Treatment of locally advanced unresectable pancreatic cancer: a 10-year experience
Introduction
Pancreatic cancer remains a highly lethal malignancy despite advances in treatment. In 2009 there were 42,470 new cases of pancreatic cancer and 35,240 deaths from the disease (1). At initial diagnosis, 50% of patients present with metastatic disease, 30% present with a locally advanced tumor, and only 20% are resectable. Surgical resection remains the only potentially curative therapy. The large number of recurrences and/or distant failures following resection suggest that microscopic metastases continue to be an obstacle to better outcomes. Patterns of spread include direct extension, lymphatic spread to regional lymph nodes, and hematogenous spread to distant sites. For all stages, the 1- and 5-year survival rates are 25% and 6%, respectively. Even for patients diagnosed with localized disease, the 5-year survival rate is only 22% (2).
Treatment of locally advanced unresectable pancreatic cancer (LAPC) has evolved to consist of chemotherapy alone or in combination with radiation, in hopes of achieving better survival. Although the reported benefits of chemoradiation (CRT) are controversial, it remains a management option for patients with LAPC. The survival advantage to a chemoradiation approach has not been consistently demonstrated (3) and there are few randomized phase III studies evaluating the role of combined modality therapy in recent years (4-10). There is thus a need to further examine the role of chemoradiation in LAPC.
In this study, we retrospectively analyzed the results of patients with LAPC treated with either CRT or chemotherapy alone over the past decade.
Materials and methods
Patients
Between December 1998 and October 2009, 253 patients with pancreatic adenocarcinoma were identified. Of these, 159 underwent treatment with CRT or chemotherapy alone. Patients with metastatic disease at presentation and those that underwent surgery for definitive resection were excluded from analysis, as were patients with islet-cell tumors and mucinous cystadenocarcinoma. The remaining 116 patients formed the study population for this Institutional Review Board-approved retrospective analysis. Baseline patient and tumor characteristics were reviewed, including age, gender, race, weight loss >10%, Eastern Cooperative Oncology Group performance status, tumor diameter (mm), tumor location, T stage, nodal status, histologic grade, and non-obstructive pre-treatment CA 19-9 levels when available. Stage was determined according to the American Joint Committee on Cancer staging system, 6th edition (11). Patient data were obtained through the tumor registry and review of medical records.
Treatment
Referral for chemoradiation was done at the discretion of the attending surgeon and/or medical oncologist after multidisciplinary discussion. Chemoradiation was offered primarily to patients with T3 or higher disease and/or with nodal involvement. These patients were deemed unresectable based on radiographic imaging, surgical consultation, and multidisciplinary consensus.
Patients who received radiation underwent CT simulation for treatment planning and received three-dimensional conformal external-beam radiation to the abdomen. Radiotherapy was delivered on linear accelerators using 6-23 MV photons. CT-based treatment planning was done using the Theraplan Plus treatment planning system (MDS Nordion, Ottawa, Ontario, Canada) and the Eclipse Treatment Planning System (Varian Medical Services, Palo Alto, CA, USA). Targets and organs at risk were contoured. Treatment field arrangements were designed to encompass targets with margin while sparing organs at risk. Planning dose constraints used were consistent with those postulated by Emami et al. (12). Toxicity from treatment was graded per Radiation Therapy Oncology group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) common toxicity criteria (13) by a single person after review of medical records.
Endpoints
Patterns of failure were defined by first relapse event, determined based on radiographic imaging, and categorized as locoregional versus distant. Progression-free survival (PFS) was calculated from date of diagnosis to date of first recurrence, date of death, or date of last follow-up. Date of first recurrence was determined based on radiologic follow-up imaging. Overall survival (OS) was calculated from date of diagnosis to date of death or last follow up.
Statistical analysis
Univariate statistical analysis was used to determine significant prognostic factors for OS and PFS. Statistical analyses for comparing groups in regards to categorical variables were performed using Fisher’s exact test. Similar comparisons for continuous variables were done using the Wilcoxon non-parametric test with exact p-values. The Kaplan-Meier method was used to obtain PFS and OS estimates. Survival was compared between groups using the log-rank test. Estimates of risk were obtained using the proportional hazard model. Values for continuous variables are given as median (range). Values for categorical data are specified as frequency. Statistical analysis was performed using SAS statistical analysis software version 9.2 (SAS Institute Inc, Cary, NC, USA). A nominal significance level of 0.05 was used.
Results
Of the 116 patients, 60 (52%) were female with a median age of 67 years (range, 43-89). Eight-four patients (72%) received chemoradiation [RT (+) group] and 32 (28%) patients received chemotherapy alone [RT (-) group]. Patient and treatment characteristics of both groups are summarized in Table 1. RT (+) and RT (-) groups were similar with respect to age, gender, percent weight loss, tumor size, T-stage, nodal status, histologic grade, pre-treatment CA 19-9, and use of gemcitabine based chemotherapy (all P=ns). The median radiation dose was 50.4 Gy (range, 32.4-60) in the RT (+) group. Patients in the RT (+) group were more likely to have an ECOG of 1-2 (96% vs. 81%, P=0.01) and experience less Grade 3-4 toxicity than the RT (-) group (19.1% vs. 45.1%, P=0.01).
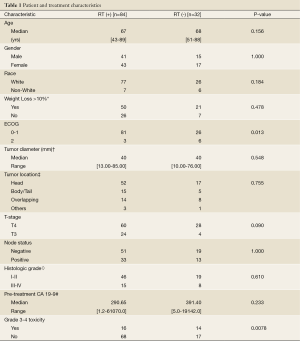
Full table
Of the 84 patients in the RT (+) group, 24 received induction chemotherapy followed by CRT and then additional chemotherapy; 41 received CRT followed by chemotherapy and 19 received CRT alone. Concurrent chemoradiation was primarily (70%) 5-fluourouracil based. The remaining 32 patients comprising the RT (-) group received chemotherapy alone with the majority (78%) receiving gemcitabine-based chemotherapy.
With a median follow-up of 11 months (range, 1.6-59.4 months), local recurrences and/or distant metastasis were observed in 53% of patients. The majority (92%) had distant metastatic disease. The most frequent site of distant metastasis was the liver (47%). Detailed patterns of failure by treatment modality are shown in Table 2.
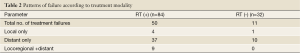
Full table
Univariate analysis showed that grade 3-4 toxicity was an adverse prognostic factor affecting PFS and OS. Other patient and treatment factors including age, tumor size, T stage, nodal status, histologic grade, pre-treatment CA 19-9, chemotherapy regimen, and the use of RT were also analyzed and are summarized in Table 3.
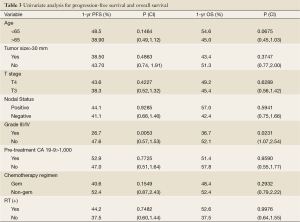
Full table
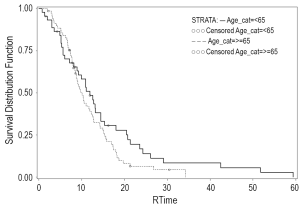
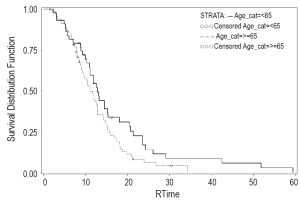
When evaluated by treatment modality, PFS was 10.9 months for the RT (+) group versus 9.1 months for the RT (-) group (Figure 1). One-year OS was 52.6% alive at one year in the RT (+) group versus 37.5% in the RT (-) group (P=0.15). Median OS was 12.5 versus 9.1 months for the RT (+) group and RT (-) groups, respectively (Figure 2).
In patients with good or excellent performance status (ECOG 0-1), subset analysis showed that PFS was 10.5 months compared to 7.6 months for the RT (+) and RT (-) groups, respectively (P=0.7574). The median OS was 12.2 months versus 7.6 months for the RT (+) groups and RT (-) groups, respectively (P=0.54) in the ECOG 0-1 subset.
Discussion
The role of combined therapy for LAPC continues to evolve. The goals of radiotherapy in LAPC include improvement in local control and palliation of pain and/or obstructive symptoms. Trials of chemoradiation versus chemotherapy alone in LAPC have reported mixed findings regarding survival and are summarized in Table 4 (4-6,9,10). In a trial conducted by the Gastrointestinal Tumor Study Group (5), the effect of concurrent chemoradiotherapy versus chemotherapy alone in LAPC was evaluated and a benefit in survival from combined modality therapy was noted. The chemoradiation arm consisted of radiation combined with 5-fluorouracil to a total dose of 54 Gy in 1.8 Gy fractions followed by maintenance streptozocin, mitomycin and 5-fluorouracil (SMF). The chemotherapy-only arm was SMF combination chemotherapy for two years or until progression. In this trial, the one-year OS was 41% in the chemoradiation arm compared to 19% in the chemotherapy-alone arm (P<0.02).
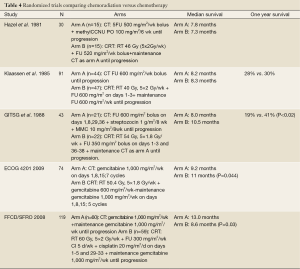
Full table
Modern chemotherapy and radiation techniques have been tested in two recent phase III trials evaluating the efficacy of chemoradiation. In the trial by the Eastern Cooperative Oncology Group (E4201), patients with LAPC were randomly assigned to chemoradiation (50.4 Gy in 28 fractions) with concurrent gemcitabine (600 mg/m2 weekly ×6) followed by 5 cycles of gemcitabine alone (1,000 mg/m2 weekly ×3 every 4 wks) versus gemcitabine alone (1,000 mg/m2 weekly ×3 every 4 wks) for 7 cycles. This trial showed that chemoradiation was associated with a slightly improved survival (11 versus 9.2 months, P=0.044) (4).
In a second recent study by Chauffert et al. reported in 2008 (10), chemoradiation was delivered to a total dose of 60 Gy concurrently with cisplatin (20 mg/m2/day, days 1-5 during weeks 1 and 5) and 5-fluorouracil (300 mg/m2/day, days 1-5 for 6 weeks). The chemotherapy-alone arm consisted of gemcitabine (1,000 mg/m2 weekly for 7 weeks). Maintenance gemcitabine (1,000 mg/m2 weekly, 3/4 weeks) was given in both arms until disease progression or toxicity. Overall survival in this trial was shorter in the chemoradiotherapy arm (13.0 vs. 8.6 months, P=0.044) and these patients experienced a higher rate of grade 3-4 toxicity compared with the chemotherapy arm (66% vs. 40% respectively; P=0.0008). A potential explanation for increased toxicity is the combination of aggressive chemotherapy delivered with concurrent radiation (60 Gy concurrent with cisplatin followed by high-dose weekly maintenance gemcitabine). Due to inferior survival in the chemoradiation arm, this study was stopped prior to planned enrollment. However, it adds to the growing body of opinion that the benefit of chemoradiation for LAPC is likely confined to a carefully selected group of patients.
We observed prolonged median survival, from 9 to 12 months, in the RT (+) group. Although not statistically significant, our limited sample size precluded our ability to detect such a difference. Retrospective power analysis revealed that it would require more than 500 patients to detect the difference between the 9 and 12 month median survival observed in the RT (-) and RT (+) groups respectively with 80% power. Excluding the study of Chauffert et al., phase II and III multi-institutional data have reported similar survival results for patients with LAPC treated with chemotherapy (range, 9.1-9.0 months) (4,14,15) and chemoradiation (range, 11.0-11.9 months) (4,16,17).
Comparison of patient characteristics between each treatment modality group [RT (+) and RT (-) groups] using the Fisher’s exact test revealed that some of the potential prognostic factors were not evenly distributed between the groups. Patients in the RT (-) group were more likely to have co-morbidities and poor performance status than those in the RT (+) group. Therefore, these patients were less likely to be selected for chemoradiation. We observed that the patients in the RT (+) experienced fewer grade 3/4 toxicities from treatment than did historical controls. Univariate analysis of patient characteristics showed that a reduced frequency of grade 3/4 toxicity predicted for improved PFS and one-year OS.
Our data suggest that chemoradiation can be delivered safely and that acceptable toxicity is achievable with strict quality assurance, multidisciplinary management, and appropriate patient selection. It also highlights the need for a consistent approach to modern radiotherapy in an anatomic region with unique planning considerations, to avoid overdosing the neighboring radiosensitive organs reported by our group previously (18-21). CT simulation and three-dimensional conformal treatment planning was used in our study. Radiotherapy up to 54 Gy was delivered over a period of 5-7 weeks using standard fractionation. No planned treatment break or altered fractionation schemes were used. Potential detrimental effects of treatment interruptions and lack of effective systemic effect during a protracted radiation course on tumor control has led to the investigation of altered fractionation schemes, including shorter courses of high-dose radiotherapy using image guidance, as well as more conformal techniques (22-27). This is an area under active investigation and needs to be tested in a randomized setting (23,24,28,29).
Although local control rates have been improved by innovations in radiation therapy, systemic failure remains a major obstacle in improving survival. In our patterns of failure analysis, we found that the majority of treatment failures in both groups occurred at distant sites. The proportion of patients with a component of distant metastasis in the RT (+) group was 92% (46 of 50) and it was 91% (10 of 11) in the RT (-) group. The need for more effective chemotherapy is suggested by the high rate of distant metastasis in the RT (+) and RT (-) groups as shown in Table 2.
Over the last 10 years, gemcitabine alone and in combination has evolved as a standard of chemotherapy in LAPC (30,31). In more recent phase I/II studies, concurrent gemcitabine with radiation has shown promise in the treatment of locally advanced unresectable disease with manageable toxicity (32-40). In some of these trials, radiation targets included elective coverage of draining lymphatics, resulting in large treatment volumes that may have contributed to the increased toxicity that was described. Conformal radiation fields combined with newer systemic agents may help to reduce toxicity of treatment. More recently, biologic agents such as erlotinib have been tested in combination with gemcitabine, with varying success (7,14,41). There is a need for clinical trials using newer systemic agents and molecular targets to evaluate their efficacy in reducing the incidence of distant metastases.
Our study is limited by its retrospective nature, small sample size, and lack of data regarding quality of life. Many of the cited studies in this patient population have not incorporated assessments of quality of life, improvement in performance status, and palliation of symptoms (4-6,9,10). These endpoints are important to consider in patients with limited survival and marginal performance status who are at increased risk for toxicity from chemoradiation. In 2002, a study in Japan looked at combined-modality therapy versus best supportive care and found that locally advanced patients who underwent treatment derived benefit in quality of life as measured by a maintained performance status (42).
An attractive strategy to facilitate patient selection for CRT is through a trial of upfront systemic therapy followed by re-assessment. Radiotherapy may offer a survival benefit in patients with disease that proves to be localized after a period of time. Many patients will progress during induction chemotherapy and may be spared the added toxicity of combined-modality therapy.
In a study by The Groupe Cooperateur Multidisciplinaire en Oncologie (GERCOR) LAP07, 181 patients were reviewed who were treated with 5-fluorouracil (5-FU) or gemcitabine-based chemotherapy for four months. Those without evidence of disease progression were given additional chemotherapy or chemoradiation. Overall survival was improved in patients who went on to receive chemoradiation (43). In our study, 24 patients received induction chemotherapy followed by CRT and then additional chemotherapy. The median survival of these patients was 14.5 months (95% CI, 11.1-18.4) compared to 11.9 months (95% CI, 9.8-12.8) for the patients who did not receive induction chemotherapy prior to chemoradiation.
In addition to appropriate patient selection, a more effective surrogate marker is needed to identify those patients most likely to benefit from additional therapy. CA19-9 is the most commonly used tumor marker in patients with pancreatic cancer. Occult metastatic disease may be suggested by rising tumor markers such as CA 19-9 during the induction period. Perioperative CA 19-9 levels have been shown to be prognostic in patients with resectable disease (44); CA 19-9 is a useful marker to incorporate into decisions regarding adjuvant therapy. Similarly, recent studies have shown that the peri-chemoradiation serum CA 19-9 level is an independent predictor of recurrence and survival after chemoradiation in LAPC (45,46).
Conclusion
Optimal management for locally advanced, unresectable pancreatic cancer continues to evolve. Chemoradiation is a management option in appropriately selected patients. Chemotherapy alone is also an option, especially for patients with marginal performance status.
Acknowledgements
The authors would like to thank William Preston, Ed.D for his assistance with manuscript preparation.
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer Statistics, 2009. CA Cancer J Clin 2009;59:225-49. [PubMed]
- American Cancer Society. Facts and Figures 2010. Atlanta, GA, American Cancer Society, 2010.
- Yang GY, Wagner TD, Fuss M, et al. Multimodality approaches in pancreatic cancer. CA Cancer J Clin 2005;55:352-67. [PubMed]
- Loehrer PJ, Powell ME, Cardenes HR, et al. A randomized phase III study of gemcitabine in combination with radiation therapy versus gemcitabine alone in patients with localized, unresectable pancreatic cancer: E4201 2008; ASCO Meeting Abstracts 2008;26:4506.
- Gastrointestinal Tumor Study Group. Treatment of locally unresectable carcinoma of the pancreas: comparison of combined modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. J Natl Cancer Inst 1988;80:751-5. [PubMed]
- Klaassen DJ, MacIntyre JM, Catton GE, et al. Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol 1985;3:373-8. [PubMed]
- Philip PA, Benedetti J, Corless CL, et al. Phase III study comparing gemcitabine plus cetuximab versus gemcitabine in patients with advanced pancreatic adenocarcinoma: Southwest Oncology Group-directed intergroup trial S0205. J Clin Oncol 2010;28:3605-10. [PubMed]
- Childs DS, Moertel C, Holbrook MA, et al. Treatment of malignant neoplasms of the gastrointestinal tract with a combination of 5-fluorouracil and radiation. Radiology 1965;84:843-8. [PubMed]
- Hazel JJ, Thirwell MP, Huggins M, et al. Multi-drug chemotherapy with and without radiation for carcinoma of the stomach and pancreas: A prospective randomized trial. J Can Assoc Radiol 1981;32:164-5. [PubMed]
- Chauffert B. Phase III trial comparing an intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer: Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 2008;19:1592-9. [PubMed]
- Greene FL, Page DL, Fleming ID, et al. The American Joint Committee on Cancer. AJCC Cancer Staging Manual. 6th ed. New York, NY: Springer-Verlag 2002.
- Emami B, Lyman J, Brown A, et al. Tolerance of normal tissue to therapeutic irradiation. Int J Radiat Oncol Biol Phys 1991;21:109-22. [PubMed]
- Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 1995;31:1341-6. [PubMed]
- Kindler HL, Niedzwiecki D, Hollis D, et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J Clin Oncol 2010;28:3617-22. [PubMed]
- Poplin E, Feng Y, Berlin J, et al. Phase III, randomized study of gemcitabine and oxaliplatin versus gemcitabine (fixed-dose rate infusion) compared with gemcitabine (30-minute infusion) in patients with pancreatic carcinoma E6201: A trial of the eastern cooperative oncology group. J Clin Oncol 2009;27:3778-85. [PubMed]
- Rich T, Harris J, Abrams R, et al. Phase II study of external irradiation and weekly paclitaxel for nonmetastatic, unresectable pancreatic cancer: RTOG-98-12. Am J Clin Oncol 2004;27:51-6. [PubMed]
- Safran H, Dipetrillo T, Iannitti D, et al. Gemcitabine, paclitaxel, and radiation for locally advanced pancreatic cancer: a Phase I trial. Int J Radiat Oncol Biol Phys 2002;54:137-41. [PubMed]
- Crane CH, Winter K, Regine WF, et al. Phase II study of bevacuzimab with concurrent capecitabine and radiation followed by maintenance gemcitabine and bevacuzimab for locally advanced pancreatic cancer: Radiation Therapy Oncology Group RTOG 0411. J Clin Oncol 2009;27:4096-102. [PubMed]
- May KS, Khushalani NI, Chandrasekhar R, et al. Analysis of clinical and dosimetric factors associated with change in renal function in patients with gastrointestinal malignancies following chemoradiation to the abdomen. Int J Radiat Oncol Biol Phys 2010;76:1193-8. [PubMed]
- Yang GY, Salerno May K, Iyer RV, et al. Renal atrophy secondary to chemoradiation treatment for abdominal malignancies. Int J Radiat Oncol Biol Phys 2010;78:539-46. [PubMed]
- Landry JC, Yang GY, Ting JY, et al. Treatment of pancreatic cancer tumors with IMRT using the volume at risk approach: Employing dose-volume histogram (DVH) and normal tissue complication probability (NTCP) to evaluate small bowel toxicity. Med Dosim 2002;27:121-9. [PubMed]
- Koong AC, Le QT, Ho A, et al. Phase I study of stereotactic radiosurgery in patients with LAPC. Int J Radiat Oncol Biol Phys 2004;58:1017-21. [PubMed]
- Koong AC, Christofferson E, Le QT, et al. Phase II study to assess the efficacy of conventionally fractionated radiotherapy followed by stereotactic radiosurgery boost in patients with locally advance pancreatic cancer. Int J Radiat Oncol Biol Phys 2005;63:320-3. [PubMed]
- Parikh SD, Burton SA, Heron DE, et al. Stereotactic radiosurgery in patients with resected pancreatic carcinomas with positive margins. Int J Radiat Oncol Biol Phys 2008;72:S272-3.
- Schellenberg D, Goodman KA, Lee F, et al. Gemcitabine chemotherapy and single-fraction stereotactic body radiotherapy for locally advance pancreatic cancer. Int J Radiat Oncol Biol Phys 2008;72:678-86. [PubMed]
- Hoyer M, Roed H, Sengelov L, et al. Phase II study on stereotactic radiotherapy of locally advanced pancreatic carcinoma. Radiother Oncol 2005;76:48-53. [PubMed]
- Mahadevan A, Shanmugam L, Kaplan I, et al. Fractionated radiosurgery for pancreas cancer. Int J Radiat Oncol Biol Phys 2007;69:S307.
- Chang BW, Saif MW. Stereotactic Body Radiation Therapy (SBRT) in Pancreatic Cancer: Is It Ready for Prime Time? JOP 2008;9:676-82. [PubMed]
- Hong TS, Ryan DP, Blaszkowsky LS, et al. Phase I study of preoperative short-course chemoradiation with proton beam therapy and capecitabine for resectable pancreatic ductal adenocarcinoma of the head. Int J Radiat Oncol Biol Phys 2011;79:151-7. [PubMed]
- Burris HA III, Moor MJ, Andersen J, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol 1997;15:2403-13. [PubMed]
- Heinemann V, Boeck S, Hinke A, et al. Meta-analysis of randomized trials: evaluation of benefit from gemcitabine-based combination chemotherapy applied in advanced pancreatic cancer. BMC Cancer 2008;8:82. [PubMed]
- Cardenes HR, Moore AM, Johnson CS, et al. A Phase II Study of gemcitabine in combination with radiation therapy with localized, unresectable, pancreatic cancer: A Hoosier Oncology Group Study. Am J Clin Oncol 2011;34:460-5. [PubMed]
- Small W Jr, Berlin J, Freedman GM, et al. Full-dose gemcitabine with concurrent radiation therapy in patients with nonmetastatic pancreatic cancer: a multicenter phase II trial. J Clin Oncol 2008;26:942-7. [PubMed]
- Wolff RA, Evans DB, Gravel DM, et al. Phase I trial of gemcitabine combined with radiation for the treatment of locally advanced pancreatic adenocarcinoma. Clin Cancer Res 2001;7:2246-53. [PubMed]
- Cengiz M, Zorlu F, Yalchin S, et al. Concurrent gemcitabine and radiotherapy for LAPC. Med Oncol 2007;24:239-43. [PubMed]
- Murphy JD, Adusumilli S, Griffith KA, et al. Full-dose gemcitabine and concurrent radiotherapy for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2007;68:801-8. [PubMed]
- Okusaka T, Ito Y, Ueno H, et al. Phase II study of radiotherapy combined with gemcitabine for LAPC. Br J Cancer 2004;91:673-7. [PubMed]
- McGinn CJ, Zalupski MM, Shureiqi I, et al. Phase I trial of radiation dose escalation with concurrent weekly full-dose gemcitabine in patients with advanced pancreatic cancer. J Clin Oncol 2001;19:4202-8. [PubMed]
- McGinn CJ, Zalupski MM. Radiation therapy with once-weekly gemcitabine in pancreatic cancer: current status of clinical trials. Int J Radiat Oncol Biol Phys 2003;56:10-5. [PubMed]
- Crane CH, Abbruzzese JL, Evans DB, et al. Is the therapeutic index better with gemcitabine-based chemoradiation than with 5-fluorouracil-based chemoradiation in locally advance pancreatic cancer? Int J Radiat Oncol Biol Phys 2002;52:1293-302. [PubMed]
- Moore MJ, Goldstein D, Hamm J, et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 2007;25:1960-6. [PubMed]
- Shinchi H, Takao S, Noma H, et al. Length and quality of survival after external-beam radiotherapy with concurrent continuous 5-fluorouracil infusion for locally unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 2002;53:146-50. [PubMed]
- Huguet F, Andre T, Hammel P. Impact of chemoradiotherapy after disease control with chemotherapy in locally advanced pancreatic adenocarcinoma in GERCOR phase II and III studies. J Clin Oncol 2007;25:326-31. [PubMed]
- Berger AC, Garcia M Jr, Hoffman JP, et al. Postresection CA 19-9 predicts overall survival in patients with pancreatic cancer treated with adjuvant chemoradiation: A prospective validation by RTOG 9704. J Clin Oncol 2008;26:5918-22. [PubMed]
- Yang G, Malik N, Chandrasekhar R, et al. Change in CA 19-9 levels after chemoradiotherapy predicts survival in patients with locally advance unresectable pancreatic cancer. ASTRO 2010 [Abstract].
- Park JK, Yoon YB, Kim YT, et al. Survival and prognostic factors of unresectable pancreatic cancer. J Clin Gastroenterol 2008;42:86-91. [PubMed]