
Bee venom protects against pancreatic cancer via inducing cell cycle arrest and apoptosis with suppression of cell migration
Introduction
As one of the most aggressive and deadliest malignancies, pancreatic cancer seriously threatens human health. The 5-year survival rate of pancreatic cancer patients is nearly 8%, and the survival rate in patients with advanced disease is only 3% (1). At present, surgical excision is the best curative treatment for patients with pancreatic cancer, but most patients do not recognize the symptoms in time, especially in the early stages (2,3). Chemotherapy is also widely used for the treatment of pancreatic cancer in the clinic, but most patients respond poorly to several chemotherapeutic drugs. The increasing incidence and mortality rates of pancreatic cancer have prompted investigations into new drug therapies for the comprehensive treatment of patients with pancreatic cancer.
It is commonly recognized that excessive cell growth is the hallmark of cancer (4). As cancer is a cell cycle disease, inhibition of inappropriate cell proliferation is the main targeted mechanism for the treatment of pancreatic cancer. In normal tissues, cells proliferate after triggering mitogenic signals for growth, while the disorder of cell cycle regulation induces abnormal proliferation in cancer tissues (5). The cell cycle includes the mitotic phase and interphase. The interphase is divided into G1 phase, S phase, and G2 phase. The regulation of the cell cycle in mammalian cells mainly depends on cyclins and cyclin-dependent kinases (CDKs) (6). Cyclins and CDKs drive cell cycle progression from G1 to S phase and G2 to M phase (7). There are 4 CDKs, namely CDK1, CDK2, CDK4, and CDK6. CDK4 and CDK6 are necessary for cell cycle progression in cancer cells but not essential in normal cells (8,9). They can interact with cyclin D to block the cell cycle. Cyclin D is usually stimulated by some growth factors and oncoproteins, and drives cell cycle progression from G1 phase to S phase (10,11). CDK1 and cyclin B1 complex regulate the entrance from the G1 phase to S phase in cell cycle progression (12).
Apoptosis regulation is complex, but it is a critical process in protecting against tumor development (13). As a programmed cell death mechanism, cell apoptosis is involved in the progression of several diseases, including cancer (14). Cell apoptosis can be triggered by the stimulation of death receptors, such as tumor necrosis factor (TNF)-receptor-1 (15,16). The death receptors initiate signaling cascades through mitochondrial dysfunction in cells, which finally results in caspase-dependent cell apoptosis (17). Numerous studies have reported that several proteins may regulate the process of cell apoptosis, mainly including the caspase family and the Bcl-2 family (18,19). The caspase family is a class of cysteine proteases and plays a pivotal role in the process of cell apoptosis. Caspase-3 and caspase-9 have attracted the most attention in the caspase family. Caspase-3 is well known for its role in the proteolytic cleavage of several proteins (18). It has been reported that some death receptors can activate caspase-8, which triggers signaling caspases and leads to the activation of caspase-3 and caspase-9 (20). The Bcl-2 family includes both anti-apoptotic proteins and pro-apoptotic proteins, and plays an important role in regulating cell apoptotic processes. For example, both Bcl-2 and Bax belong to the Bcl-2 family, but Bcl-2 is an anti-apoptotic protein while Bax is a pro-apoptotic protein (21).
Bee venom is a mixture of enzymes, peptides, and amines. The main composition of bee venom includes melittin, catecholamines, polyamines, histamine, and phospholipase A2 (22). Bee venom has been widely used as an anti-inflammatory agent and pain reliever. Bee venom therapy is used to ameliorate some human disease such as Parkinson’s disease, Alzheimer's disease, spinal cord injury, and so on. Notably, melittin and its extract hybrid peptides have demonstrated anti-tumor effects in tumor-derived cell lines (23-26). It has been reported that crude bee venom and melittin exhibited the inhibition capability in several cancer cell lines such as melanoma, colon, breast, and cervical cancer cells (27-30). Due to the wide range of biological properties, bee venom attracts more and more attention. However, so far, little is known about the effects of bee venom on pancreatic cancer cell lines. In this study, the effects of bee venom on cell proliferation, the cell cycle, cell apoptosis, and cell migration were evaluated in pancreatic cancer cell lines. We present the following article in accordance with the MDAR reporting checklist (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-222/rc).
Methods
Bee venom
Lyophilized bee venom was obtained from North China Pharmaceutical Company Ltd. (Hebei, China). Lyophilized bee venom was dissolved in phosphate-buffered saline (PBS) at different concentrations and stored in the refrigerator for further use.
Cell culture
The human pancreatic cancer cell line PANC-1 and the human metastatic pancreatic cancer cell line AsPC-1 were purchased from the National Collection of Authenticated Cell Cultures in China. PANC-1 cells were cultured routinely in Dulbecco’s modified Eagle’s medium (DMEM; Gibco, USA) supplemented with 10% fetal bovine serum (FBS), and AsPC-1 cells were cultured in RPMI 1640 media (Gibco, USA) with 10% FBS at 37 ℃ with 5% CO2. The culture medium was changed every 2 days.
Cytotoxicity assay
Cell viability was measured by the Cell Counting Kit-8 (CCK-8) assay following the manufacturer’s protocol. Briefly, PANC-1 cells or AsPC-1 cells were seeded in 48-well plates with their corresponding culture medium. After cultivation, cells were stimulated with 1, 5, 10, and 20 µg/mL bee venom for 1 day, and an equal volume of PBS was added to the wells as the control group. Then, CCK-8 solution was added in each well and incubated for 1 h at 37 ℃ before measuring the absorbance at 450 nm by a microtiter plate reader (Molecular Devices, USA).
Cell cycle assay
After treatment with different concentrations of bee venom for 24 h, cells were collected and centrifuged at 1,000 g for 5 min. Cells were harvested and washed with PBS 3 times. Then, the cells were incubated with DNA staining solution and permeabilization solution from the Cell Cycle Staining Kit (Multisciences Biotech Co., Ltd., China) for 30 min according to the manufacturer’s instructions. Samples were routinely subjected to the cell cycle assay using a CytoFLEX instrument (Beckman Coulter, USA) with an excitation wavelength of 535 nm and an emission wavelength of 615 nm for detection. Data were automatically analyzed by the Modfit LT software for Windows (version 3.1).
Apoptosis analysis
To detect cell apoptosis, cells were harvested after incubation with or without bee venom. Cells were treated with EDTA-free trypsin and centrifuged at 1,000 g for 5 min. Then, cells were resuspended and washed in cold PBS twice. After being stained with Annexin V-FITC and permeabilization solution avoiding light at room temperature for 30 min, cells were analyzed by the CytoFLEX instrument (Beckman Coulter, USA) to measure cell apoptosis rate.
Cell scratch test
Cells were seeded into a 6-well plate with 1×105 cells/well. After reaching a 90% confluence state, a single scratch wound was made by a sterile plastic 10 µL micropipette tip. Then, cells were washed with PBS and cultured in FBS-free DMEM with different concentrations of bee venom. Images of each scratch were captured at 0, 12, 24, and 36 h with the Nikon ECLIPSE Ts2 light microscope (Nikon Corporation, Tokyo, Japan) at 100× magnification. The scratch was analyzed to evaluate migration by Image J software.
Terminal deoxynucleotidyl transferase (TdT) dUTP Nick-End Labeling (TUNEL) assay
The TUNEL assay was performed according to the manufacturer’s protocol. Briefly, after treatment with 5 and 10 µg/mL of bee venom, cells were incubated at 37 ℃ for 24 h. Then, cells were washed with PBS and fixed in 4% paraformaldehyde for 20 min. The fixed cells were incubated with 0.2% Triton X-100 and washed with PBS 3 times. Following incubation with TdT equilibration buffer, cells were incubated for 1 h avoiding light with TdT enzyme. The cells were stained with 4',6-diamidino-2-phenylindole (DAPI) for 5 min and captured using a Nikon ECLIPSE Ts2 light microscope (Nikon Corporation, Tokyo, Japan) at 100× magnification.
Transwell migration analysis
Transwell migration analysis was performed as protocol (31). Transwell inserts (Corning, New York, United States) with an 8 µm pore filter were used in this study. Firstly, 1×105 cells were seeded with serum-free DMEM in the upper chamber of the insert. Meanwhile, 600 µL complete DMEM was added to the lower chamber as the chemoattractant. After incubation for 48 h at 37 ℃, cells on the upper surface of the filter membrane were wiped off. Cells on the lower surface were fixed with 4% paraformaldehyde. After washing twice, cells were stained with 0.2% crystal violet solution (G-Clone, Beijing, China) and imaged by the Nikon ECLIPSE Ts2 light microscope (Nikon Corporation, Tokyo, Japan) at 200× magnification. Five random fields were examined and counted to calculate the average number of migrated cells.
Western blot analysis
Cells were treated with or without bee venom at different concentrations and homogenized in cold RIPA lysis buffer containing 1% phosphatase inhibitor cocktail and 1% phenylmethanesulfonyl fluoride (Beyotime Institute of Biotechnology, Haimen, China). After measuring the protein concentrations by the Pierce™ BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA), equal amounts of protein were loaded on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and transblotted onto 0.22 µm polyvinylidene fluoride membranes. Nonspecific proteins were blocked using 5% fat-free milk at room temperature for 1 h. The membranes were incubated with primary antibodies at 4 ℃ overnight. The primary antibodies are as follows: caspase-3 (1:1,000; Biosynthesis Biotechnology Co., Ltd., Beijing, China), caspase-9 (1:1,000; Cell Signaling Technology, Inc., Danvers, MA, USA), PARP (1:1,000; Cell Signaling Technology), Bcl-2 (1:500; Biosynthesis Biotechnology), Bax (1:500; Biosynthesis Biotechnology), cyclin A (1:1,000; Cell Signaling Technology), cyclin B (1:1,000; Cell Signaling Technology), cyclin D1 (1:1,000; Cell Signaling Technology), CDK1 (1:1,000; Biosynthesis Biotechnology), CDK2 (1:1,000; Biosynthesis Biotechnology), CDK4 (1:1,000; Biosynthesis Biotechnology), p53 (1:1,000; Cell Signaling Technology), p21 (1:1,000; Cell Signaling Technology), and GAPDH (1:10,000; Cell Signaling Technology). Membranes were washed with phosphate-buffered saline with Tween 20 (PBST) and incubated with horseradish peroxidase (HRP)-conjugated secondary antibodies. The target bands were visualized and analyzed by the ZETA ECL Select Western Blotting Detection Reagent (ZETA Life Inc., USA) using the Bio-Rad ChemiDoc™ XRS System (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Results were normalized to GAPDH and presented as fold changes with setting the values of control group as one.
Statistical analysis
Data were shown as means ± standard deviations. Differences between groups were analyzed using one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. P values <0.05 were considered to be statistically significant. Statistical analysis was performed by GraphPad Prism 5.0 (GraphPad Software, San Diego, CA, USA).
Results
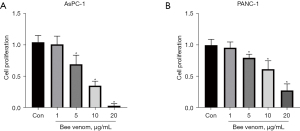
The effect of bee venom on pancreatic cancer cell proliferation
We first detected the chemosensitivity of pancreatic cancer cell lines AsPC-1 and PANC-1 to bee venom via the CCK-8 assay. In the pre-experiment, we did a range of bee venom concentrations from 0.01 to 100 µg/mL, and we used 1, 5, 10, and 20 µg/mL bee venom in the further experiments. Results showed that cell proliferation was suppressed by bee venom at doses of 5, 10, and 20 µg/mL, while the dose of 1 µg/mL had little effect on AsPC-1 cells (Figure 1A). Similarly, bee venom also significantly inhibited cell viability in a dose-dependent manner in PANC-1 cells (Figure 1B). Due to the better effects in AsPC-1 cells than in PANC-1 cells, we used the AsPC-1 cell line for subsequent experiments. Cell proliferation results suggested the anti-tumoral effect of bee venom in protecting against pancreatic cancer.
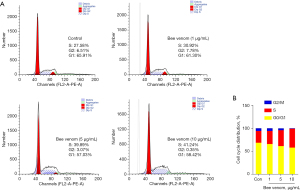
Bee venom treatment induces S phase arrest in pancreatic cancer cells
To further investigate the effect of bee venom on pancreatic cancer repopulation, we analyzed the cell cycle in vitro. AsPC-1 cells were treated with bee venom at different doses. As shown in Figure 2, the percentages of G2/M, S, and G0/G1 phase cells were 6.51%, 27.58%, and 65.91%, respectively, in the control group. Notably, bee venom treatment markedly reduced the percentage of G2/M and G0/G1 phase cells along with enhancing the percentage of S phase cells in a dose-dependent manner, indicative of S phase arrest of AsPC-1 cells after treatment with bee venom.
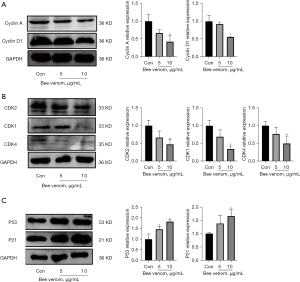
Effect of bee venom on the expression of cell cycle proteins
The effect of bee venom on cell cycle proteins was also analyzed in AsPC-1 cells. As it is well established that cell cycle progression is mainly regulated by cyclins and CDKs, we measured the expression of cyclin A and cyclin D1. Results showed that bee venom drastically suppressed both cyclin A and cyclin D1 protein expression (Figure 3A). In line with this finding, bee venom also inhibited CDK1 and CDK4, with slightly decreased CDK2 expression (Figure 3B). In addition, higher protein expression of p21 and p53 was observed in bee venom treated cells compared with the control group (Figure 3C), suggesting that the inhibition of cell cycle protein expression may be dependent on p21/p53.
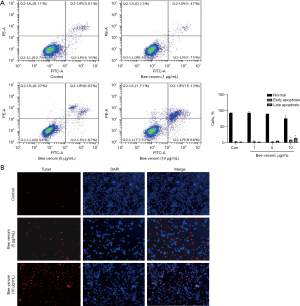
Bee venom treatment induces cell apoptosis in pancreatic cancer cells
Due to the effects of bee venom on cell proliferation and the cell cycle, we investigated the effect of bee venom on cell apoptosis. Flow cytometry was used to detect the percentage of apoptotic cells. As shown in Figure 4A, the percentages of early-stage apoptotic [Annexin V-positive and propidium iodide (PI)-negative] and late-stage apoptotic (Annexin V- and PI-positive) cells in the control group were 4.1% and 3.01%, respectively. Different from the control group, 10 µg/mL bee venom treatment increased both early apoptosis and late apoptosis, while 1 µg/mL and 5 µg/mL bee venom had little effect (Figure 4A). In line with this finding, the TUNEL assay was used to detect apoptotic cells. Results showed that there were more TUNEL-positive cells in the bee venom stimulated group compared with the control group (Figure 4B). These data indicated that bee venom could accelerate cell apoptosis in pancreatic cancer cells.
Effect of bee venom on the expression of cell apoptosis proteins
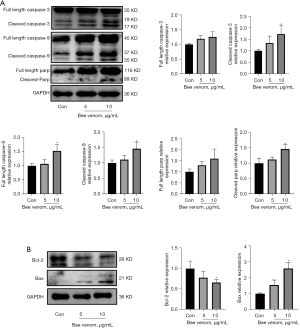
It is well known that the caspase family plays a critical role in regulating cell apoptosis (32). In this study, we measured caspase-3 and caspase-9 expression in AsPC-1 cells. As shown in Figure 5A, bee venom treatment markedly increased both cleaved caspase-3 and cleaved caspase-9 expression in a dose-dependent manner. Concurrently, the caspase family proteins activate PARP cleavage, which is also a hallmark of apoptosis (33). Cleaved fragments of PARP were observed in the bee venom treatment group, especially the 10 µg/mL bee venom treatment group (Figure 5A). Apart from the caspase family, bee venom also elevated Bax expression and suppressed the protein expression of Bcl-2 (Figure 5B). These results suggest the promotive effects of bee venom on pancreatic cancer cell apoptosis.
Bee venom treatment suppresses cell migration in pancreatic cancer cells
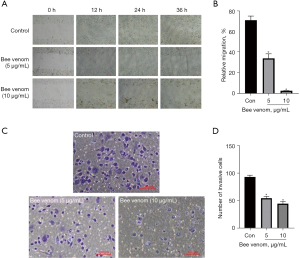
Cell invasion and migration play critical roles in cancer progression and metastasis. The scratch wound healing assay was performed to measure the anti-migratory effect of bee venom on pancreatic cancer cells (Figure 6A). Results showed that the 36 h migration ratio reached 71.26% compared to the 0 h ratio in the control group; however, the bee venom treated group had a remarkably stronger migration inhibitory effect, especially the 10 µg/mL bee venom group (Figure 6B). Correspondingly, Transwell assay results demonstrated that bee venom treatment effectively decreased the migrating cell number, especially the 10 µg/mL bee venom treatment group, which reached a 52.15% reduction compared with the control group (Figure 6C,6D).
Discussion
As a natural product with biological activity, bee venom from bee stings has been widely used in the clinic for the treatment of diseases involving inflammation and pain (34,35). So far, the safety of bee venom is still pending for its application. The adverse side effect includes skin problems, pain, immunological systemic reactions, nonspecific reactions, and so on (36). Though some side effects of bee venom have been reported, it has been well known that bee venom is effective for the treatment of some cancers (29,37). As a potential approach of cancer therapy, bee venom should be further investigated. The present study aimed to investigate the effect of bee venom on pancreatic cancer cell lines. Results showed that bee venom significantly suppressed pancreatic cancer cell proliferation, inducing cell cycle arrest in S phase. Furthermore, cell apoptosis and cell migration were also evaluated, and results demonstrated that bee venom treatment markedly promoted cell apoptosis and inhibited the migration of pancreatic cancer cells. All these data suggest the anti-tumoral effect of bee venom in protecting against pancreatic cancer.
A previous study demonstrated that bee venom exhibited cytotoxicity in a duration-dependent and dose-dependent manner in the human lung cancer cell line NCI-H1299 using the MTT assay (38). Consistent with previous study (38), in the present study we showed that bee venom can effectively inhibit the proliferation of pancreatic cancer cell lines AsPC-1 and PANC-1. These data suggest the potential of bee venom in protecting against cancer. In addition, flow cytometric analysis of the cell cycle confirmed the alteration of the cell cycle induced by bee venom, indicating that the inhibition of cell proliferation may be associated with cell cycle proteins.
As a transcription factor, p53 plays a pivotal role in tumor suppression (39). Several stress signals, such as DNA damage and hypoxia, among others, can activate p53 by post-translational modifications (40). Activated p53 binds with the p53 consensus DNA-binding elements and regulates the transcriptional expression of its downstream signals, leading to changes in cellular processes including cell cycle arrest and apoptosis. It has been reported that p53 knockout mice can develop normally but are more susceptible to spontaneous tumors (41). Consistently, p53 has also been found to be involved in human cancers, and approximately 50% of human cancers harbor mutations in p53 (42-44). In the present study, we found that bee venom enhanced the protein expression of p53 and p21, suggesting the anti-tumor effect of bee venom on pancreatic cancer cells. P21 has been demonstrated to be the consequence of p53 activation and p21 induction by p53 leads to CDK2 inhibition. el-Deiry et al. reported the relationship between p21 and p53, and quickly the p53-p21 axis has widely become recognized as the central pathway for the cell cycle (45). It is well known that p21 is a CDK inhibitor. P21 can suppress the activity of cyclin-CDK complexes, which results in cell cycle arrest. Additionally, p21 can regulate cellular senescence through p53-independent and p53-dependent pathways (46,47). In line with previous reports, in the present study we found that bee venom significantly elevated p21 expression, which indicated the anti-tumor effect of bee venom.
Besides cell cycle regulation, cell apoptotic death is also an important indicator of cancer progression. Both the Bcl-2 family and caspase family are involved in cell apoptosis. Bcl-2 can combine with Bax to form a heterodimer, which inhibits cell death and promotes cancer cell proliferation (19). The ratio of Bax to Bcl-2 is widely recognized as an indicator which reflects susceptibility to cell apoptosis. It has been demonstrated that bee venom treatment upregulated Bax expression and downregulated Bcl-2 expression in the human lung cancer cell line NCI-H1299 (38). As the integral components of the apoptotic signaling pathway, caspases induce the production of apoptotic morphological features. The present study showed that bee venom had an inhibitory effect on Bcl-2 expression and a promotive effect on Bax expression. In addition, we also found elevated expression of cleaved caspase-3 and cleaved caspase-9.
To conclude, our study revealed that bee venom can effectively inhibit tumor progression in pancreatic cancer cells. The anti-tumor effect of bee venom may be associated with cell cycle arrest, inducing cell apoptosis and inhibiting cell migration. Our study provides evidence for the anti-tumor effect of bee venom on pancreatic cancer, but further investigation is needed to determine the underlying mechanism.
Acknowledgments
Funding: This work was supported by S&T Program of Hebei (No. 20273001D).
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-222/rc
Data Sharing Statement: Available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-222/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jgo.amegroups.com/article/view/10.21037/jgo-22-222/coif). WH is an employee of North China Pharmaceutical Co., Ltd. The other authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Zhang YL, Zhang X, Miao XZ, et al. Coptisine suppresses proliferation and inhibits metastasis in human pancreatic cancer PANC-1 cells. J Asian Nat Prod Res 2020;22:452-63. [Crossref] [PubMed]
- Kamisawa T, Wood LD, Itoi T, et al. Pancreatic cancer. Lancet 2016;388:73-85. [Crossref] [PubMed]
- Ryan DP, Hong TS, Bardeesy N. Pancreatic adenocarcinoma. N Engl J Med 2014;371:1039-49. [Crossref] [PubMed]
- Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discov 2022;12:31-46. [Crossref] [PubMed]
- Bertoli C, Skotheim JM, de Bruin RA. Control of cell cycle transcription during G1 and S phases. Nat Rev Mol Cell Biol 2013;14:518-28. [Crossref] [PubMed]
- Murray AW. Recycling the cell cycle: cyclins revisited. Cell 2004;116:221-34. [Crossref] [PubMed]
- Malumbres M. Cell cycle-based therapies move forward. Cancer Cell 2012;22:419-20. [Crossref] [PubMed]
- Yu Q, Sicinska E, Geng Y, et al. Requirement for CDK4 kinase function in breast cancer. Cancer Cell 2006;9:23-32. [Crossref] [PubMed]
- Hu MG, Deshpande A, Enos M, et al. A requirement for cyclin-dependent kinase 6 in thymocyte development and tumorigenesis. Cancer Res 2009;69:810-8. [Crossref] [PubMed]
- Liao DJ, Thakur A, Wu J, et al. Perspectives on c-Myc, Cyclin D1, and their interaction in cancer formation, progression, and response to chemotherapy. Crit Rev Oncog 2007;13:93-158. [Crossref] [PubMed]
- Kim JK, Diehl JA. Nuclear cyclin D1: an oncogenic driver in human cancer. J Cell Physiol 2009;220:292-6. [Crossref] [PubMed]
- Wu ZZ, Chien CM, Yang SH, et al. Induction of G2/M phase arrest and apoptosis by a novel enediyne derivative, THDA, in chronic myeloid leukemia (K562) cells. Mol Cell Biochem 2006;292:99-105. [Crossref] [PubMed]
- Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res 2011;30:87. [Crossref] [PubMed]
- Pistritto G, Trisciuoglio D, Ceci C, et al. Apoptosis as anticancer mechanism: function and dysfunction of its modulators and targeted therapeutic strategies. Aging (Albany NY) 2016;8:603-19. [Crossref] [PubMed]
- Canbay A, Friedman S, Gores GJ. Apoptosis: the nexus of liver injury and fibrosis. Hepatology 2004;39:273-8. [Crossref] [PubMed]
- Rust C, Gores GJ. Apoptosis and liver disease. Am J Med 2000;108:567-74. [Crossref] [PubMed]
- Kumar S. Caspase function in programmed cell death. Cell Death Differ 2007;14:32-43. [Crossref] [PubMed]
- Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997;326:1-16. [Crossref] [PubMed]
- Korsmeyer SJ. BCL-2 gene family and the regulation of programmed cell death. Cancer Res 1999;59:1693s-700s. [PubMed]
- Tummers B, Green DR. Caspase-8: regulating life and death. Immunol Rev 2017;277:76-89. [Crossref] [PubMed]
- Edlich F. BCL-2 proteins and apoptosis: Recent insights and unknowns. Biochem Biophys Res Commun 2018;500:26-34. [Crossref] [PubMed]
- Oršolić N. Bee venom in cancer therapy. Cancer Metastasis Rev 2012;31:173-94. [Crossref] [PubMed]
- Winder D, Günzburg WH, Erfle V, et al. Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem Biophys Res Commun 1998;242:608-12. [Crossref] [PubMed]
- Li B, Gu W, Zhang C, et al. Growth arrest and apoptosis of the human hepatocellular carcinoma cell line BEL-7402 induced by melittin. Onkologie 2006;29:367-71. [PubMed]
- Chu ST, Cheng HH, Huang CJ, et al. Phospholipase A2-independent Ca2+ entry and subsequent apoptosis induced by melittin in human MG63 osteosarcoma cells. Life Sci 2007;80:364-9. [Crossref] [PubMed]
- Liu S, Yu M, He Y, et al. Melittin prevents liver cancer cell metastasis through inhibition of the Rac1-dependent pathway. Hepatology 2008;47:1964-73. [Crossref] [PubMed]
- Liu X, Chen D, Xie L, et al. Effect of honey bee venom on proliferation of K1735M2 mouse melanoma cells in-vitro and growth of murine B16 melanomas in-vivo. J Pharm Pharmacol 2002;54:1083-9. [Crossref] [PubMed]
- Zheng J, Lee HL, Ham YW, et al. Anti-cancer effect of bee venom on colon cancer cell growth by activation of death receptors and inhibition of nuclear factor kappa B. Oncotarget 2015;6:44437-51. [Crossref] [PubMed]
- Jung GB, Huh JE, Lee HJ, et al. Anti-cancer effect of bee venom on human MDA-MB-231 breast cancer cells using Raman spectroscopy. Biomed Opt Express 2018;9:5703-18. [Crossref] [PubMed]
- Kim DH, Lee HW, Park HW, et al. Bee venom inhibits the proliferation and migration of cervical-cancer cells in an HPV E6/E7-dependent manner. BMB Rep 2020;53:419-24. [Crossref] [PubMed]
- Senger DR, Perruzzi CA, Streit M, et al. The alpha(1)beta(1) and alpha(2)beta(1) integrins provide critical support for vascular endothelial growth factor signaling, endothelial cell migration, and tumor angiogenesis. Am J Pathol 2002;160:195-204. [Crossref] [PubMed]
- Favaloro B, Allocati N, Graziano V, et al. Role of apoptosis in disease. Aging (Albany NY) 2012;4:330-49. [Crossref] [PubMed]
- Acar V, Couto Fernandez FL, Buscariolo FF, et al. Immunohistochemical Evaluation of PARP and Caspase-3 as Prognostic Markers in Prostate Carcinomas. Clin Med Res 2021;19:183-91. [Crossref] [PubMed]
- Kwon YB, Lee HJ, Han HJ, et al. The water-soluble fraction of bee venom produces antinociceptive and anti-inflammatory effects on rheumatoid arthritis in rats. Life Sci 2002;71:191-204. [Crossref] [PubMed]
- Kim KS, Choi US, Lee SD, et al. Effect of bee venom on aromatase expression and activity in leukaemic FLG 29.1 and primary osteoblastic cells. J Ethnopharmacol 2005;99:245-52. [Crossref] [PubMed]
- Park JH, Yim BK, Lee JH, et al. Risk associated with bee venom therapy: a systematic review and meta-analysis. PLoS One 2015;10:e0126971. [Crossref] [PubMed]
- Wehbe R, Frangieh J, Rima M, et al. Bee Venom: Overview of Main Compounds and Bioactivities for Therapeutic Interests. Molecules 2019;24:2997. [Crossref] [PubMed]
- Jang MH, Shin MC, Lim S, et al. Bee venom induces apoptosis and inhibits expression of cyclooxygenase-2 mRNA in human lung cancer cell line NCI-H1299. J Pharmacol Sci 2003;91:95-104. [Crossref] [PubMed]
- Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature 2000;408:307-10. [Crossref] [PubMed]
- Levine AJ, Hu W, Feng Z. The P53 pathway: what questions remain to be explored? Cell Death Differ 2006;13:1027-36. [Crossref] [PubMed]
- Yamamoto M, Tsukamoto T, Sakai H, et al. p53 knockout mice (-/-) are more susceptible than (+/-) or (+/+) mice to N-methyl-N-nitrosourea stomach carcinogenesis. Carcinogenesis 2000;21:1891-7. [Crossref] [PubMed]
- Freed-Pastor WA, Prives C. Mutant p53: one name, many proteins. Genes Dev 2012;26:1268-86. [Crossref] [PubMed]
- Muller PA, Vousden KH. Mutant p53 in cancer: new functions and therapeutic opportunities. Cancer Cell 2014;25:304-17. [Crossref] [PubMed]
- Duffy MJ, Synnott NC, Crown J. Mutant p53 as a target for cancer treatment. Eur J Cancer 2017;83:258-65. [Crossref] [PubMed]
- el-Deiry WS, Harper JW, O'Connor PM, et al. WAF1/CIP1 is induced in p53-mediated G1 arrest and apoptosis. Cancer Res 1994;54:1169-74. [PubMed]
- Abbas T, Dutta A. p21 in cancer: intricate networks and multiple activities. Nat Rev Cancer 2009;9:400-14. [Crossref] [PubMed]
- Qian Y, Chen X. Tumor suppression by p53: making cells senescent. Histol Histopathol 2010;25:515-26. [PubMed]
(English Language Editor: C. Betlazar-Maseh)


