 150.0 (69.5–410.8); P=0.11] (cm3), and baseline platelet counts {162 [77–441] vs. 166 [69–532]; P=0.90} (K/µL) between the two groups.
Table 1
| Characteristics | Bevacizumab cohort (n=73) | Non-bevacizumab cohort (n=80) | P value |
|---|---|---|---|
| Age, median [range], y | 63 [51–79] | 58 [53–89] | 0.12 |
| Sex, No. (%) | 0.50 | ||
| Female | 24 (32.9) | 31 (38.8) | |
| Male | 49 (67.1) | 49 (61.2) | |
| BMI, median (range), kg/m2 | 21.1 (15.5–29.2) | 21.5 (15.9–33.8) | 0.64 |
| Tumor site, No. (%) | 0.33 | ||
| Colon | 45 (61.6) | 43 (53.8) | |
| Rectum | 28 (38.4) | 37 (46.2) | |
| Chemotherapy cycles, median [range] | 6 [6–24] | 6 [6–16] | 0.68 |
| Baseline spleen size, median (range), cm3 | 152.2 (74.1–529.6) | 150.0 (69.5–410.8) | 0.11 |
| Baseline platelet count, median [range], K/μL | 162 [77–441] | 166 [69–532] | 0.90 |
BMI, body mass index.
Bevacizumab had an impact on the rate of thrombocytopenia from chemotherapy
The 6-month cumulative incidence rates of splenic enlargement of 10% or greater (23.3% vs. 55%; P=0.002; Figure 1A) and cumulative incidence rates of thrombocytopenia (43.8% vs. 57.5%; P=0.203; Figure 1B) were lower in the bevacizumab group than the non-bevacizumab group, however there were no statistical differences for the cumulative incidence rates of thrombocytopenia (Table 2). The multivariate Cox proportional hazard regression model indicated that bevacizumab and chemotherapy regimen were independent prognostic factors for the patients (Table 3). In the bevacizumab-treated cohort, the 6-month cumulative incidence rates of thrombocytopenia (31.7% vs. 59.4%; P=0.20) and splenic enlargement of 10% or greater (19.5% vs. 28.1%; P=0.59) were lower in the oxaliplatin group than the irinotecan group, however there were no statistical differences. In the non-bevacizumab treatment cohort, the incidence rates of splenic enlargement were similar between the two groups (66.7% vs. 26.1%; P=0.08), while the rates of thrombocytopenia were lower in the irinotecan group (77.2% vs. 8.7%; P<0.01) (Table 2).
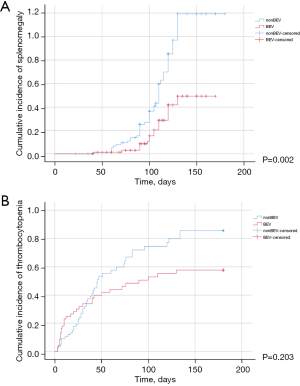
Table 2
| Outcomes | Bevacizumab cohort (n=73) | Non-bevacizumab cohort (n=80) | P value | |||||
|---|---|---|---|---|---|---|---|---|
| Oxaliplatin-based chemo (n=41) | Irinotecan-based chemo (n=32) | P value | Oxaliplatin-based chemo (n=57) | Irinotecan-based chemo (n=23) | P value | |||
| Six-month cumulative incidence rates of splenic enlargement of 10%, % [No.] | 23.3 [17] | 55 [44] | 0.01 | |||||
| Six-month cumulative incidence rates of thrombocytopenia, % [No.] | 43.8 [32] | 57.5 [46] | 0.40 | |||||
| Six-month cumulative incidence rates of splenic enlargement of 10%, % [No.] | 19.5 [8] | 28.1 [9] | 0.59 | 66.7 [38] | 26.1 [6] | 0.08 | ||
| Six-month cumulative incidence rates of thrombocytopenia, % [No.] | 31.7 [13] | 59.4 [19] | 0.20 | 77.2 [44] | 8.7 [2] | <0.01 | ||
| Six-month cumulative incidence rates of grade 1 thrombocytopenia, % [No.] | 22.0 [9] | 46.9 [15] | 0.16 | 57.9 [33] | 8.7 [2] | 0.01 | ||
| Six-month cumulative incidence rates of grade 2 thrombocytopenia, % [No.] | 9.8 [4] | 12.5 [4] | 1.00 | 19.3 [11] | 0 | 0.06 | ||
| Six-month cumulative incidence rates of grade 3 thrombocytopenia, % [No.] | 0 | 0 | – | 0 | 0 | – | ||
Table 3
| Characteristics | Cumulative incidence of splenomegaly | Cumulative incidence of thrombocytopenia | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | ||
| Age | 0.99 | 0.97–1.01 | 0.42 | 1.01 | 0.99–1.03 | 0.36 | |
| Sex | 1.50 | 0.85–2.65 | 0.16 | 0.98 | 0.61–1.58 | 0.94 | |
| BMI | 1.02 | 0.93–1.12 | 0.68 | 1.04 | 0.96–1.13 | 0.39 | |
| Bevacizumab or not | 0.43 | 0.24–0.76 | <0.01 | 0.76 | 0.48–1.20 | 0.23 | |
| Oxaliplatin or Irinotecan | 1.93 | 1.03–3.63 | 0.04 | 1.84 | 1.06–3.18 | 0.03 | |
HR, hazard ratio; CI, confidence interval; BMI, body mass index.
Bevacizumab decreased the rate of thrombocytopenia from oxaliplatin
Of the 98 patients treated with oxaliplatin and fluoropyrimidine, 41 received bevacizumab. Baseline characteristics for the two groups, including age, sex, tumor site, BMI, chemotherapy cycles, spleen size, and platelet count, were similar (Table 4). The median age of patients was 63 [51–79] years old for the bevacizumab cohort, and 63 [53–80] years old for non-bevacizumab cohort (P=0.83). Sex (P=1.00) and tumor sites (P=0.84) of patients were equally distributed in the two groups. The median values of BMI were 20.9 (15.5–28.2) for the bevacizumab group and 21.3 (15.9–26.4) for the non-bevacizumab group (P=0.62). No significant differences were found in chemotherapy cycles {6 [6–24] vs. 6 [6–15]; P=0.33}, baseline spleen sizes [174.4 (76.4–382.8) vs. 208.0 (76.7–525.6); P=0.35] (cm3), and baseline platelet counts {159 [77–441] vs. 166 [69–461]; P=0.84} (K/µL) between the two groups. In patients treated with oxaliplatin, the 6-month cumulative incidence rates of splenic enlargement of 10% or greater were lower in the bevacizumab-treated cohort than in the non-bevacizumab cohort (19.5% vs. 66.7%; P<0.001; Figure 2A-2C). There was no splenic enlargement of more than 20% in the bevacizumab-treated cohort. The median time of splenic enlargement of 10% or greater in patients treated with oxaliplatin was longer in the bevacizumab cohort, but the difference was not significant [120 d (4.0 m) vs. 103 d (3.4 m); P=0.85]. The 6-month cumulative incidence rates of thrombocytopenia were lower in the bevacizumab-treated cohort than in the non-bevacizumab cohort (31.7% vs. 77.2%; P<0.001; Figure 2D), especially for grade 1 thrombocytopenia (22.0% vs. 57.9%; P=0.02). The difference was not significant for that of grade 2 thrombocytopenia (9.8% vs. 19.3%; P=0.40), and there was no grade 3 thrombocytopenia. The median time to thrombocytopenia was shorter in the bevacizumab-treated cohort than in the non-bevacizumab cohort [23 d (0.8 m) vs. 33 d (1.1 m); P=0.02]. In patients with liver metastasis, the 6-month cumulative incidence rates of splenic enlargement of 10% or greater (26.1% vs. 60.6%; P=0.14) and thrombocytopenia (43.5% vs. 75.8%; P=0.27) were similar in the bevacizumab-treated cohort and the non-bevacizumab cohort. In patients without liver metastasis, the 6-month cumulative incidence rates of splenic enlargement of 10% or greater (11.1% vs. 75.0%, P=0.01) and thrombocytopenia (16.7% vs. 79.2%, P=0.03) were lower in the bevacizumab-treated cohort than in the non-bevacizumab cohort. When comparing the bevacizumab and non-bevacizumab-treated cohorts in patients with splenic enlargement of more than 10%, the 6-month cumulative incidence rates of thrombocytopenia were similar [50.0% (4/8) vs.78.9% (30/38); P=0.54]. In patients with reduced splenic volume, the incidence rates were also similar [35.0% (7/20) vs. 67.7% (10/15); P=0.37] (Table 5).
Table 4
| Characteristics | Oxaliplatin-based chemotherapy (n=98) | Irinotecan-based chemotherapy (n=55) | |||||
|---|---|---|---|---|---|---|---|
| Bevacizumab cohort (n=41) |
Non-bevacizumab cohort (n=57) | P value | Bevacizumab cohort (n=32) |
Non-bevacizumab cohort (n=23) | P value | ||
| Age, median [range], y | 63 [51–79] | 63 [53–80] | 0.83 | 58 [53–79] | 62 [55–89] | 0.18 | |
| Sex, No. (%) | 0.27 | ||||||
| Female | 13 (31.7) | 19 (33.3) | 1.00 | 11 (34.4) | 12 (52.2) | ||
| Male | 28 (68.3) | 38 (66.7) | 21 (65.6) | 11 (47.8) | |||
| BMI, median (range), kg/m2 | 20.9 (15.5–28.2) | 21.3 (15.9–26.4) | 0.62 | 21.7 (16.8–29.2) | 22.9 (18.7–33.8) | 0.09 | |
| Tumor site, No. (%) | 0.41 | ||||||
| Colon | 24 (58.5) | 31 (54.4) | 0.84 | 21 (65.6) | 12 (52.2) | ||
| Rectum | 17 (41.5) | 26 (45.6) | 11 (34.4) | 11 (47.8) | |||
| Chemotherapy cycles, median [range] | 6 [6–24] | 6 [6–15] | 0.33 | 6 [6–19] | 7 [6–16] | 0.23 | |
| Baseline spleen size, median (range), cm3 | 174.4 (76.4–382.8) | 208.0 (76.7–525.6) | 0.35 | 160.1 (83.5–529.6) | 153.6 (101.9–263.7) | 0.18 | |
| Baseline platelet count, median [range], K/μL | 159 [77–441] | 166 [69–461] | 0.84 | 164 [86–309] | 183 [102–532] | 0.55 | |
BMI, body mass index.
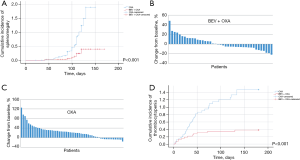
Table 5
| Outcomes | Oxaliplatin-based chemotherapy (n=98) | Irinotecan-based chemotherapy (n=55) | |||||
|---|---|---|---|---|---|---|---|
| Bevacizumab cohort (n=41) | Non-bevacizumab cohort (n=57) | P value | Bevacizumab cohort (n=32) | Non-bevacizumab cohort (n=23) | P value | ||
| Six-month cumulative incidence rates of splenic enlargement of 10%, % [No.] | 19.5 [8] | 66.7 [38] | 0.01 | 28.1 [9] | 26.1 [6] | 1.00 | |
| Six-month cumulative incidence rates of splenic enlargement of 20%, % [No.] | 0 | 50.1 [29] | 0.00 | 18.8 [6] | 17.4 [4] | 1.00 | |
| Six-month cumulative incidence rates of splenic enlargement of 30%, % [No.] | 0 | 27.9 [12] | <0.01 | 6.3 [2] | 8.7 [2] | 1.00 | |
| Median time to splenic enlargement of 10% or greater, d (m) | 120 (4.0) | 103 (3.4) | 0.85 | 130 (4.3) | 140 (4.7) | 0.80 | |
| Six-month cumulative incidence rates of thrombocytopenia, % [No.] | 31.7 [13] | 77.2 [44] | 0.02 | 59.4 [19] | 8.7 [2] | 0.01 | |
| Six-month cumulative incidence rates of grade 1 thrombocytopenia, % [No.] | 22.0 [9] | 57.9 [33] | 0.02 | 46.9 [15] | 8.7 [2] | 0.04 | |
| Six-month cumulative incidence rates of grade 2 thrombocytopenia, % [No.] | 9.8 [4] | 19.3 [11] | 0.40 | 12.5 [4] | 0 | 0.15 | |
| Six-month cumulative incidence rates of grade 3 thrombocytopenia, % (No.) | 0 | 0 | – | 0 | 0 | – | |
| Median time to thrombocytopenia, d (m) | 23 (0.8) | 33 (1.1) | 0.02 | 10 (0.3) | 64 (2.1) | 0.01 | |
| Six-month cumulative Incidence rates of splenic enlargement of 10%, % [No.] | |||||||
| Patients with liver metastasis | 26.1 [6] | 60.6 [20] | 0.14 | 36.4 [4] | 20.0 [2] | 0.66 | |
| Patients without liver metastasis | 11.1 [2] | 75.0 [18] | 0.01 | 23.8 [5] | 30.8 [4] | 1.00 | |
| Six-month cumulative Incidence rates of thrombocytopenia, % [No.] | |||||||
| Patients with liver metastasis | 43.5 [10] | 75.8 [25] | 0.27 | 63.6 [7] | 10.0 [1] | 0.11 | |
| Patients without liver metastasis | 16.7 [3] | 79.2 [19] | 0.03 | 57.1 [12] | 7.7 [1] | 0.07 | |
| Six-month cumulative Incidence rates of thrombocytopenia, % [No.] | |||||||
| Patients with splenic enlargement of more than 10% | 50.0 [4] | 78.9 [30] | 0.54 | 66.7 [6] | 9.1 [1] | 0.09 | |
| Patients with reduced splenic volume | 35.0 [7] | 67.7 [10] | 0.37 | 50.0 [7] | 8.3 [1] | 0.12 | |
Bevacizumab increased the rate of thrombocytopenia from irinotecan
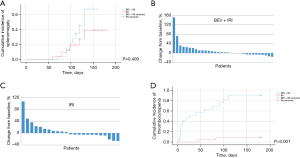
Of the 55 patients treated with irinotecan and fluoropyrimidine, 32 received bevacizumab. Baseline characteristics for the two groups, including age, sex, tumor site, BMI, chemotherapy cycles, spleen size, and platelet count, were similar (Table 4). The median age of patients was 58 [53–79] years old for the bevacizumab cohort, and 62 [55–89] years old for non-bevacizumab cohort (P=0.18). Sex (P=0.27) and tumor sites (P=0.41) of patients were equally distributed in the two groups. The median values of BMI were 21.7 (16.8–29.2) for the bevacizumab group and 22.9 (18.7–33.8) for the non-bevacizumab group (P=0.09). No significant differences were found in chemotherapy cycles {6 [6–19] vs. 7 [6–16]; P=0.23}, baseline spleen sizes [160.1 (83.5–529.6) vs. 153.6 (101.9–263.7); P=0.18] (cm3), and baseline platelet counts {164 [86–309] vs. 183 [102–532]; P=0.55} (K/µL) between the two groups. In patients treated with irinotecan, the 6-month cumulative incidence rates of splenic enlargement of 10% or greater [28.1% vs. 26.1%; P=0.409; Figure 3A-3C] and median time to splenic enlargement [130 d (4.3 m) vs. 140 d (4.7 m); P=0.80] were similar between the bevacizumab and non-bevacizumab cohorts. When we used a higher threshold of 20% (18.8% vs. 17.4%, P=1.00) or 30% (6.3% vs. 8.7%, P=1.00), the 6-month cumulative incidence rates were also similar between the bevacizumab and non-bevacizumab cohorts. The 6-month cumulative incidence rates of thrombocytopenia were higher in the bevacizumab-treated cohort than in the non-bevacizumab cohort (59.4% vs. 8.7%; P<0.001; Figure 3D). The difference was significant for that of grade 1 thrombocytopenia (46.9% vs. 8.7%; P=0.04). There was no grade 2 thrombocytopenia in the non-bevacizumab treatment cohort, and no grade 3 thrombocytopenia was found in either of the cohorts. The median time to thrombocytopenia was shorter in the bevacizumab-treated cohort [10 d (0.3 m) vs. 64 d (2.1 m); P=0.01]. With or without liver metastasis, the 6-month cumulative incidence rates of splenic enlargement of 10% or greater (with liver metastasis: 36.4% vs. 20.0%, P=0.66; without liver metastasis: 23.8% vs. 30.8%, P=1.00) and thrombocytopenia (with liver metastasis: 63.6% vs. 10.0%, P=0.11; without liver metastasis: 57.1% vs. 7.7%, P=0.07) were similar in the bevacizumab-treated and non-bevacizumab cohorts. When comparing the bevacizumab and non-bevacizumab cohorts in patients with splenic enlargement of more than 10%, the 6-month cumulative incidence rates of thrombocytopenia were similar [66.7% (6/9) vs. 9.1% (1/11); P=0.09]. In patients with reduced splenic volume, the incidence rates were also similar [50.0% (7/14) vs. 8.3% (1/12); P=0.12] (Table 5).
Discussion
Human schistosomiasis is a snail-borne disease caused by Schistosoma worms and is a serious public health problem worldwide. Schistosomiasis threatens 800 million people in 78 countries. China has carried out active control measures over the past six decades and has made great achievements. However, schistosomiasis remains a serious issue in China. The eggs of Schistosoma worms deposited in liver tissues elicit a granulomatous response that leads to periportal fibrosis, liver cirrhosis, portal hypertension, splenomegaly, and thrombocytopenia, the reason of which might be increased splenic clearance, suppression of platelet production in the bone marrow, sequestration of platelets in the liver and anti-platelet antibodies mediated destruction (17). The baseline platelet counts of patients in this study were lower than that of Overman et al.’s study (median platelet count: 162 vs. 320 in the bevacizumab cohort, 166 vs. 304 in the non-bevacizumab cohort; range of platelet count: 77–441 vs. 161–1,013 in the bevacizumab cohort, 69–532 vs. 151–887 in the non-bevacizumab cohort) (K/µL) (15). This may be because schistosomiasis which leads to liver fibrosis, splenomegaly, and thrombocytopenia is endemic in Kunshan city. Our patients were more likely to encounter thrombocytopenia during chemotherapy, therefore they had an increased risk of bleeding, treatment delay, and chemotherapy dose reduction or discontinuation, and a greater blood transfusion requirement. These factors mean that evaluating the effect of treatment regimen on platelet count was critically important for the patients in our study.
This study showed that bevacizumab decreases the rate of thrombocytopenia from oxaliplatin-based chemotherapy but increases the rate from irinotecan-based chemotherapy. Bevacizumab decreased the rate of splenic enlargement from oxaliplatin-based chemotherapy but did not change that from irinotecan-based chemotherapy. Results were similar when we stratified patients with splenic enlargement and shrinkage.
Thrombocytopenia is a prominent side effect during oxaliplatin treatment and it occurs in up to 70% of patients. Bone marrow suppression, hypersplenism, non-immune microangiopathy, and immune-mediated thrombocytopenia are the main mechanisms of thrombocytopenia (18). Thrombocytopenia could also be associated with splenic enlargement as a result of oxaliplatin-induced HSI (19,20). Sinusoidal injury is a disruption of sinusoidal endothelium that leads to subsequent collagen deposition in the perisinusoidal space and veno-occlusive fibrosis. It may result in portal hypertension and splenomegaly with associated thrombocytopenia (21,22). Bevacizumab has been found to protect against HSI by reducing the extent and incidence of sinusoidal dilatation and reducing the incidence of perisinusoidal fibrosis and hepatocellular necrosis (23), while also reducing the rate of thrombocytopenia and the frequency of splenomegaly (24). These previous findings are consistent with our current results. We found that the 6-month cumulative incidence rates of splenic enlargement and thrombocytopenia were lower in the bevacizumab-treated cohort than in the non-bevacizumab cohort. In patients without liver metastasis, we drew similar conclusions. In patients with liver metastasis, the rates were lower in the bevacizumab-treated cohort, while the differences were not significant. This may be because that the protective effect of bevacizumab on oxaliplatin-induced splenomegaly was attenuated by liver metastasis, or because only a small number of patients were involved in this study.
Irinotecan, a synthetic analog of camptothecin, is a topoisomerase I inhibitor. Its dominant hematologic side effect is neutropenia, and thrombocytopenia, when it occurs, is usually mild. Reports showed that bevacizumab increases the incidence of thrombocytopenia from irinotecan-based chemotherapy (5% vs. 0%) in patients with mCRC (13,14). However, other studies showed that the addition of bevacizumab to irinotecan-based chemotherapy did not alter the incidence of thrombocytopenia (25-27). Our study shows that, in patients treated with irinotecan, the 6-month cumulative incidence rates of thrombocytopenia were much higher in the bevacizumab-treated cohort than in the non-bevacizumab cohort. For patients with or without liver metastasis, the rates were both higher in the bevacizumab-treated cohort, though the differences were not significant. It is possible that this was caused by the small number of patients that were involved in this study. However, thrombocytopenia can cause platelet dysfunction and consuming it could shorten the platelet half-life and activate the compensatory mechanisms of the bone marrow caused by bevacizumab. These may be the reasons for the increased incidence of thrombocytopenia in the bevacizumab cohort treated with irinotecan.
Our study adds evidence to the protective effect of bevacizumab as a surrogate for HSI that results in thrombocytopenia in mCRC patients in oxaliplatin-induced splenomegaly. The data suggests that we can gain a better understanding of platelet counts by monitoring the changes in spleen size during oxaliplatin-based chemotherapy. Additional work is needed to identify patients at the greatest risk of oxaliplatin-induced HSI as they would benefit from bevacizumab because of its ability to reduce the rate of thrombocytopenia. This study also shows that bevacizumab increases the incidence of thrombocytopenia from irinotecan. More studies are also needed to identify patients at the greatest risk of thrombocytopenia, as dose reduction or regimen adjustment should be considered for them. With a better understanding of the impact of treatment regimens on platelet counts, we can select regimens that are more suitable for patients, since patients with a low baseline platelet count are more likely to encounter thrombocytopenia.
To minimize investigator bias in this study, the evaluator that calculated the spleen size was blinded to the chemotherapy regimens. However, our study still has limitations. First, this is a retrospective study, and there might be bias due to unidentified confounders. The decision to use bevacizumab was made by the treating physician who may have been influenced by additional factors. Second, although the patients involved in this study had a treatment history for schistosomiasis, they did not necessarily have splenomegaly, and it was not easy to assess the hepatic fibrosis statements for each patient. Third, the number of patients in this study was relatively small. Fourth, when treatment was delayed, dose adjustments were not only based on platelet count, but also other clinical and laboratory factors. We were unable to isolate all the impacts of thrombocytopenia upon chemotherapy dose intensity. Fifth, the interaction of the impact of schistosomiasis on liver fibrosis and thrombocytopenia treated with oxaliplatin was unknown. Studies with randomized prospective and larger cohorts are needed to confirm our findings.
Conclusions
Bevacizumab decreased the rate of thrombocytopenia in the oxaliplatin-based group; however, it increased thrombocytopenia in the irinotecan-based group. For mCRC patients with a history of schistosomiasis, especially for patients with lower platelet count, choosing a regimen of bevacizumab with oxaliplatin is potentially effective and safer. Further studies are required to verify our findings.
Acknowledgments
Funding: This work was supported by the National Natural Science Foundation (No. 82072712); National Natural Science Foundation (No. 81773192); the Natural Science Foundation of Jiangsu Province (No. BK20171248); Jiangsu Youth Medical Talents Project (No. QNRC2016527); Jiangsu Province “333 Project” Research Projects (No. 2016-III-0367); The Foundation of tumor clinical and basic research team (No. KYC005); the Kunshan Science and Technology Program (No. KS1528); and the Suzhou Science and Technology plan project (No. KJXW2019064).
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-207/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-207/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-207/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This study was approved by the Affiliated Kunshan Hospital of Jiangsu University Institutional Review Board (No. 2022-03-006-K01). Individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Wu W, Feng A, Huang Y. Research and control of advanced schistosomiasis japonica in China. Parasitol Res 2015;114:17-27. [Crossref] [PubMed]
- Li-Juan Z, Zhi-Min X, Si-Min D, et al. Endemic status of schistosomiasis in People’s Republic of China in 2017. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi 2018;30:481-8. [PubMed]
- Cook KM, Figg WD. Angiogenesis inhibitors: current strategies and future prospects. CA Cancer J Clin 2010;60:222-43. [Crossref] [PubMed]
- Almogbil HH, Nasrallah FP, Zderic V. Feasibility of Therapeutic Ultrasound Application in Topical Scleral Delivery of Avastin. Transl Vis Sci Technol 2021;10:2. [Crossref] [PubMed]
- Catalano V, Bergamo F, Cremolini C, et al. Clinical impact of first-line bevacizumab plus chemotherapy in metastatic colorectal cancer of mucinous histology: a multicenter, retrospective analysis on 685 patients. J Cancer Res Clin Oncol 2020;146:493-501. [Crossref] [PubMed]
- Wang H, Guo J, Wang T, et al. Efficacy and safety of bevacizumab in the treatment of adult gliomas: a systematic review and meta-analysis. BMJ Open 2021;11:e048975. [Crossref] [PubMed]
- Jaiswal V, Jain E, Hitawala G, et al. Bevacizumab and Sinus Venous Thrombosis: A Literature Review. Cureus 2021;13:e19471. [Crossref] [PubMed]
- Lombardi P, Rossini D, Crespi V, et al. Bevacizumab-induced hypertension as a predictor of clinical outcome in metastatic colorectal cancer: An individual patient data-based pooled analysis of two randomized studies and a systematic review of the literature. Cancer Treat Rev 2022;103:102326. [Crossref] [PubMed]
- Quintanilha JCF, Wang J, Sibley AB, et al. Bevacizumab-induced hypertension and proteinuria: a genome-wide study of more than 1000 patients. Br J Cancer 2022;126:265-74. [Crossref] [PubMed]
- Baxter NN, Fischer HD, Richardson DP, et al. A Population-Based Study of Complications After Colorectal Surgery in Patients Who Have Received Bevacizumab. Dis Colon Rectum 2018;61:306-13. [Crossref] [PubMed]
- Wichelmann TA, Abdulmujeeb S, Ehrenpreis ED. Bevacizumab and gastrointestinal perforations: a review from the FDA Adverse Event Reporting System (FAERS) database. Aliment Pharmacol Ther 2021;54:1290-7. [Crossref] [PubMed]
- Dong J, Meng X, Li S, et al. Risk of Adverse Vascular Events in Patients with Malignant Glioma Treated with Bevacizumab Plus Irinotecan: A Systematic Review and Meta-Analysis. World Neurosurg 2019;130:e236-43. [Crossref] [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [Crossref] [PubMed]
- Hurwitz HI, Fehrenbacher L, Hainsworth JD, et al. Bevacizumab in combination with fluorouracil and leucovorin: an active regimen for first-line metastatic colorectal cancer. J Clin Oncol 2005;23:3502-8. [Crossref] [PubMed]
- Overman MJ, Ferrarotto R, Raghav K, et al. The Addition of Bevacizumab to Oxaliplatin-Based Chemotherapy: Impact Upon Hepatic Sinusoidal Injury and Thrombocytopenia. J Natl Cancer Inst 2018;110:888-94. [Crossref] [PubMed]
- Mizrahi JD, Overman MJ. Bevacizumab as a chemoprotectant: reducing oxaliplatin induced hepatic sinusoidal injury. Oncotarget 2018;9:34857-8. [Crossref] [PubMed]
- Song LG, Wu XY, Sacko M, et al. History of schistosomiasis epidemiology, current status, and challenges in China: on the road to schistosomiasis elimination. Parasitol Res 2016;115:4071-81. [Crossref] [PubMed]
- Jardim DL, Rodrigues CA, Novis YAS, et al. Oxaliplatin-related thrombocytopenia. Ann Oncol 2012;23:1937-42. [Crossref] [PubMed]
- May D, Djonov V, Zamir G, et al. A transgenic model for conditional induction and rescue of portal hypertension reveals a role of VEGF-mediated regulation of sinusoidal fenestrations. PLoS One 2011;6:e21478. [Crossref] [PubMed]
- Kopetz S, Lesslie DP, Dallas NA, et al. Synergistic activity of the SRC family kinase inhibitor dasatinib and oxaliplatin in colon carcinoma cells is mediated by oxidative stress. Cancer Res 2009;69:3842-9. [Crossref] [PubMed]
- Tajima H, Ohta T, Miyashita T, et al. Oxaliplatin-based chemotherapy induces extravasated platelet aggregation in the liver. Mol Clin Oncol 2015;3:555-8. [Crossref] [PubMed]
- Simpson AL, Leal JN, Pugalenthi A, et al. Chemotherapy-induced splenic volume increase is independently associated with major complications after hepatic resection for metastatic colorectal cancer. J Am Coll Surg 2015;220:271-80. [Crossref] [PubMed]
- Martins J, Alexandrino H, Oliveira R, et al. Sinusoidal dilation increases the risk of complications in hepatectomy for CRCLM - Protective effect of bevacizumab and diabetes mellitus, serum gamma-glutamyltranspeptidase as predictive factor. Eur J Surg Oncol 2016;42:713-21. [Crossref] [PubMed]
- Overman MJ, Maru DM, Charnsangavej C, et al. Oxaliplatin-mediated increase in spleen size as a biomarker for the development of hepatic sinusoidal injury. J Clin Oncol 2010;28:2549-55. [Crossref] [PubMed]
- Stathopoulos GP, Batziou C, Trafalis D, et al. Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology 2010;78:376-81. [Crossref] [PubMed]
- Guan ZZ, Xu JM, Luo RC, et al. Efficacy and safety of bevacizumab plus chemotherapy in Chinese patients with metastatic colorectal cancer: a randomized phase III ARTIST trial. Chin J Cancer 2011;30:682-9. [Crossref] [PubMed]
- Cao R, Zhang S, Ma D, et al. A multi-center randomized phase II clinical study of bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) compared with FOLFIRI alone as second-line treatment for Chinese patients with metastatic colorectal cancer. Med Oncol 2015;32:325. [Crossref] [PubMed]
(English Language Editor: C. Mullens)


