Diagnosis and management of patients with malignant peritoneal mesothelioma
Introduction
Malignant peritoneal mesothelioma (MPM) represents approximately 7% to 10% of all mesothelioma diagnoses with the majority being the pleural variant. It is a rare cancer that is eventually fatal is most afflicted individuals. There are approximately 800 new cases of MPM diagnosed annually in the United States with both males and females having an equal incidence of the disease (1-3). There are several risk factors that have been implicated in the development of MPM; data indicating a strong association between asbestos exposure and the development of disease have been known for decades (4).
The first description of MPM was likely in a case report from over 100 years ago in which a 32-year-old male miller who presented with abdominal pain and ascites was described (5). The authors reported that the patient harbored a condition that was an extensive and diffuse intraperitoneal neoplastic process not amenable to surgical resection. He was treated palliatively and succumbed to disease one year later. Fifty years later, a review of the literature identified only 13 pathologically confirmed cases of MPM (6). However, after that detailed description of the tumor’s pathological features, there was a marked increase in the number of subsequently documented cases. In 1972, Moertel published a comprehensive review of the subject and described the clinical presentation, histological features, and biological behavior of 169 cases documented in the literature (7). One of the earliest studies evaluating a regimen specifically for MPM patients was published by Antman and colleagues in 1983 (8). In that report, 18 treatment naïve patients with MPM were treated with a multi-disciplinary regimen including cytoreduction (CRS) and a systemically administered doxorubicin containing regimen. Fourteen had measurable or evaluable disease and six (43%) had a measurable response. The median survival in the six responding patients was 22 months while survival for the remaining eight patients who had stable or progressive disease was 5 months. The doxorubicin containing regimens were associated with significant toxicity.
Over the next 30 years, a number of institutional reviews established the foundation for CRS and hyperthermic intraperitoneal chemotherapy (HIPEC) as the preferred first-line therapy in selected patients due to the long term survival associated with the procedure and the limited benefit of systemic therapy. More recently, there have been remarkable discoveries into the molecular biology of MPM that will undoubtedly serve as the springboard for more effective intervention. It has been shown that the tumor suppressor gene, BAP-1 is mutated in high frequency in patients with MPM and that BAP-1 mutation may predispose individuals to the development of MPM (9,10). This review will cover the current concepts in the diagnosis and management of patients with this condition.
Diagnosis and initial evaluation
Patients with MPM usually present with signs and symptoms that reflect a diffuse progressive abdominal condition; these symptoms include bloating and abdominal pain which are frequently caused by ascites as well as early satiety, weight loss, and decrease in energy (8,11,12). In some cases, a palpable abdominal mass has been described on examination. In contrast to the pleural form of the disease, genders are afflicted equally and the median age at diagnosis is approximately 50 years although the condition can arise in teenagers and the elderly (13).
Two hallmark features of the disease are the heterogeneity of it biological behavior, that is, the rate of disease progression is highly variable, and its propensity to remain confined to the abdominal cavity throughout the course of disease (14). Extra-abdominal disease is unusual and when it occurs is manifested by disease progression either into the pleural space (via direct extension or trans-diaphragmatic lymphatics) or extra-abdominal lymph node metastasis (15). Extra-abdominal disease will usually occur when there is long-standing and advanced disease in the abdomen (16,17). Nevertheless, almost all patients succumb to the consequences of disease progression in the abdominal cavity.
The diagnosis of MPM should be considered in any individual with evidence of a diffuse malignant process in the abdomen on initial clinical evaluation. It is confirmed based on suspicion of findings on cross-sectional imaging and tissue biopsy with appropriate immunohistochemical staining. Computed tomography (CT) scan is the imaging modality most commonly used although magnetic resonance imaging (MRI) using specific acquisition protocols may be increasingly used in the future (18,19). The value of positron emission tomography (PET) or PET/CT in initial diagnosis or staging is not clear (20). Radiographically, the disease may or may not be associated with ascites. There are usually diffuse omental masses, mesenteric nodules or nodularity, or parietal peritoneal thickening (21). Generally, the most favorable findings are ascites associated with minimal soft tissue masses and preserved normal anatomy of the small bowel and its mesentery (Figure 1). The most unfavorable radiographic findings include the absence of ascites and large diffuse nodular thickening of the peritoneal surfaces with marked distortion of the normal architecture of the bowel (Figure 2). Intermediate CT findings include an imageable layer of tumor on the small bowel and its mesentery. CT findings consistent with bowel obstruction are very ominous (19). CT scan is important not only to assess the extent of disease and assist in treatment planning but may also provide findings that distinguish MPM from other malignancies that can present with peritoneal dissemination such as stomach, pancreas, colon, and ovarian neoplasms.
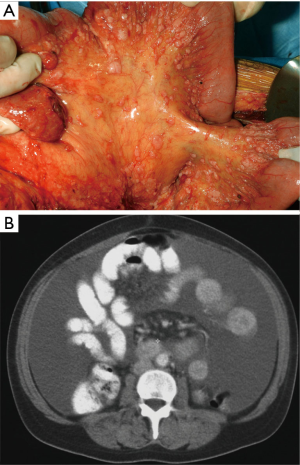
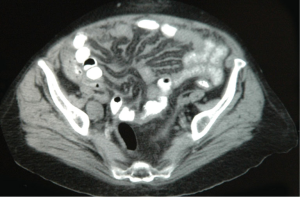
Tumor specimens may be obtained through diagnostic laparoscopy or CT guided biopsy; diagnostic laparoscopy has the added advantage of allowing for direct visualization of tumor burden and, consequently, the identification of patients whose disease is amenable to operative intervention. Abdominal paracentesis may be diagnostic; however, there are usually only scant numbers of malignant cells present in ascites for diagnosis. Upper and lower endoscopy should be performed as clinically indicated.
There are three histological subtypes of MPM: epithelioid, sarcomatoid, and the mixed/biphasic type; the epithelioid subtype is the most common and associated with the best prognosis (Figure 3) (22-25). A panel of immunohistochemical antibodies must be employed to definitively establish the diagnosis. Antibodies that usually stain positive in MPM and are most commonly used are calretinin, cytokeratin 5/6, and vimentin. Others that may be used include epithelial membrane antigen (EMA), and Wilms tumor 1 (WT-1). CEA, B 72.3, MOC-31, and Ber-EP4 are used to exclude a carcinoma from another primary source (22-25). It is recommended that two or more mesothelial immunohistochemical markers be used when establishing the diagnosis of MPM (26).
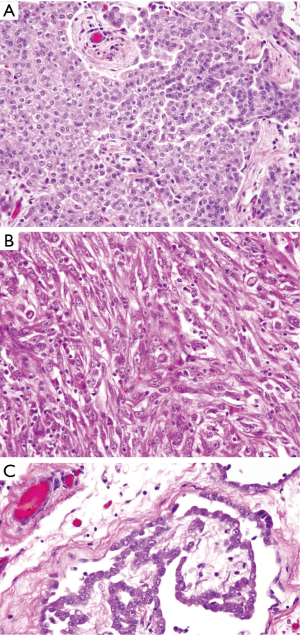
Serum laboratory studies may reveal an elevated cancer antigen (CA)-125; however, this marker alone is not tumor specific and is typically best used to monitor for disease recurrence or progression in those with a confirmed diagnosis (16,27). Consequently, morbidity and mortality from DMPM occurs from regional disease progression secondary to progressive intestinal obstruction and cachexia (11). The median survival in untreated patients is approximately 6 months after diagnosis (28).
Staging and patient selection for operative CRS and HIPEC
First-line intervention for selected patients with MPM is widely acknowledged to be operative CRS and HIPEC with chemotherapy. Patient selection for CRS and HIPEC is influenced by the overall physiological health of the patient and a careful estimation regarding the ability to achieve a complete or near complete cytoreduction. The peritoneal cancer index (PCI), which is derived from radiographic imaging or intraoperative evaluation codifies the extent of disease by assigning an extent of disease score of 0 to 3 to 13 different regions of the abdominal cavity (29). In general a PCI of less than 20 is considered low to moderate burden disease, 21 to 30 as moderate, and above 30 as high burden of disease.
Based on the diffuse nature of MPM and its propensity to remain confined to and progress within the abdominal cavity, a traditional TNM staging system has limited utility. One such system has been proposed that stratifies the PCI into quartiles (1-10, 11-20, 21-30, and above 30) as a surrogate for T-stages 1 to 4. The presence of intra-abdominal nodal disease constitutes N disease and any extra-abdominal disease is M (30). This staging system shows an association with decreasing survival with increasing stage (Table 1).
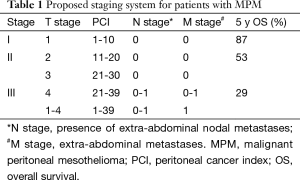
Full table
CRS and HIPEC for patients with MPM
Most data detailing outcomes in selected patients undergoing CRS and HIPEC or EPIC (early post-operative intraperitoneal chemotherapy) are retrospective single of multi-center reports. In selected patients the morbidity and mortality range from 30-40% and 2-4%, respectively (13,15,29,31). Median overall survival ranges from 30 to 92 months and this most likely reflects variations in patient selection rather that differences in therapeutic approach (13-15). The best survival has been uniformly observed in patients for whom a complete or near complete (minimal residual disease) can be achieved; this has been codified as completeness of cytoreduction (CCR) score 0 or 1, respectively. Patients who have limited or diffuse gross residual disease are scored as a CCR 2 or 3, respectively; outcomes for these patients are significantly worse.
Two large multi-center studies have been reported (Table 2) (29,31). The largest is a multi-institutional review that combined data from 29 centers worldwide and included 405 MPM patients; although the goal of operative CRS was complete resection of all visible disease, the selection criteria for CRS and HIPEC were not uniformly defined and a variety of intraperitoneal chemotherapeutic agents were utilized during HIPEC including cisplatin, mitomycin C, and doxorubicin (29). The median actuarial overall survival was 53 months with three and five-year survival rates were 60 and 47 percent, respectively. A second multi-institutional report that included 211 patients treated at three centers in the United States (which were not part of the previous study) showed an actuarial overall survival of 38 months and 5- and 10-year survival rates of 41 and 26 percent, respectively (31). All patients underwent CRS and HIPEC using either cisplatin or mitomycin C. Both studies evaluated potential prognostic factors associated with improved survival. Factors independently associated with improved survival in both studies included a CCR score of 0 or 1 and histologic grade of tumor. In the second study, the use of cisplatin versus mitomycin C during HIPEC was associated with improved survival ; interestingly, the benefit was most marked in those who had a CC 0 or 1, and there was no benefit to HIPEC with either agent in patients who had a suboptimal surgical cytoreduction (CC >2). The association between improved survival and the use of cisplatin has also been reported by another group (33).
Additional studies, mostly single center reports have provided additional information related to the use of CRS and HIPEC in patients with MPM. Based on reports from the University of Maryland, the degree of tissue invasion based on histopathology appears to be an important predictor of aggressive tumor biological behavior and shortened patient survival (24,25). In an analysis of factors associated with outcome in 73 patients treated with CRS and HIPEC factors independently associated with poor outcome included sarcomatoid growth pattern, degree of tissue invasion (codified at the time of histopathologic analysis as absent (0), into stroma (I), into fat (II), or into adjacent structures (III)), and surgical CCR score of >2.
Several studies have reported on variations of treatment regimens such as neoadjuvant or adjuvant systemic chemotherapy or prolonged intra-peritoneal administration of chemotherapy after CRS. Based on the uncontrolled nature of these reports it is conclude that more ambitious treatment regimens offer any additional benefit over CRS and HIPEC alone. An aggressive multimodality two-staged operative approach for patients with MPM has been reported (34). In this phase II trial, 27 patients had initial CRS and placement of an IP catheter, followed by IP cisplatin, doxorubicin, and gamma interferon for four months. Subsequently, a second laparotomy was performed with attempted complete cytoreduction of residual disease and HIPEC using cisplatin and mitomycin C, followed by whole abdominal radiotherapy (RT) in approximately half of patients. There were no perioperative deaths; grade 3 or 4 toxicities included small bowel obstruction, fistula, chemical peritonitis, and catheter infection in one patient each, and ototoxicity in two patients. The median overall survival was 70 months, with a three-year survival of 67 percent. The Washington Hospital Center has reported outcomes of a study using adjuvant IP pemetrexed and IV cisplatin for six cycles after CRS and HIPEC in ten patients with MPM (35). Treatment was well tolerated, and there was an apparent pharmacokinetic advantage to IP pemetrexed. No long term survival data are reported. Investigators at NCI Milan reported outcomes in 116 MPM patients, some of whom received neoadjuvant and/or adjuvant systemic chemotherapy in addition to HIPEC (36). There was no association between use of chemotherapy and CCR, operative morbidity, or survival. Several studies have shown that HIPEC can palliate malignant ascites in over 90% of patients with MPM, even in those who have suboptimal or no surgical cytoreduction (37,38).
Two recent population based studies have been reported on the use of surgical intervention in patients with MPM (Table 2). In an analysis of 1,591 patients diagnosed with MPM between 1973 and 2010 obtained from the Surveillance, Epidemiology, and End Results database (32), a number of parameters associated with increased risk of shortened survival were identified including advancing age, male gender, histology (biphasic versus epithelioid), and extent of disease. The study also found that surgical resection was associated with improved survival overall, that survival after surgical resection improved over time, and that currently almost 57% of individuals with a diagnosis of MPM do not undergo any type of surgical resection. Together the data suggest that over time improvement in patient selection has resulted in better outcomes after surgical resection but that many individuals who may be good candidates for surgical resection are not presented with that option. A meta-analysis of 20 publications reporting on 1,047 patients with MPM undergoing CRS and HIPEC reported a complete or near complete cytoreduction (CC0 or CC1) in 67% of patients (13). The estimated 5-year survival in the cohort was 42%; treatment factors associated with improved survival included the use of early post-operative intraperitoneal chemotherapy (EPIC) and the use of cisplatin alone or in combination during HIPEC or EPIC. Some of the publications included in the meta-analysis were sequential reports from one institution which may have included overlapping cohorts of patients.

Full table
For patients with recurrence after initial CRS/HIPEC, repeat CRS and HIPEC in selected patients is associated with long term survival. Two studies have shown that in selected patients, outcome after repeat CRS and HIPEC is associated with long term benefit. A study from the Washington Hospital Center reported the outcomes of 44 patients out of 205 with MPM who underwent a second CRS and HIPEC (35). Median overall survival was 54 months in those undergoing a second DRX and HIPEC versus 77 months for those after initial CRS and HIPEC. Notably, the ability to achieve a complete or near complete (CC0 or CC1) resection was significantly lower in patients undergoing a second CRS and HIPEC highlighting the need for careful patient selection.
Systemic therapy for patients with MPM
Systemic chemotherapy for patients with MPM is most commonly use in those who are not good operative candidates. Two reports have presented the results of an expanded access program that evaluated pemetrexed alone or in combination with cisplatin for patients with MPM who were deemed surgically unresectable (39,40). Patients received pemetrexed alone or in combination with cisplatin for six cycles or until disease progression. The response rates for chemotherapy naïve patients versus those who had previously received chemotherapy were similar (25% and 23.3%, respectively). The median survival of patients who received pemetrexed alone was 8.7 months compared to 13.1 months for patients who received the combination regimen. The rate of disease control (CR + PR + SD) among all patients was 71.2%. The disease control rate and similar response rates between chemotherapy naïve and previously treated patients indicate that pemetrexed has reasonable clinical activity as a first or second line chemotherapeutic agent. As a result of these studies, pemetrexed with cisplatin has been widely adopted as the preferred initial chemotherapeutic regimen for DMPM patients with surgically unresectable disease.
An alternate regimen has been tested and reported from phase II trial which evaluated the efficacy of pemetrexed and gemcitabine in surgically unresectable and chemotherapy naive patients with (41). Patients received this combination regimen for six cycles or until disease progression. The overall response rate with this regimen was 15% with an estimated one year survival rate of 67.5%. The median time to disease progression was 10.4 months. Unfortunately, the toxicity associated with this regimen was significant; 25% of patients did not finish the planned course of therapy and there was one treatment related death. Despite the similar disease control rates and the longer median overall survival for patients in this study compared to those who received the pemetrexed/cisplatin combination in the previous study, the severe toxicity associated with this regimen limits its clinical utility for patients with MPM.
There are limited other systemic options for patients with MPM although current research studies may alter the landscape in this regard very markedly. Although a high proportion of MPM tumors express or over-express the epidermal growth factor receptor (EGFR) (42); data from a phase II study using an EGFR inhibitor have not demonstrated clinically meaningful activity (43). Other reports have shown that the phosphatidylinositol-3-kinase and mammalian target of rapamycin (P13K/mTOR) signaling pathways may be targets in this disease but no clinical data have been published (44,45). The use of bevacizumab with systemic chemotherapy showed no benefit in a multi-center trial random assignment trial (46,47). A monoclonal antibody that targets the cytotoxic T-lymphocyte antigen 4 (CTLA4), was evaluated in a phase II trial in patients with previously-treated pleural or peritoneal mesothelioma (48). In 29 evaluable patients the overall response rate was low (7 percent) but disease-control was seen in 31 percent of patients with a median progression-free survival of six months. The overall toxicity profile was favorable.
Summary and conclusions
MPM is a rare and diffuse condition of the peritoneal cavity. In selected patients, CRS and HIPEC has been associated with long term survival; factors associated with the best outcome are female gender, age less than 60 years, favorable histology, and complete resection of all visible disease at operation (CCR 0). Systemic chemotherapy has some efficacy but is usually held in reserve for patients who are not good operative candidates. Immune checkpoint inhibitors and other targeted agents are in clinical development.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: An update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576-88. [PubMed]
- Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973-2005. Cancer Causes Control 2009;20:935-44. [PubMed]
- Larson T, Melnikova N, Davis SI, et al. Incidence and descriptive epidemiology of mesothelioma in the United States, 1999-2002. Int J Occup Environ Health 2007;13:398-403. [PubMed]
- Selikoff IJ, Churg J, Hammond EC. Relation between exposure to asbestos and mesothelioma. N Engl J Med 1965;272:560-5. [PubMed]
- Miller J, Wynn H. Malignant tumor arising from endothelium of peritoneum, and producing mucoid ascitic fluid. J Pathol Bacteriol 1908;12:267-78.
- Winslow DJ, Taylor HB. Malignant peritoneal mesotheliomas: a clinicopathological analysis of 12 fatal cases. Cancer 1960;13:127-36. [PubMed]
- Moertel CG. Peritoneal mesothelioma. Gastroenterology 1972;63:346-50. [PubMed]
- Antman KH, Pomfret EA, Aisner J, et al. Peritoneal mesothelioma: Natural history and response to chemotherapy. J Clin Oncol 1983;1:386-91. [PubMed]
- Alakus H, Yost SE, Woo B, et al. BAP1 mutation is a frequent somatic event in peritoneal malignant mesothelioma. J Transl Med 2015;13:122. [PubMed]
- Napolitano A, Pellegrini L, Dey A, et al. Minimal asbestos exposure in germline BAP1 heterozygous mice is associated with deregulated inflammatory response and increased risk of mesothelioma. Oncogene 2015. [Epub ahead of print]. [PubMed]
- Antman K, Shemin R, Ryan L, et al. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965-1985. J Clin Oncol 1988;6:147-53. [PubMed]
- Sugarbaker PH, Welch LS, Mohamed F, et al. A review of peritoneal mesothelioma at the Washington Cancer Institute. Surg Oncol Clin N Am 2003;12:605-21. xi. [PubMed]
- Helm JH, Miura JT, Glenn JA, et al. Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Malignant Peritoneal Mesothelioma: A Systematic Review and Meta-analysis. Ann Surg Oncol 2015;22:1686-93. [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [PubMed]
- Magge D, Zenati MS, Austin F, et al. Malignant peritoneal mesothelioma: prognostic factors and oncologic outcome analysis. Ann Surg Oncol 2014;21:1159-65. [PubMed]
- Alexander HR, Hanna N, Pingpank JF. Clinical results of cytoreduction and HIPEC for malignant peritoneal mesothelioma. Cancer Treat Res 2007;134:343-55. [PubMed]
- Antman KH, Blum RH, Greenberger JS, et al. Multimodality therapy for malignant mesothelioma based on a study of natural history. Am J Med 1980;68:356-62. [PubMed]
- Low RN, Barone RM. Combined diffusion-weighted and gadolinium-enhanced MRI can accurately predict the peritoneal cancer index preoperatively in patients being considered for cytoreductive surgical procedures. Ann Surg Oncol 2012;19:1394-401. [PubMed]
- Yan TD, Haveric N, Carmignani CP, et al. Computed tomographic characterization of malignant peritoneal mesothelioma. Tumori 2005;91:394-400. [PubMed]
- Deraco M, Bartlett D, Kusamura S, et al. Consensus statement on peritoneal mesothelioma. J Surg Oncol 2008;98:268-72. [PubMed]
- Park JY, Kim KW, Kwon HJ, et al. Peritoneal mesotheliomas: clinicopathologic features, CT findings, and differential diagnosis. AJR Am J Roentgenol 2008;191:814-25. [PubMed]
- Cerruto CA, Brun EA, Chang D, et al. Prognostic significance of histomorphologic parameters in diffuse malignant peritoneal mesothelioma. Arch Pathol Lab Med 2006;130:1654-61. [PubMed]
- Husain AN, Colby TV, Ordóñez NG, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: a consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med 2009;133:1317-31. [PubMed]
- Lee M, Alexander HR, Burke A. Diffuse mesothelioma of the peritoneum: a pathological study of 64 tumours treated with cytoreductive therapy. Pathology 2013;45:464-73. [PubMed]
- Liu S, Staats P, Lee M, et al. Diffuse mesothelioma of the peritoneum: correlation between histological and clinical parameters and survival in 73 patients. Pathology 2014;46:604-9. [PubMed]
- Ordóñez NG. Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol 2000;24:598-606. [PubMed]
- Baratti D, Kusamura S, Martinetti A, et al. Prognostic value of circulating tumor markers in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2007;14:2300-8. [PubMed]
- Kaya H, Sezgı C, Tanrıkulu AC, et al. Prognostic factors influencing survival in 35 patients with malignant peritoneal mesothelioma. Neoplasma 2014;61:433-8. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [PubMed]
- Yan TD, Deraco M, Elias D, et al. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database*. Cancer 2011;117:1855-63. [PubMed]
- Alexander HR Jr, Bartlett DL, Pingpank JF, et al. Treatment factors associated with long-term survival after cytoreductive surgery and regional chemotherapy for patients with malignant peritoneal mesothelioma. Surgery 2013;153:779-86. [PubMed]
- Miura JT, Johnston FM, Gamblin TC, et al. Current trends in the management of malignant peritoneal mesothelioma. Ann Surg Oncol 2014;21:3947-53. [PubMed]
- Blackham AU, Shen P, Stewart JH, et al. Cytoreductive surgery with intraperitoneal hyperthermic chemotherapy for malignant peritoneal mesothelioma: mitomycin versus cisplatin. Ann Surg Oncol 2010;17:2720-7. [PubMed]
- Hesdorffer ME, Chabot JA, Keohan ML, et al. Combined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol 2008;31:49-54. [PubMed]
- Ihemelandu C, Bijelic L, Sugarbaker PH. Iterative Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy for Recurrent or Progressive Diffuse Malignant Peritoneal Mesothelioma: Clinicopathologic Characteristics and Survival Outcome. Ann Surg Oncol 2015;22:1680-5. [PubMed]
- Deraco M, Baratti D, Hutanu I, et al. The role of perioperative systemic chemotherapy in diffuse malignant peritoneal mesothelioma patients treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2013;20:1093-100. [PubMed]
- Randle RW, Swett KR, Swords DS, et al. Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 2014;21:1474-9. [PubMed]
- Bartlett DL, Buell JF, Libutti SK, et al. A Phase I Trial of continuous hyperthermic peritoneal perfusion with tumor necrosis factor and cisplatin in the treatment of peritoneal carcinomatosis. Cancer 1998;83:1251-61. [PubMed]
- Jänne PA, Wozniak AJ, Belani CP, et al. Open-label study of pemetrexed alone or in combination with cisplatin for the treatment of patients with peritoneal mesothelioma: outcomes of an expanded access program. Clin Lung Cancer 2005;7:40-6. [PubMed]
- Carteni G, Manegold C, Garcia GM, et al. Malignant peritoneal mesothelioma-Results from the International Expanded Access Program using pemetrexed alone or in combination with a platinum agent. Lung Cancer 2009;64:211-8. [PubMed]
- Simon GR, Verschraegen CF, Jänne PA, et al. Pemetrexed plus gemcitabine as first-line chemotherapy for patients with peritoneal mesothelioma: final report of a phase II trial. J Clin Oncol 2008;26:3567-72. [PubMed]
- Foster JM, Radhakrishna U, Govindarajan V, et al. Clinical implications of novel activating EGFR mutations in malignant peritoneal mesothelioma. World J Surg Oncol 2010;8:88. [PubMed]
- Govindan R, Kratzke RA, Herndon JE 2nd, et al. Gefitinib in patients with malignant mesothelioma: a phase II study by the Cancer and Leukemia Group B. Clin Cancer Res 2005;11:2300-4. [PubMed]
- Kanteti R, Dhanasingh I, Kawada I, et al. MET and PI3K/mTOR as a potential combinatorial therapeutic target in malignant pleural mesothelioma. PLoS One 2014;9:e105919. [PubMed]
- Varghese S, Chen Z, Bartlett DL, et al. Activation of the phosphoinositide-3-kinase and mammalian target of rapamycin signaling pathways are associated with shortened survival in patients with malignant peritoneal mesothelioma. Cancer 2011;117:361-71. [PubMed]
- Dowell JE, Dunphy FR, Taub RN, et al. A multicenter phase II study of cisplatin, pemetrexed, and bevacizumab in patients with advanced malignant mesothelioma. Lung Cancer 2012;77:567-71. [PubMed]
- Kindler HL, Karrison TG, Gandara DR, et al. Multicenter, double-blind, placebo-controlled, randomized phase II trial of gemcitabine/cisplatin plus bevacizumab or placebo in patients with malignant mesothelioma. J Clin Oncol 2012;30:2509-15. [PubMed]
- Calabrò L, Morra A, Fonsatti E, et al. Tremelimumab for patients with chemotherapy-resistant advanced malignant mesothelioma: an open-label, single-arm, phase 2 trial. Lancet Oncol 2013;14:1104-11. [PubMed]


