Assessment of clinical benefit and quality of life in patients undergoing cytoreduction and Hyperthermic Intraperitoneal Chemotherapy (HIPEC) for management of peritoneal metastases
Background
Once peritoneal metastases occur from gastrointestinal cancers or mesothelioma, morbidity and mortality are almost always secondary to disease progression within the abdominal cavity. Patients experience progressive abdominal distention due to tumor growth and malignant ascites, pain, early satiety, and ultimately experience profound cachexia and inanition (1). The condition is characterized grossly by diffuse tumor nodules on the peritoneal surfaces; the omentum is a favored site for development of extensive bulky metastases which is typically referred to as “omental caking” on preoperative imaging studies. Tissue is usually obtained by laparoscopic or percutaneous biopsy and the histologic features of the tumor combined with other clinical, laboratory, or imaging findings can successfully identify the tumor site of origin. According to the multicenter EVOCAPE I study (2), the median survival in patients with peritoneal metastases was 5.2 months for those with advanced colorectal cancer (n=118) and 3.1 months for those with advanced gastric cancer (n=125). Despite significant advances in the development of more efficacious systemic chemotherapy for many GI cancers, most notably colorectal cancer; systemic treatment is associated with potentially severe toxicity in many patients and median survival is still less than two years (Table 1). Mesothelioma is very rare with 200-400 new cases diagnosed annually in US, its incidence is increasing and expected to reach a peak in 2020 in Europe (3).

Full table
Systemic chemotherapy for advanced GI cancers and mesothelioma
It is important to briefly review the efficacy and toxicity of various systemic chemotherapy regimens commonly used for patients with advanced GI cancers or mesothelioma to provide context and better understand the potential role of cytoreduction surgery (CRS) and HIPEC. Over the past 6 years there have been several new chemotherapeutic and biological agents that have been approved by the FDA for treatment of patients with advanced colorectal cancer. One common regimen is 5-fluorouracil, leucovorin, and oxaliplatin (FOLFOX) combined with bevacizumab. Saltz et al. reported results of a 2×2 factorial design random assignment trial comparing capcitebine and oxaliplatin to FOLFOX with or without bevacizumab (4). A response rate greater than 50% and a median actuarial survival longer than 20 months were reported for those treated with FOLFOX and bevacizumab; however, less than half of all patients completed the full course of planned therapy. In addition to the toxicities associated with chemotherapy, a recent study showed that the fatal adverse events (FAEs) associated with bevacizumab and chemotherapy was 2.9% (5). Compared with chemotherapy alone, the addition of bevacizumab was associated with an increased risk of FAEs, with a relative risk of 1.33. This association varied significantly with chemotherapeutic agents, such as taxanes or platinum agents, but not with tumor types or bevacizumab doses. The most common causes of FAEs were hemorrhage (23.5%), neutropenia (12.2%), and gastrointestinal tract perforation (7.1%).
In patients with malignant peritoneal mesothelioma the use of pemetrexed and cisplatin has been widely used; however, the overall response rate is approximately 20% and the duration of response is less than 12 months (6). In a Phase III clinical study of chemotherapy in malignant peritoneal mesothelioma patients, pemetrexed and cisplatin resulted in grade 3 or 4 neutropenia in 27.9% and grade 3 or 4 leukopenia in 17.7%) (7). The incidence of grade 3/4 neutropenia was significantly higher among none or partial vitamin supplementation patients (41.4%) compared with full supplementation patients. Fourteen patients who received pemetrexed/cisplatin died while on study therapy or within 30 days of the last dose of study drug, compared with eight patients who received cisplatin alone (6.2% vs. 3.6%). The incidence of nausea, vomiting, fatigue, diarrhea, dehydration and stomatitis were significantly higher in the pemetrexed and cisplatin group.
Taken together, these data show that systemic chemotherapy and biological therapy regimens commonly accepted as standard of care for patients with advanced GI cancers and MPM have considerable toxicity and mortality. Toxicities can be cumulative as in the case of oxaliplatin and severe as noted with bevacizumab. Patients typically receive protracted courses of therapy in order to enjoy continued clinical benefit and not systemic regimens have been shown to be curative in the setting of metastatic disease.
CRS and HIPEC
In the past, the role of operation in the management of patients with cancer has been mainly to cure localized cancers, to provide staging information, and for palliation in patients with pain, bleeding or obstruction (Table 2). Pseudomyxoma peritonei, malignant mesothelioma and peritoneal carcinomatosis from gastrointestinal cancers have been considered incurable conditions for which the role of surgical intervention was limited (1). However, over the last two decades, multi-modality treatments have evolved for patients with peritoneal carcinomatosis and the utilization of cytoreduction combined with hyperthermic intraperitoneal chemotherapy (HIPEC) has been increasingly used with therapeutic intent (Figure 1). Most data regarding its efficacy and morbidity have been derived from single center reviews or more recently from combined institutional databases (8-11).
Full table
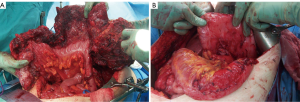
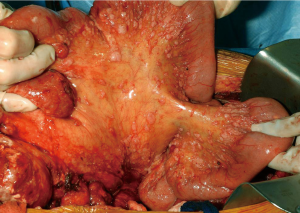
CRS requires a combination of standard surgical maneuvers designed to completely resect or ablate all gross disease in the peritoneum (Table 3). The various operative procedures may include parietal and visceral peritonectomy, greater omentectomy, splenectomy, cholecystectomy, ablation of tumor deposits on the liver capsule, small bowel resection, colonic and rectal resection, partial gastrectomy, lesser omentectomy, hysterectomy, ovariectomy, and urinary bladder or ureteral resection (Figure 2). The aim of CRS is to obtain a complete macroscopic cytoreduction; after resection a score estimating the completeness of cytoreduction or CCR is used and defined as: CCR-0, no residual peritoneal tumor nodules; CCR-1, residual tumor nodules <2.5 mm; CCR-2, residual tumor nodules between 2.5 mm and 2.5 cm; or CCR-3 residual tumor nodules >2.5 cm or a confluence of unresectable tumor nodules at any site (12).
Full table
For the performance of HIPEC a circuit pump consisting of a reservoir heat exchanger and roller pump is necessary to circulate the perfusate via several drains place into the peritoneal cavity. The intraperitoneal temperature should reach 41-42 °C by using leading to an inflow temperature of about 43. HIPEC can be performed in open or closed abdomen technique. One of the leading advantages of the open technique is a better control of the intraperitoneal circulation and uniform distribution of the cytostatic agents (Table 3). However, the disadvantage is the increased risk of exposure to health care personnel when compared with the closed abdomen technique. Mitomycin-c is the most common agent used during HIPEC in the treatment of peritoneal carcinomatosis patients from appendiceal and colorectal cancers. It is an antitumor antibiotic, with approximately 90% of the drug absorbed within the 90 minutes intraperitoneal irrigation. Cisplatin is an alkylating agent used in treating gastric cancer, ovarian cancer and diffuse malignant peritoneal mesothelioma (13).
In selected patients with minimal disease burden or in those undergoing staging laparoscopy, laparoscopic HIPEC can be performed. The benefit of such approach is based on avoiding inherent complications related to major abdominal incision. Laparoscopy is usually associated with less postoperative pain, hospital stay and an earlier capacity to return to work.
Mortality and mrbidity of CRS and HIPEC
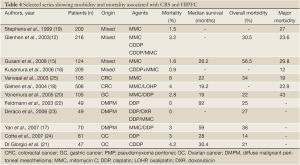
The morbidity and mortality after CRS and HIPEC range from 12% to 41% and from 0% to 8%, respectively (12-24) (Table 4, Figure 3). Morbidity can be divided into surgery-related and chemotherapy-related events. Common surgical complications are bleeding, postoperative bowel obstruction, anastomotic leakage, wound infection, pulmonary embolism and venous thrombosis. Morbidity related with cytostatic agents used in HIPEC is rare but includes leucopenia, anemia, thrombocytopenia, and liver or renal toxicity.
Full table
Numerous reports over the past 20 years indicate that mortality and morbidity following cytoreduction with HIPEC is decreasing because of improvements of surgical technique and patient selection criteria (Table 5). Two recent publications addressing mortality and morbidity provide informative data. Glehen and et al. conducted a retrospective multicenter cohort study in French-speaking institutions to evaluate toxicity and principal prognostic factors after cytoreduction surgery and HIPEC (14). One thousand two hundred ninety patients from 25 institutions who underwent 1,344 procedures between 1989 and 2007 were included; HIPEC was performed in 1,154 procedures. Colorectal adenocarcinoma (40.5%), pseudomyxoma peritonei (23.3%), gastric adenocarcinoma (12.3%), peritoneal mesothelioma (6.8%), and appendiceal adenocarcinoma (3.9%) were the principal origins of carcinomatosis. The overall morbidity and mortality rates were 33.6% and 4.1%, respectively. The median survival was 30 months for patients with colorectal cancer, 9 months for patients with gastric cancer, 41 months for patients with peritoneal mesothelioma, and 77 months for patients with cancer from appendiceal adenocarcinoma.
Full table
Gusani et al. reported low mortality in patients treated at a single institution (15). A total of 122 patients underwent 124 cytoreduction and HIPEC procedures. R-0 resection was achieved in 28.7% of cases, R-1 in 54.9%, and R-2 in 16.4%. Median operative time was 460 minutes (range, 250-840 minutes) and median blood loss was 1,150 mL (range, 10-14,000 mL). Grade 3 or 4 morbidity was seen in 29.8% of cases, with overall morbidity 56.5%. Two-year survival was 66.7% for appendiceal cancer patients; however, 5-year survival was 36.7%. The number of anastomoses and extent of cytoreduction were independent prognostic variables for major morbidity. In-hospital and 30-day mortality rates were 0% and 1.6%, respectively.
Survival following CRS and HIPEC
In 2003, Verwaal et al. reported results of a small prospective randomized controlled trial from the Netherlands Cancer Institute including 105 colorectal cancer patients who had peritoneal metastases or positive cytology from ascites who were treated with CRS and HIPEC versus intravenous 5-fluorouracil (Table 4). This trial was updated with a minimal follow-up of 6 years in 2008 (25,26). The study showed a disease-specific survival of 22.2 months after CRS and HIPEC that was significantly better than the survival of 12.6 months after standard systemic chemotherapy. Elias et al. reported a cohort controlled study that compared outcomes of 48 patients treated with systemic chemotherapy to 48 who underwent CRS and HIPEC for peritoneal metastases for colorectal cancer (27). Most clinical and pathological variables were well matched; the median actuarial overall survivals were 23.9 months in the chemotherapy treated group and 62.7 months in the CRS and HIPEC group. The differences were statistically significant; the outcome of the CRS and HIPEC treated group was better than most other reports of this treatment approach in this patient population.
The largest single study providing outcomes in patients following CRS and HIPEC in patients with colorectal cancer was reported by Glehen and et al. That study included 506 patients treated at 28 institutions operated between 1987 and 2002 (18). The morbidity and mortality rates were 22.9% and 4%, respectively. The overall median survival was 19.2 months compared to the 62.7 months median survival reported by Elias et al. Patients in whom CRS was complete had a median survival of 32.4 months compared with 8.4 months for patients in whom complete CRS was not possible.
Survival is only marginally improved by CRS and HIPEC in selected patients with peritoneal metastases from gastric cancer and is approximately 9 months (18,20). Patients with ovarian cancer who have undergone CRS and HIPEC had median survival rates ranging from 28 to 46 months and 5-year survival rates from 15% to 50% (21,24). For patients with diffuse malignant peritoneal mesothelioma (DMPM), a rare disease with relatively low incidence, median overall survivals between 34 and 92 months and 5-year survival rates from 33% to 59%, respectively, have been reported (17,23). Since patients with peritoneal metastases have a poor prognosis and limited longevity, measuring quality of life (QoL) endpoints for patients after CRS and HIPEC is very important.
Health related quality of life after CRS and HIPEC
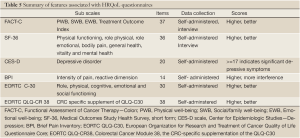
The concept of health-related quality of life (HRQOL) and its determinants have evolved since the 1980s to encompass those aspects of overall quality of life that can be clearly shown to affect health—either physical or mental (28-30). HRQoL is a broad multi-dimensional concept that usually includes self-reported measures of physical and mental health. Several measures have been used to assess HRQoL and related concepts of functional status. However, there is no substitute for a standard QOL questionnaire properly administered.
The HRQoL construct is measurable in that its dimensions can be assessed reliably over time and have been shown to be valid with reference to other validated instruments (Table 5). There are at least four areas can be measured in quality of life assessment: physical well-being, social/family well-being, functional well-being and emotional well-being. There are other important factors in patient’s life that may modify how they experience their overall QOL, such as spirituality, financial and support resources, psychological resilience and sexuality. FACT-C and SF-36 are the mostly used questionnaires to estimate QoL in patients after CRS and HIPEC. The FACT-C scale (Functional Assessment of Cancer Therapy—Colon) is a self-reported questionnaire, consisting of the FACT-G general version with 28 items plus 9 items for the colon subscale (31). It consists of subscales measuring physical well-being (PWB), functional well-being (FWB), social/family well-being (SWB), emotional well-being (EWB), and a Treatment Outcome Index (TOI). The TOI is calculated by adding PWB, FWB, and the colon cancer subscale. The FACT can be either self-administered or used in an interview format and is easily completed in 5 to 10 minutes. A higher score indicates a better QoL. The SF-36 (Medical Outcomes Study Health Survey, short form) is a 36-item generic health measure. It assesses the physical functioning, role physical, role emotional, bodily pain, general health, vitality and mental health (32). Scores range from 0 to 100. A higher score indicates better functioning. While these measures have been widely used and extensively validated in clinical settings and special population studies, their length often makes them impractical to use in population surveillance.
In addition to the FACT-C and SF-36, there are other instruments to evaluate patient’s QoL. The CES-D scale (Center for Epidemiologic Studies—Depression) is a 20-item self-report measure having a high sensitivity and positive predictive value for detecting depressive disorders (33). A score of >=17 indicates that the patients has significant depressive symptoms and would be categorized as a possible case of depression. Brief Pain Inventory (BPI) is a 14-item, self-report questionnaire used to provide information on the intensity of pain (the sensory dimension) as well as the degree to which pain interferes with function (the reactive dimension) (34). The Eastern Cooperative Oncology Group (ECOG) performance status are used by doctors and researchers to assess how a patient’s disease is progressing, assess how the disease affects the daily living abilities of the patient, and determine appropriate treatment and prognosis (35). The score runs from 0 to 5, with 0 denoting perfect health and 5 denoting death. European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 (EORTC QLQ-C30) is a 30-item self-reporting questionnaire developed to assess the quality of life of cancer patients. It is grouped into five functional subscales (role, physical, cognitive, emotional and social functioning). The Colorectal Cancer Module 38 (EORTC QLQ-CR38) is the CRC-specific supplementation of the QLQ-C30. Its 38 items cover symptoms and side effects from different treatment modalities, body image, sexuality, and future perspective. It was tested in 117 Dutch colorectal patients and was found to yield good reliability and validity (36).
The amount of data published relating to the HRQoL in patients after CRS and HIPEC are very limited (37-45) (Table 6). Ideally, data should be derived from a prospectively designed study in which patients receive a pre-surgery assessment of QoL as the baseline. Postoperative assessments are then conducted at various time points ranges and compared with the baseline score. With each patient serving as their own control relatively small studies can be used to identify statistically significant differences in HRQoL over time. The research group at Wake Forest University has published results of several studies relating to the QoL in patients after CRS and HIPEC. Their initial study was in 64 patients treated by CRS and HIPEC in 2001 (37). The authors used FACT-C to assess QoL and they found significant decrease of physical, emotional and functional, and well-being scores with an increase relative to baseline levels during follow-up at 3,6 and 12 months. Most patients returned to baseline or better levels of functioning within 3 months post-treatment. Seventy-four percent of patients resumed greater than 50% of their normal activities one year after surgery. Depressive symptoms were observed at base line and different time points. The patterns were similar to those of patients following bone marrow transplantation (38).

Full table
The same research group subsequently published the largest HRQoL study in patients treated by CRS and HIPEC from 1998 to 2005 which included 96 patients (39). Patients completed a questionnaire before and after surgery at 3, 6 and 12 months. Similar assessment instruments were used (FACT-C, SF-36, CES-D, BPI, ECOG). Quality of life and pain scores improved from baseline to 12 months. Physical functioning changed over the 12-month study period with improvement recorded at 6 months. Depressive symptoms were common as 25% of patients had symptoms. The authors concluded that acceptable QoL, return of functional status, and reduced pain can be attained between 3 and 6 months following treatment although some deficits in general health remains. The highest scores on general health subscale are lower than the scores in general population.
McQuellon et al. also studied long term survivor ship in 17 patients (40). They were interviewed from 3.1 to 8.0 years after treatment. Sixty-two percent described their health as excellent or very good. No limitations on moderate activity were reported in 94% of cases. Functional well-being, physical well-being and FACT total were significantly improved and demonstrated that long-term survivors of peritoneal carcinomas after CRS and HIPEC can return to a good life of quality.
Appendiceal cancer is also research interest for investigators. QoL for patients with disseminated peritoneal cancer of appendiceal cancer were studied by McQuellon et al. Fifty-eight patients with a mean age 52.4 years were assessed before surgery. Overall survival at 1 year was 78.7%. Emotional well-being improved over the study period, while physical well-being and physical functioning declined at 3 months and then improved to near baseline levels at 6 and 12 months. Depressive symptoms and some physical limitations remain in surviving patients. Percentage of patients with depressive symptoms ranges from 24% to 33% in baseline, 3, 6, and 12 months (41). The authors conclude survival in appendix cancer patients with peritoneal cancer is good, although complications may affect short-form recovery. However, half of patients dropped out of the study.
In Hill et al.’s recently published paper a total 62 patients who underwent HIPEC for peritoneal carcinomatosis of colonic origin were studied (42). Questionnaires were completed preoperatively and after surgery at 3, 6, and 12 months. The authors used FACT-C, Brief Pain Inventory (BPI), SF-36, CES-D, and the ECOG Performance Status Rating to estimate their QoL. Median overall survival was 18 months, with 71.3% survival at 1 year. Emotional well-being scores significantly improved after HIPEC. Social/family wellbeing and the colon subscale of the FACT worsened at 3 months, but recovered at 6 months. CES-D scores showed 33%-50% of patients reported depressive symptom. Pain scores increased above base line at 3 months, but decreased below base line at 6 and 12 months. 47% of patients reported normal activity according to their performance status.
Long-term functioning in patients following CRS and HIPEC has also been studied by Schmidt et al. who evaluated QoL in 67 patients using the EORTC QLQ-C30 questionnaire with an average post-treatment time of 4 years (range 1-8 years) (43). The mean score for global health status of long-term survivors was 62.6, which was significantly decreased when compared with the general Norwegian population (73.3). The authors showed functional status, particularly the role and the social functioning, were impaired because of presence of ostomies, fatigue, insomnia, or pain. These data indicated that QoL may be adversely affected following CRS and HIPEC.
Per Jess et al. reported a clinical study of 23 patients underwent CRS and early postoperative intraperitoneal chemotherapy for pseudomyxoma peritonei (44). Patients were followed in clinic 3, 6, 12, 18, and 24 months after surgery. Short Form-36, together with the two symptom-specific instruments-- EORTC-C30, and EORTC-CR38 were used to assess the quality of life. Seventy percent of patients had one or more complications during or after surgery, but all had recovered; 14% had an asymptomatic recurrence detected within two years. No significant decrease was observed in the scores on the Short Form-36 Questionnaire scales of physical dimension and role physical three months after surgery, only returning to normal after six months. No measurable decrease in QoL was found after 12 and 18 months.
Tuttle et al. studied 35 consecutive patients with peritoneal metastases enrolled in a prospective trial from 2001 to 2005. Before treatment and then at 4-month postoperative intervals, the authors used the FACT-C, FACT-G and TOI instrument to assess the patients quality of life (45). Quality of life measurements returned to baseline 4 months after treatment and were significantly improved at 8 and 12 months. Functional well being scores and emotional well being scores improved significantly at 8 and 12 months when compared to baseline. Patients treated by MMC dose >30 mg were significantly more likely to have an adverse event compared to low dose MMC treated patients. In their study, many patients were still receiving systemic chemotherapy 4 months after CRS and HIPEC which decreased their quality of life scores. The authors found the QoL of patients after CRS and HIPEC at 12 months is similar to the QoL of colorectal cancer patients who underwent curative resection of primary tumors.
Summary
Peritoneal metastases from cancer are a common and unfortunate pattern of recurrent metastatic disease for many cancers arising from the gastrointestinal tract or the peritoneal lining. Despite advance in chemotherapy survival is limited; many patients suffer from a marked morbidity from tumor progression in the abdominal cavity. CRS and HIPEC provide a promising and potentially therapeutic option for selected patients with peritoneal surface metastases. Short term mortality and morbidity have been reduced in recent years because of better patient selection and improvements in operative technique and post-operative management. Because CRS and HIPEC have associated morbidity it is important to assess the success of treatment in terms of both quality and longevity of life. In most clinical studies, patient HRQoL status returns to baseline and is generally improve for up to a year after treatment.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Glockzin G, Ghali N, Lang SA, et al. Peritoneal carcinomatosis. Surgical treatment, including hyperthermal intraperitoneal chemotherapy. Chirurg 2007;78:1100, 1102-6, 1108-10.
- Rusch VW. A proposed new international TNM staging system for malignant pleural mesothelioma. From the International Mesothelioma Interest Group. Chest 1995; 108:1122-8.
- Neumann V, Muller KM, Fischer M. Peritoneal mesothelioma--incidence and etiology. Pathologe 1999;20:169-76.
- Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumb in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9.
- Ranpura V, Hapani S, Wu S. Treatment-related mortality with bevacizumab in cancer patients: a meta-analysis. JAMA 2011;305:487-94.
- Obasaju CK, Ye Z, Wozniak AJ, et al. Single-arm, open label study of pemetrexed plus cisplatin in chemotherapy naive patients with malignant pleural mesothelioma: outcomes of an expanded access program. Lung Cancer 2007;55:187-94.
- Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol 2003;21:2636-44.
- Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol 1989;16:83-97.
- Loggie BW, Fleming RA, McQuellon RP, et al. Cytoreduction surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of gastrointestinal origin. Am Surg 2000;66:561-8.
- Stewart JH 4th, Shen P, Levine EA. Intraperitoneal hyperthermic chemotherapy for peritoneal surface malignancy: current status and future directions. Ann Surg Oncol 2005;12:765-77.
- Sugarbaker PH. Cytoreduction surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 2001;27:239-43.
- Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreduction surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive procedures. Ann Surg Oncol 2003;10:863-9.
- Yan TD, Cao CQ, Munkholm-Larsen S. A pharmacological review on intraperitoneal chemotherapy for peritoneal malignancy. World J Gastrointest Oncol 2010;2:109-16.
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreduction surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18.
- Gusani NJ, Cho SW, Colovos C, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol 2008;15:754-63.
- Kusamura S, Younan R, Baratti D, et al. Cytoreduction surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006;106:1144-53.
- Yan TD, Welch L, Black D, et al. A systematic review on the efficacy of cytoreduction surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Oncol 2007;18:827-34.
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreduction surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92.
- Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality analysis of 200 treatments with cytoreduction surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the coliseum technique. Ann Surg Oncol 1999;6:790-6.
- Yonemura Y, Kawamura T, Bandou E, et al. Treatment of peritoneal dissemination from gastric cancer by peritonectomy and chemohyperthermic peritoneal perfusion. Br J Surg 2005;92:370-5.
- Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreduction surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315-25.
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7.
- Deraco M, Nonaka D, Baratti D, et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreduction surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 2006;13:229-37.
- Cotte E, Glehen O, Mohamed F, et al. Cytoreduction surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg 2007;31:1813-20.
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43.
- Verwaal VJ, Bruin S, Boot H, et al. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 2008;15:2426-32.
- Mahteme H, Hansson J, Berglund A, et al. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer 2004;90:403-7.
- Centers for Disease Control: “Measuring healthy days: Population assessment of health related quality of life.” 2000.
- Gandek B, Sinclair SJ, Kosinski M, et al. Psychometric evaluation of the SF-36 health survey in Medicare managed care. Health Care Financ Rev 2004;25:5-25.
- McHorney CA. Health status assessment methods for adults: past accomplishments and future challenges. Annu Rev Public Health 1999;20:309-35.
- Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 1993;11:570-9.
- Ware JE, Snow KK, Kosinski M, et al. “SF-36 Health Survey Manual and Interpretation Guide.” Boston: New England Medical Center, 1997.
- Radloff LS. The CES-D Scale. Applied Psychological Measurement 1977;1:385-401.
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore 1994;23:129-38.
- Zubrod CG, Schneiderman M, Frei E, et al. Appraisal of methods for the study of cancer in man: Comparative therapeutic trial of nitrogen mustard and triethylene thiophoramide. J Chron Dis 1960;11:7-33.
- Sprangers MA, te VA, Aaronson NK. The construction and testing of the EORTC colorectal cancer-specific quality of life questionnaire module (QLQ-CR38). European Organization for Research and Treatment of Cancer Study Group on Quality of Life. Eur J Cancer 1999;35:238-47.
- McQuellon RP, Loggie BW, Fleming RA, et al. Quality of life after intraperitoneal hyperthermic chemotherapy (IPHC) for peritoneal carcinomatosis. Eur J Surg Oncol 2001;27:65-73.
- McQuellon RP, Russell GB, Rambo TD. Quality of life and psychological distress of bone marrow transplant recipients: The time trajectory to recovery over the first year. Bone Marrow Transplant 1998;21:477-86.
- McQuellon RP, Danhauer SC, Russell GB, et al. Monitoring health outcomes following cytoreduction surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol 2007;14:1105-13.
- McQuellon RP, Loggie BW, Lehman AB, et al. Long-term survivorship and quality of life after cytoreduction surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. Ann Surg Oncol 2003;10:155-62.
- McQuellon RP, Russell GB, Shen P, et al. Survival and health outcomes after cytoreduction surgery with intraperitoneal hyperthermic chemotherapy for disseminated peritoneal cancer of appendiceal origin. Ann Surg Oncol 2008;15:125-33.
- Hill AR, McQuellon RP, Russell GB, et al. Survival and Quality of Life Following Cytoreduction Surgery Plus Hyperthermic Intraperitoneal Chemotherapy for Peritoneal Carcinomatosis of Colonic Origin. Ann Surg Oncol 2011;18:3673-9.
- Schmidt U, Dahlke MH, Klempnauer J, et al. Perioperative morbidity and quality of life in long-term survivors following cytoreduction surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol 2005;31:53-8.
- Jess P, Iversen LH, Nielsen MB, et al. Quality of life after cytoreduction surgery plus early intraperitoneal postoperative chemotherapy for pseudomyxoma peritonei: a prospective study. Dis Colon Rectum 2008;51:868-74.
- Tuttle TM, Zhang Y, Greeno E, et al. Toxicity and quality of life after cytoreduction surgery plus hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 2006;13:1627-32.


