Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy
Introduction
Historically, cancers that spread within the peritoneal cavity were deemed fatal. Systemic chemotherapy has little, if any, effect on improving survival when malignancies spread to the peritoneum. This is due to the poor blood supply to the peritoneal surface with low penetration into tumor nodules thereby preventing eradication or substantial hindrance to tumor growth. Surgical debulking can palliate symptoms; however, there is inevitable gross or microscopic disease left behind, and little survival benefit. Surgery also disrupts the tumor mass and disseminates cancer cells throughout the peritoneal cavity. Moreover, post-operative adhesion formation is an ideal place for cancer cells to hide and proliferate in a rich protein environment.
The rational for administration of intraperitoneal chemotherapy is to have direct contact of cytotoxic drugs to the tumors themselves, without reliance on blood supply to the area. The use of maximal removal of the gross disease leaving only microscopic cancer cells followed by immediate intraperitoneal chemotherapy with or without hyperthermia, has been shown by multiple phase II trials over the past two decades to prolong survival compared to historical data (1-14).
In 1955, Weissberger first introduced the concept of intraperitoneal chemotherapy to treat peritoneal tumors as a local disease. In 1978, Dedrick studied the depth of tissue penetration by different drugs and identified a group of cytotoxic drugs that can penetrate 1-3 mm into tissue. This gave rise to the notion that tumor deposits need to be 2.5 mm or less for intraperitoneal chemotherapy to have some effect (15,16). For this reason, any intraperitoneal chemotherapy requires a good cytoreduction prior to its use. Sugarbaker, in the 1990s, mirrored these concepts to develop a treatment option of surgical peritonectomy and organ removal followed by intraperitoneal chemotherapy (17).
Malignancies most likely to spread to the peritoneum include appendix [including pseudomyxoma peritonei (PMP)], colon, gastric, ovarian, and peritoneal mesothelioma.
This review details chemotherapeutic agent selection and modality of treatment for peritoneal-based malignancies.
Chemotherapy and surface properties for intraperitoneal administration
Intraperitoneal administration of chemotherapy is designed to maximize the chemotherapeutic dose delivered to peritoneal tumor nodules while minimizing systemic toxicity. To accomplish this, the cytotoxic drugs physical properties should include large, high molecule weight, hydrophilic, and ionized compounds. These properties take advantage of the plasma-peritoneal barrier to allow for higher concentrations of cytotoxic agents to be administered intraperitoneally when compared to systemic administration (18,19). These drugs then enter the tumor nodules by passive diffusion. For the small amount of cytotoxic drug that does get into the systemic circulation, its bioavailability is short lived due to first-pass hepatic metabolism or renal excretion.
Drug concentrations in the peritoneal cavity and systemically can be measured by the area under the curve (AUC). AUC is a measure of drug exposure, calculated by taking the integral of plasma concentration versus time, ∫([drug, plasma] × Dt). An AUC ratio of intraperitoneal concentration to plasma concentration time reflects how much of the drug is preserved in the peritoneal cavity and how much was absorbed into the systemic circulation (20). A large peritoneal to plasma ratio is important to maintain high concentrations in the abdomen with few systemic toxicities.
The characteristics of commonly used intraperitoneal agents are highlighted in Table 1 (15,21).
The timing of the intraperitoneal chemotherapy is important. Administration immediately after cytoreduction allows the entire abdominal cavity to be bathed with the perfusate. This prevents compartmentalization (and inadequate exposure of the entire peritoneal cavity) due to post-operative adhesion formation. The two timeframes for administration of intraperitoneal chemotherapy are intra-operative via hyperthermic intraperitoneal chemotherapy (HIPEC) and early post-operative intraperitoneal chemotherapy (EPIC).
HIPEC and EPIC
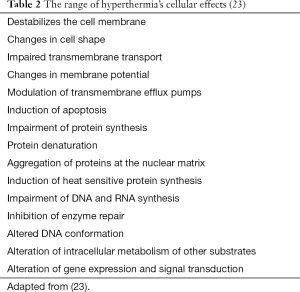
Spratt et al. in 1980, using a canine model, described the use of HIPEC to eradicate peritoneal-based cancers (22). HIPEC is the administration of chemotherapy at optimal temperatures between 42-43 °C. Synergy between heat and drug cytotoxicity starts at 39 °C and falls off at 43 °C. Temperatures above 44 °C cause apoptosis in normal cells. Table 2 shows the effects of hyperthermia on cells (23). For HIPEC to be useful, the cytotoxic drugs need to act synergistically with hyperthermia. The majority of chemotherapy drugs used for HIPEC is cell cycle non-specific and act synergistically with hyperthermia.
In the early 1990s, Sugarbaker introduced the model of cytoreductive surgery with systematic peritonectomies with or without organ removal followed by HIPEC (24). This revolutionary approach to remove all gross disease and kill the remaining microscopic disease was the beginning of regional therapies for peritoneal based tumors.
While HIPEC is performed immediately after the cytoreduction in the operating room for 60-120 minutes, EPIC is administered post-operative day #1 with continued daily therapy for 5-7 days. During EPIC, the chemotherapy solution dwells for 23 hours and then is drained for 1 hour prior to re-administration. The cytotoxic drugs selected are usually cell cycle specific which requires for longer periods of cell contact with the chemotherapy to get cell death (25).
Tumors treated with intraperitoneal chemotherapy
Appendix tumors and pseudomyxoma peritonei (PMP)
PMP is a clinical syndrome of gelatinous mucinous ascites with a characteristic pattern of peritoneal spread. The mucin produced by these tumors is a glycoprotein encoded for by the MUC family of genes. Histologically these tumors were originally classified by Ronnett and Misdraji with the nomenclature changing over the years (26,27). The most recent classifications for these tumors include low-grade mucinous appendiceal neoplasms for acellular mucin production or low-grade mucinous adenocarcinoma. The spectrum of PMP in the literature has also changed over the past years to include high-grade mucinous adenocarcinomas as well as adenocarcinoid tumors. All of these tumors act similarly in regards to distribution within the peritoneal cavity with varying incidences of invasion and hematological spread for the more aggressive histologies.
Mitomycin C (MMC)
The current standard for patients with PMP is cytoreductive surgery with HIPEC. MMC is the most widely used and studied drug for HIPEC. This is due to several characteristics of MMC that make it ideal for HIPEC application: a satisfactory AUC ratio of intraperitoneal concentrations and plasma concentration times time; large-sized molecule that is not rapidly absorbed systemically; stability at high temperatures and synergistic effect with heat; and compatibility with other drugs to allow combination therapy. It is the drug of choice for appendiceal, colorectal, and gastric (in combination with other drugs) malignancies (25,28,29).
MMC is an antitumor antibiotic isolated from the broth of a Streptomyces species. It is commonly used intravesical for bladder tumors as well as to prevent scaring during glaucoma surgery. It has a favorable toxicity profile with very little systemic absorption and is rapidly cleared by the kidneys. Toxicity effects are usually additive so multiple administrations does increase the risk of renal injury and pulmonary fibrosis. Hemolytic uremic syndrome has been seen with systemic use, however, it has not been reported in the HIPEC literature (30). HIPEC with MMC causes neutropenia in 40% of patients but the majority are minor (31). More worrisome is the effect on wound healing which can cause bowel perforation and anastomotic dehiscence (32,33). For patients that undergo a low anterior resection during cytoreduction, the leak rate is 25-30%. It is recommended that a diverting loop ileostomy be performed.
The typical dosing of MMC varies within the literature from 15 mg/m2 in 1.5 L/m2 of perfusate to a single dose of 35 mg/m2 (10,29,34). The most common regimen consists of 40 mg MMC at 42 °C for 90-120 minutes (13). It is usually administered in two separate doses: 30 mg given for the first hour once temperatures of the perfusate reach 42 °C with a second dose of 10 mg added at 60 minutes. When EPIC is planned, it has been suggested to decrease the dose (however, there is no data to suggest an optimal dose reduction).
5-fluorouracil (5-FU)
5-FU alone or in combination is used systemically for the majority of gastrointestinal tumors. It is the most commonly used agent for EPIC in appendiceal and other gastrointestinal tumors. 5-FU is an antimetabolite type of chemotherapy. This class of cytotoxic drugs is cell cycle specific and appears very similar to normal substances within the cell. It is a purine antagonist and interferes with cellular metabolism to prevent cell division.
5-FU is administered one day after cytoreductive surgery with HIPEC through operatively-placed drains in the abdominal cavity. A dose of 650 mg/m2 of 5-FU is infused in a hypertonic, high molecular weight solution to decrease the clearance from the abdominal cavity (35). The dwell time is 23 hours and then 1 hour for drainage. This is repeated every day for 5 days (36). Systemic toxicity is low even with much higher doses than typical systemic infusion because of single-pass metabolism through the liver. Patients with liver dysfunction need to have dose adjustments.
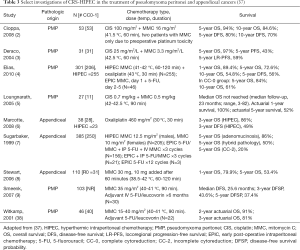
Other drugs that can be used intraperitoneally for appendiceal tumors include doxorubicin, cisplatin (CIS), oxaliplatin, and carboplatin. Table 3 shows treatment regimen and survival for HIPEC with and without EPIC (2-9,37,38).
Full table
Colorectal tumors
The American Cancer Society estimates there will be 93,000 new cases of colon cancer and 39,610 new cases of rectal cancers in 2015. It will be the third most common cancer diagnosed in the United States. It is expected to cause about 49,700 deaths during 2015 making it the second most common cause of cancer deaths. Approximately 10% of patients have peritoneal spread at the time diagnosis despite advances in early detection and is the second leading cause of death in patients with colorectal cancer (39).
The mainstay of treatment has been systemic chemotherapy for patients with metastatic colorectal cancer. Great progress has been made with the addition of different regimens including FOLFOX, FOLFIRI and biological targeted therapies such as bevacizumab and cetuximab. With these advances, the median overall survival (OS) for patients with colorectal cancer with peritoneal dissemination increased from 6 to 24 months (40-45). When HIPEC is employed, MMC is the most common intraperitoneal chemotherapy given for colorectal cancer; however, oxaliplatin use has been advocated in large part from the European literature.
Oxaliplatin
Oxaliplatin is an alkylating agent, specifically a metal salt that is cell-cycle non-specific. It binds to DNA crosslinking which prevents DNA replication. It is most commonly used systemically in combination with 5-FU and leucovorin (FOLFOX regimen) for gastrointestinal malignancies. It has a low AUC ratio so has a higher chance of being absorbed systemically in a short period of time.
Elias et al. were the first to report on its use during HIPEC, using a dose of 460 mg/m2 of oxaliplatin with a dwell time of 30 minutes (46). This is a very high does which if given systemically would be extremely toxic. They also administered 400 mg/m2 5-FU intravenously just prior to starting the HIPEC. This bidirectional manner gave a higher cytotoxic effect to the cancer cells by passive diffusion of the oxaliplatin and the vast capillary network allowing the systemic 5-FU to enter the tumor nodules. The group at Wake Forest reported on a phase I trial using 200 mg/m2 of oxaliplatin as the maximum tolerated dose for a 2-hour perfusion (47).
EPIC has also be used with 5-FU in a similar fashion described previously.
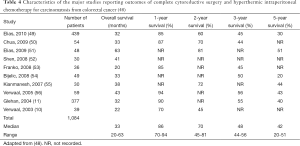
Outcomes with HIPEC for colorectal cancer are very promising, although there is only one phase III study in the current literature, and it is therefore not yet a mainstay of treatment for carcinomatosis from colorectal cancers (Table 4) (48).
Full table
Malignant peritoneal mesothelioma (MPM)
MPM is a rare disease usually caused from asbestos exposure. It is an aggressive loco-regional disease with the coalescence of small tumor nodules forming large plaques that constrict abdominal organs. Patients develop severe abdominal pain and bowel obstructions. In the past, MPM was treated with a combination of systemic chemotherapy, palliative surgery, and in some cases total abdominal radiation. Rarely patients responded to these treatments and median survival was 12 months (17,57-59).
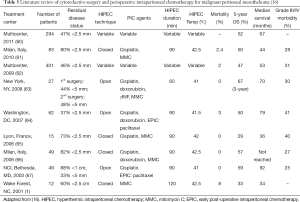
Cytoreductive surgery with HIPEC +/- EPIC has been used extensively for MPM. The median survival has been 34-92 months which is greatly improved over previous treatments (Table 5) (17).
Full table
The most common drugs used for MPM during HIPEC are MMC, doxorubicin, and CIS. For EPIC, paclitaxel is most commonly used.
Doxorubicin
Doxorubicin is an antitumor antibiotic which has a good profile for intraperitoneal use. It is a large molecule with high AUC ratio of intraperitoneal to plasma. Doxorubicin is stable and synergist with hyperthermia. It is metabolized as a single pass through liver to decrease systemic effects. Doxorubicin is compatible with multiple different drugs including CIS and MMC. The mechanism of antineoplastic activity includes DNA intercalation, inhibition of topoisomerase II, and formation of oxygen free radicals. Use of a pegylated liposomal modification of doxorubicin for IP treatment has also been studied. Compared to unaltered doxorubicin, pegylated liposomal doxorubicin has a longer half-life and reduced cardiotoxicity. The drug is released at a lower rate and is able to achieve higher concentrations within the tumor. Additionally, the process of pegylation of liposomes decreases immune identification and the ultimate destruction of the agent by the mononuclear phagocyte system (68,69).
When used intraperitoneal at high doses, doxorubicin causes a severe inflammatory reaction to the peritoneum. Patients develop severe pain and eventually peritoneal fibrosis with bowel obstructions. A dose escalation study with pharmacokinetic monitoring of intraperitoneal doxorubicin demonstrated that a total dose of 15 mg/m2 resulted in a thin layering of fibrosis which was not extensive enough to cause abdominal pain or intestinal obstruction (70). Another indication to use doxorubicin with HIPEC is for its sclerosing effect in malignant ascites. It can be very effective when used for this indication.
Most commonly doxorubicin is combined with CIS for the additive effect of both drugs.
Cisplatin (CIS)
CIS, like oxaliplatin is an alkylating agent specifically a metal salt. It is used intraperitoneally for ovarian cancer, gastric cancer, and MPM. It has a molecular weight of 300 but a low AUC ratio intraperitoneal to plasma. It works synergistically with hyperthermia as well as other multiple drugs including MMC and doxorubicin. At high doses (systemic or intraperitoneal) renal and ototoxicity can occur due to the low intraperitoneal to plasma AUC ratio. Care must be given when administering CIS to protect the kidneys of the heavy metal binding in the renal tubules by using a metal binding agent specifically sodium thiosulfate and amifostine.
Most common regimen to treat MPM with HIPEC is doxorubicin 15 mg/m2 and CIS 50 mg/m2 at a temperature above 41.5 °C for 90 minutes (67).
Paclitaxel
Paclitaxel is a plant alkaloid specifically a taxane. Taxanes come from the bark of the Pacific yew tree. It is cell-cycle specific by preventing mitoses. It stabilizes microtubules so they are unable to depolymerize for free tubulin. It is a large molecule through with a very high AUC ratio of intraperitoneal to plasma.
Paclitaxel’s most frequent uses are during EPIC for gastric cancer, diffuse peritoneal mesothelioma or ovarian cancer or in treatment of malignant ascites. Typical doses range from 60 mg/m2 to as high as 175 mg/m2. It has also been given during HIPEC in combination with CIS for ovarian cancer at similar doses. A 6% hetastarch carrier solution can be used to diminish the clearance from the peritoneal cavity (71,72).
Gastric cancer
Gastric cancer is the 4th most common cancer worldwide and the second leading cause of cancer death (73). At the time of potential curative resection, up to 20% may have peritoneal carcinomatosis present (74). In fact, peritoneal dissemination is more frequent than hematogenous spread with 40% of gastric cancer deaths have liver metastasis while 53-60% have peritoneal carcinomatosis (75). Patients with hematologic spread of disease treated with systemic chemotherapy have a median survival of 7 months however with peritoneal spread the median survival is 1-3 months (76-78).
Due to the high rate of peritoneal disease in gastric cancer, many programs have attempted to treat the local regional spread with intraperitoneal chemotherapy. MMC and CIS are the most commonly used cytotoxic drugs used in HIPEC while 5-FU for EPIC (79). There are multiple different intraperitoneal treatment protocols being studied to help improve survival.
The GYMSSA study was a prospective randomized trial to compare a promising new systemic chemotherapy regimen to cytoreductive surgery with HIPEC followed by systemic chemotherapy for patients with carcinomatosis for gastric cancer (80). The systemic chemotherapy used in both arms was FOLFOXIRI (irinotecan, leucovorin, oxaliplatin, and 5-FU). One treatment arm (SA) was administered systemic chemotherapy every 14 days for 12 cycles. Day 1 irinotecan 165 mg/m2 given over 90 min followed by leucovorin 200 mg/m2 and oxaliplatin 85 mg/m2 over 2 hours. 5-FU 3,200 mg/m2 was then given over 48 hours as a continuous infusion. Patients in the second treatment arm (GYMS) underwent gastrectomy, metastasectomy of liver or lung if needed, cytoreductive surgery and HIPEC. HIPEC was administered with oxaliplatin 460 mg/m2 at 41 °C for 30 minutes, Bidirectional treatment using 5-FU 400 mg/m2 and leucovorin 20 mg/m2 given just prior to perfusion to enhance the intraperitoneal oxaliplatin. Patients were then started on FOLFOXIRI 8 weeks after surgery. Median survival in the SA arm was 4.3 months and the GYMS arm 11.3 months with 4 of 9 patients living longer than 12 months.
Catumaxomab is a new drug being evaluated in phase II/III randomized trials. It is a rat-mouse hybrid monoclonal antibody that is being used in patients with malignant ascites for gastric cancer (81). Two studies have shown that catumaxomab improves progression free survival in patients with gastric carcinomatosis with median of 71 vs. 44 days and might improve survival in gastrointestinal antiepithelial cell adhesion molecule positive tumors (82,83).
Another interesting approach is a multimodal strategy with neoadjuvant intraperitoneal and systemic chemotherapy (NIPS), CRS + HIPEC and EPIC (84,85). The thought is to reduce tumor burden before surgery with NIPS in patients with positive peritoneal cytology washings. This is a bidirectional chemotherapy that attacks peritoneal disease from both the peritoneum and from subperitoneal blood vessels. This is then followed by cytoreductive surgery with HIPEC and then EPIC.
The NIPS technique uses 60 mg/m2 of oral S-1 for 21 days, followed by one week of rest. On days 1, 8, and 15, 30 mg/m2 of taxotere and 30 mg/m2 of CIS in 500 cc normal saline are administered into the abdomen. S-1 is an oral agent that is converted to 5-FU in the body. It contains gimeracil, which helps to inhibit the degradation of 5-FU in the body, and oteracil, which helps to reduce gastrointestinal side effects. It is not approved in the United States. Authors recommend two cycles of NIPS to achieve a negative cytology. Complications of NIPS are low with some bone marrow suppression, renal toxicity, and intraabdominal port infection. This study shows a negative washing cytology after a positive washing in 41 out of 79 patients (63%) (86).
Ovarian cancer
Ovarian cancer is the fifth leading cause of cancer death in females, with an estimated 22,000 women in the United States being diagnosed, accounting for 15,500 deaths (87). The most common route of spread is by exfoliation of malignant cells into peritoneum. While ovarian cancer is often responsive to optimal cytoreductive surgery (no residual disease or tumor nodules <1 cm) and platinum-based chemotherapy, there remains a high rate of recurrence and poor long-term survival.
Gynecological Oncology Group (GOG) #172 was the first study showing a better overall survival with a combination of systemic and intraperitoneal chemotherapy (88). Subsequent studies confirmed these results (89-91). The most common IP chemotherapy agent used is CIS (100 mg/m2) delivered every three weeks over six cycles.
When HIPEC is used for ovarian cancer it can either be at the initial surgery as front line treatment, consolidative therapy, or for recurrent disease. However there is no standard chemotherapy protocol for HIPEC including the cytotoxic drugs used or the dosages. CIS is the most common cytotoxic drug with MMC, mitoxantrone, carboplatin, doxorubicin, and gemcitabine also being used (Table 6).

Full table
When EPIC is used after HIPEC, paclitaxel is the most common drug administered each day for 5 days.
The future
As regional therapies become more accepted for the treatment of peritoneal based malignancies, new treatment regimens emerge. Bidirectional therapy as well as and NIPS are approaches that have yet to be fully explored. The use of immunotherapy and molecular targeted therapy are two other avenues that are still in their infancy for systemic use and might have the possibilities of being used in the future for intraperitoneal protocols.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Loggie BW, Fleming RA, McQuellon RP, et al. Prospective trial for the treatment of malignant peritoneal mesothelioma. Am Surg 2001;67:999-1003. [PubMed]
- Cioppa T, Vaira M, Bing C, et al. Cytoreduction and hyperthermic intraperitoneal chemotherapy in the treatment of peritoneal carcinomatosis from pseudomyxoma peritonei. World J Gastroenterol 2008;14:6817-23. [PubMed]
- Deraco M, Baratti D, Inglese MG, et al. Peritonectomy and intraperitoneal hyperthermic perfusion (IPHP): a strategy that has confirmed its efficacy in patients with pseudomyxoma peritonei. Ann Surg Oncol 2004;11:393-8. [PubMed]
- Elias D, Gilly F, Quenet F, et al. Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 2010;36:456-62. [PubMed]
- Loungnarath R, Causeret S, Bossard N, et al. Cytoreductive surgery with intraperitoneal chemo-hyperthermia for the treatment of pseudomyxoma peritonei: a prospective study. Dis Colon Rectum 2005;48:1372-9. [PubMed]
- Marcotte E, Sideris L, Drolet P, et al. Hyperthermic intraperitoneal chemotherapy with oxaliplatin for peritoneal carcinomatosis arising from appendix: preliminary results of a survival analysis. Ann Surg Oncol 2008;15:2701-8. [PubMed]
- Sugarbaker PH, Chang D. Results of treatment of 385 patients with peritoneal surface spread of appendiceal malignancy. Ann Surg Oncol 1999;6:727-31. [PubMed]
- Stewart JH, Shen P, Russell GB, et al. Appendiceal neoplasms with peritoneal dissemination: outcomes after cytoreductive surgery and intraperitoneal hyperthermic chemotherapy. Ann Surg Oncol 2006;13:624-34. [PubMed]
- Smeenk RM, Verwaal VJ, Antonini N, et al. Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg 2007;245:104-9. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-43. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-92. [PubMed]
- Yan TD, Black D, Savady R, et al. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol 2006;24:4011-9. [PubMed]
- Cao C, Yan TD, Black D, et al. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2009;16:2152-65. [PubMed]
- Glehen O, Gilly FN, Boutitie F, et al. Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy: a multi-institutional study of 1,290 patients. Cancer 2010;116:5608-18. [PubMed]
- Witkamp AJ, de Bree E, Van Goethem R, et al. Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 2001;27:365-74. [PubMed]
- Goodman MD, editor. Regional Therapeutics for Advanced Malignancies. New Delhi: Jaypee Brothers Medical Publishers, 2012:43-57.
- Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res 1996;82:79-100. [PubMed]
- Yan TD, Coa CQ, Munkholom-Larson S, et al. A pharmacological review on intraperitoneal chemotherapy for. World J Gastrointest Oncol 2010;2:109-16. [PubMed]
- Torres IJ, Litterst CL, Guarino AM, et al. Transport of model compounds across the peritoneal membrane in the rats. Pharmacology 1978;17:330-40. [PubMed]
- Yan TD, Stuart OA, Yoo D, et al. Perioperative intraperitoneal chemotherapy for peritoneal surface malignancy. J Transl Med 2006;4:17. [PubMed]
- Kusamura S, Dominique E, Baratti D, et al. Drugs, carrier solutions and temperature in hyperthermic intraperitoneal chemotherapy. J Surg Oncol 2008;98:247-52. [PubMed]
- Spratt JS, Adcock RA, Muskovin M, et al. Clinical Delivery System for Intraperitoneal Hyperthermic Chemotherapy. Cancer Res 1980;40:256-60. [PubMed]
- Hildebrandt B, Wust P, Ahlers O, et al. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol 2002;43:33-56. [PubMed]
- Sugarbaker PH. Peritonectomy procedures. Ann Surg 1995;221:29-42. [PubMed]
- Sugarbaker PH, Graves T, DeBruijn EA, et al. Early postoperative intraperitoneal chemotherapy as an adjuvant therapy to surgery for peritoneal carcinomatosis from gastrointestinal cancer: pharmacological studies. Cancer Res 1990;50:5790-4. [PubMed]
- Ronnett BM, Kurman RJ, Shmookler BM, et al. The morphologic spectrum of ovarian metastases of appendiceal adenocarcinomas: A clinicopathologic and immunohistochemical analysis of tumors often misinterpreted as primary ovarian tumors or metastatic tumors from other gastrointestinal sites. Am J Surg Pathol 1997;21:1144-55. [PubMed]
- Misdraji J, Yantiss RK, Graeme-Cook FM, et al. Appendiceal mucinous neoplasms: a clinicopathologic analysis of 107 cases. Am J Surg Pathol 2003;27:1089-103. [PubMed]
- Fernandez-Trigo V, Stuart AO, Stephens AD, et al. Surgically directed chemotherapy: heated intraperitoneal lavage with mitomycin C. In: Sugarbaker PH, editor. Peritoneal Carcinomatosis: Drugs and Diseases. Boston, MA: Kluwer Academic Publishers, 1996:51-61.
- Sugarbaker PH, Morab JT, Carmignania P, et al. Update on chemotherapeutic agents utilized for perioperative intraperitoneal chemotherapy. Oncologist 2005;10:112-22. [PubMed]
- van Ruth S, Mathot RA, Sparidans RW, et al. Population pharmacokinetics and pharmacodynamics of mitomycin during intraoperative hyperthermic intraperitoneal chemotherapy. Clin Pharmacokinet 2004;43:131-43. [PubMed]
- Kemmel V, Mercoli HA, Meyer N, et al. Mitomycin C Pharmacokinetics as Predictor of Severe Neutropenia in Hyperthermic Intraperitoneal Therapy. Ann Surg Oncol 2015. [Epub ahead of print]. [PubMed]
- Stephens AD, Alderman R, Chang D, et al. Morbidity and mortality of 200 treatments with cytoreductive surgery and hyperthermic intraoperative intraperitoneal chemotherapy using the Coliseum technique. Ann Surg Oncol 1999;6:790-6. [PubMed]
- Glehen O, Osinsky D, Cotte E, et al. Intraperitoneal chemohyperthermia using a closed abdominal procedure and cytoreductive surgery for the treatment of peritoneal carcinomatosis: morbidity and mortality analysis of 216 consecutive patients. Ann Surg Oncol 2003;10:863-9. [PubMed]
- van Ruth S, Verwaal VJ, Zoetmulder FA. Pharmacokinetics of intraperitoneal mitomycin C. Surg Oncol Clin N Am 2003;12:771-80. [PubMed]
- Pestieau SR, Schnake KJ, Stuart OA, et al. Impact of carrier solutions on pharmacokinetics of intraperitoneal chemotherapy. Cancer Chemother Pharmacol 2001;47:269-76. [PubMed]
- Sugarbaker PH, editor. Management of peritoneal surface malignancy using intraperitoneal chemotherapy and cytoreductive surgery. A manual for physicians and nurses, third edition. Michigan: The Ludann Company Grand Rapids, 1998.
- Mcpartland S, Goodman MD. Pathophysiology of Peritoneal Malignancies and Modalities of Treatment. In: Goodman MD, editor. Regional Therapeutics for Advanced Malignancies. New Delhi: Jaypee Brothers Medical Publishers, 2012:3-17.
- Witkamp AJ, de Bree E, Kaag MM, et al. Extensive surgical cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in patients with pseudomyxoma peritonei. Br J Surg 2001;88:458-63. [PubMed]
- Ihemelandu CU, Shen P, Stewart J, et al. Management of Peritoneal Carcinomatosis from Colorectal Cancer. Semin Oncol 2011;38:568-75. [PubMed]
- Saltz LB, Cox JV, Blanke C, et al. Irinotecan plus fluorouracil and leucovorin for metastatic colorectal cancer. Irinotecan Study Group. N Engl J Med 2000;343:905-14. [see comment]. [PubMed]
- Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med 2004;350:2335-42. [see comment]. [PubMed]
- Colucci G, Gebbia V, Paoletti G, et al. Phase III randomized trial of FOLFIRI versus FOLFOX4 in the treatment of advanced colorectal cancer: a multicenter study of the Gruppo Oncologico Dell'Italia Meridionale. J Clin Oncol 2005;23:4866-75. [PubMed]
- Cassidy J, Clarke S, Diaz-Rubio E, et al. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol 2008;26:2006-12. [PubMed]
- Porschen R, Arkenau HT, Kubicka S, et al. Phase III study of capecitabine plus oxaliplatin compared with fluorouracil and leucovorin plus oxaliplatin in metastatic colorectal cancer: a final report of the AIO Colorectal Study Group. J Clin Oncol 2007;25:4217-23. [PubMed]
- Saltz LB, Clarke S, Diaz-Rubio E, et al. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol 2008;26:2013-9. [PubMed]
- Elias D, Bonnay M, Puizillou JM, et al. Heated intra-operative intraperitoneal oxaliplatin after complete resection of peritoneal carcinomatosis: pharmacokinetics and tissue distribution. Ann Oncol 2002;13:267-72. [PubMed]
- Stewart JH, Shen P, Russell G, et al. A phase I trial of oxaliplatin for intraperitoneal hyperthermic chemoperfusion for the treatment of peritoneal surface dissemination from colorectal and appendiceal cancers. Ann Surg Oncol 2008;15:2137-45. [PubMed]
- Chua TC, Esquivel J. Carcinomatosis for colon Cancer. In: Goodman MD, editor. Regional Therapeutics for Advanced Malignancies. New Delhi: Jaypee Brothers Medical Publishers, 2012:33-42.
- Elias D. Peritoneal Colorectal Carcinomatosis Treated With Surgery and Perioperative Intraperitoneal Chemotherapy: Retrospective Analysis of 523 Patients From a Multicentric French Study. J Clin Oncol 2010;28:63-8. [PubMed]
- Chua TC, Yan TD, Ng KM, et al. Significance of Lymph Node Metastasis in Patients with Colorectal Cancer Peritoneal Carcinomatosis. World J Surg 2009;33:1488-94. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-5. [PubMed]
- Shen P, Thai K, Stewart JH, et al. Peritoneal surface disease from colorectal cancer: comparison with the hepatic metastases surgical paradigm in optimally resected patients. Ann Surg Oncol 2008;15:3422-32. [PubMed]
- Franko J, Gusani NJ, Holtzman MP, et al. Multivisceral resection does not affect morbidity and survival after cytoreductive surgery and chemoperfusion for carcinomatosis from colorectal cancer. Ann Surg Oncol 2008;15:3065-72. [see comment]. [PubMed]
- Bijelic L, Yan TD, Sugarbaker PH. Treatment failure following complete cytoreductive surgery and perioperative intraperitoneal chemotherapy for peritoneal dissemination from colorectal or appendiceal mucinous neoplasms. J Surg Oncol 2008;98:295-9. [PubMed]
- Kianmanesh R, Scaringi S, Sabate JM, et al. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg 2007;245:597-603. [PubMed]
- Verwaal VJ, van Ruth S, Witkamp A, et al. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005;12:65-71. [PubMed]
- Markman M, Kelsen D. Efficacy of cisplatin-based intraperitoneal chemotherapy as treatment of malignant peritoneal mesothelioma. J Cancer Res Clin Oncol 1992;118:547-50. [PubMed]
- Neumann V, Muller KM, Fischer M. Peritoneal mesothelioma--incidence and etiology. Pathologe 1999;20:169-76. [PubMed]
- Eltabbakh GH, Piver MS, Hempling RE, et al. Clinical picture, response to therapy, and survival of women with diffuse malignant peritoneal mesothelioma. J Surg Oncol 1999;70:6-12. [PubMed]
- Yan TD, Deraco M. A novel tumor-node-metastasis (TNM) staging system of diffuse malignant peritoneal mesothelioma using outcome analysis of a multi-institutional database. Cancer 2011;117:1855-63. [PubMed]
- Baratti D, Kusamura S, Cabras AD, et al. Lymph node metastases in diffuse malignant peritoneal mesothelioma. Ann Surg Oncol 2010;17:45-53. [PubMed]
- Yan TD, Deraco M, Baratti D, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 2009;27:6237-42. [PubMed]
- Hesdorffer ME, Chabot JA, Keohan ML, et al. ombined resection, intraperitoneal chemotherapy, and whole abdominal radiation for the treatment of malignant peritoneal mesothelioma. Am J Clin Oncol 2008;31:49-54. [PubMed]
- Yan TD, Brun EA, Cerruto CA, et al. Prognostic indicators for patients undergoing cytoreductive surgery and perioperative intraperitoneal chemotherapy for diffuse malignant peritoneal mesothelioma. Ann Surg Oncol 2007;14:41-9. [PubMed]
- Brigand C, Monneuse O, Mohamed F, et al. Peritoneal mesothelioma treated by cytoreductive surgery and intraperitoneal hyperthermic chemotherapy: results of a prospective study. Ann Surg Oncol 2006;13:405-12. [PubMed]
- Deraco M, Nonaka D, Baratti D, et al. Prognostic analysis of clinicopathologic factors in 49 patients with diffuse malignant peritoneal mesothelioma treated with cytoreductive surgery and intraperitoneal hyperthermic perfusion. Ann Surg Oncol 2006;13:229-37. [PubMed]
- Feldman AL, Libutti SK, Pingpank JF, et al. Analysis of factors associated with outcome in patients with malignant peritoneal mesothelioma undergoing surgical debulking and intraperitoneal chemotherapy. J Clin Oncol 2003;21:4560-7. [PubMed]
- Harrison LE, Bryan M, Pliner L, et al. Phase I trial of pegylated liposomal doxorubicin with hyperthermic intraperitoneal chemotherapy in patients undergoing cytoreduction for advanced intra-abdominal malignancy. Ann Surg Oncol 2008;15:1407-13. [PubMed]
- Sadzuka Y, Hirota S, Sonobe T. Intraperitoneal administration of doxorubicin encapsulating liposomes against peritoneal dissemination. Toxicol Lett 2000;116:51-9. [PubMed]
- Sugarbaker PH. Early postoperative intraperitoneal adriamycin as an adjuvant treatment for visceral and retroperitoneal sarcoma. In: Sugarbaker PH, editor. Peritoneal Carcinomatosis: Drugs and Diseases. Boston: Kluwer, 1996:7-14.
- Kuh HJ, Jang SH, Wientjes MG, et al. Determinants of paclitaxel penetration and accumulation in human solid tumor. J Pharmacol Exp Ther 1999;290:871-80. [PubMed]
- Mohamed F, Marchettini P, Stuart OA, et al. A comparison of hetastarch and peritoneal dialysis solution for intraperitoneal chemotherapy delivery. Eur J Surg Oncol 2003;29:261-5. [PubMed]
- Bertuccio P, Chatenoud L, Levi F, et al. Recent patterns in gastric cancer: A global overview. Int J Cancer 2009;125:666-73. [PubMed]
- Ikeguchi M, Oka A, Tsujitani S, et al. Relationship between area of serosal invasion and intraperitoneal free cancer cells in patients with gastric cancer. Anticancer Res 1994;14:2131-4. [PubMed]
- Okines A, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2010;21:v50-4. [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: Results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-63. [PubMed]
- Pyrhönen S, Kuitunen T, Nyandoto P, et al. Randomized comparison of fluorouracil, epidoxorubicin and methotrexate (FEMTX) plus supportive care with supportive care alone in patients with non-resectable gastric cancer. Br J Cancer 1995;71:587-91. [PubMed]
- Hanazaki K, Mochizuki Y, Machida T, et al. Post-operative chemotherapy in non-curative gastrectomy for advanced gastric cancer. Hepatogastroenterology 1999;46:1238-43. [PubMed]
- Gill RS, Al-Adra DP, Nagendran J, et al. Treatment of gastric cancer with peritoneal carcinomatosis by cytoreductive surgery and HIPEC: A systemic review of survival, mortality and morbidity. J Surg Oncol 2011;104:692-8. [PubMed]
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trail. J Surg Oncol 2014;110:275-84. [PubMed]
- Montori G, Coccolini F, Ceresoli M, et al. The Treatment of Peritoneal Carcinomatosis in Advanced Gastric Cancer: State of the Art. Int J Surg Oncol 2014;2014:912418-14.
- Heiss MM, Murawa P, Koralewski P, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: results of a prospective randomized phase II/III trial. Int J Cancer 2010;127:2209-21. [PubMed]
- Ströhlein MA, Lordick F, Rüttinger D, et al. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie 2011;34:101-8. [PubMed]
- Yonemura Y, Endou Y, Shinbo M, et al. Safety and efficacy of bidirectional chemotherapy for treatment of patients with peritoneal dissemination from gastric cancer: selection for cytoreductive surgery. J Surg Oncol 2009;100:311-6. [PubMed]
- Yonemura Y, Elnemr A, Endou Y, et al. Multidisciplinary therapy for treatment of patients with peritoneal carcinomatosis from gastric cancer. World J Gastrointest Oncol 2010;2:85-97. [PubMed]
- Yonemura Y, Elnemr A, Endou Y, et al. Effects of Neoadjuvant Intraperitoneal/Systemic Chemotherapy (Bidirectional Chemotherapy) for the Treatment of Patients with Peritoneal Metastasis from Gastric Cancer. Int J Surg Oncol 2012;2012:148420.
- Siegel R, Naishadham D, Jemal A. Cancer statistics. CA Cancer J Clin 2012;62:10-29. [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006;354:34-43. [PubMed]
- Markman M, Bundy BN, Alberts DS, et al. Phase III trial of standard-dose intravenous cisplatin plus paclitaxel versus moderately high-dose carboplatin followed by intravenous paclitaxel and intraperitoneal cisplatin in small-volume stage III ovarian carcinoma: an intergroup study of the Gynecologic Oncology Group, Southwestern Oncology Group, and Eastern Cooperative Oncology Group. J Clin Oncol 2001;19:1001-7. [PubMed]
- Alberts DS, Liu PY, Hannigan EV, et al. Intraperitoneal cisplatin plus intravenous cyclophosphamide versus intravenous cisplatin plus intravenous cyclophosphamide for stage III ovarian cancer. N Engl J Med 1996;335:1950-5. [PubMed]
- Walker JL, Armstrong DK, Huang HQ, et al. Intraperitoneal catheter outcomes in a phase III trial of intravenous versus intraperitoneal chemotherapy in optimal stage III ovarian and primary peritoneal cancer: a Gynecologic Oncology Group study. Gynecol Oncol 2006;100:27-32. [PubMed]
- Cotte E, Glehen O, Mohamed F, et al. Cytoreductive surgery and intraperitoneal chemo-hyperthermia for chemo-resistant and recurrent advanced epithelial ovarian cancer: prospective study of 81 patients. World J Surg 2007;31:1813-20. [PubMed]
- Deraco M, Kusamura S, Virzi S, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy as upfront therapy for advanced epithelial ovarian cancer: multi-institutional phase-II trial. Gynecol Oncol 2011;122:215-20. [PubMed]
- Ceelen WP, Van Nieuwenhove Y, Van Belle S, et al. Cytoreduction and hyperthermic intraperitoneal chemoperfusion in women with heavily pretreated recurrent ovarian cancer. Ann Surg Oncol 2012;19:2352-9. [PubMed]
- Tentes AA, Kakolyris S, Kyziridis D, et al. Cytoreductive surgery combined with hyperthermic intraperitoneal intraoperative chemotherapy in the treatment of advanced epithelial ovarian cancer. J Oncol 2012;2012:358341.
- Di Giorgio A, Naticchioni E, Biacchi D, et al. Cytoreductive surgery (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in the treatment of diffuse peritoneal carcinomatosis from ovarian cancer. Cancer 2008;113:315-25. [PubMed]
- Lim MC, Kang S, Choi J, et al. Hyperthermic intraperitoneal chemotherapy after extensive cytoreductive surgery in patients with primary advanced epithelial ovarian cancer: interim analysis of a phase II study. Ann Surg Oncol 2009;16:993-1000. [PubMed]
- Pomel C, Ferron G, Lorimier G, et al. Hyperthermic intra-peritoneal chemotherapy using oxaliplatin as consolidation therapy for advanced epithelial ovarian carcinoma. Results of a phase II prospective multicentre trial. CHIPOVAC study. Eur J Surg Oncol 2010;36:589-93. [PubMed]
- Roviello F, Pinto E, Corso G, et al. Safety and potential benefit of hyperthermic intraperitoneal chemotherapy (HIPEC) in peritoneal carcinomatosis from primary or recurrent ovarian cancer. J Surg Oncol 2010;102:663-70. [PubMed]
- Fagotti A, Costantini B, Vizzielli G, et al. HIPEC in recurrent ovarian cancer patients: morbidity-related treatment and long-term analysis of clinical outcome. Gynecol Oncol 2011;122:221-5. [PubMed]
- Helm CW, Randall-Whitis L, Martin RS 3rd, et al. Hyperthermic intraperitoneal chemotherapy in conjunction with surgery for the treatment of recurrent ovarian carcinoma. Gynecol Oncol 2007;105:90-6. [PubMed]
- Lentz SS, Miller BE, Kucera GL, et al. Intraperitoneal hyperthermic chemotherapy using carboplatin: a phase I analysis in ovarian carcinoma. Gynecol Oncol 2007;106:207-10. [PubMed]
- Bae JH, Lee JM, Ryu KS, et al. Treatment of ovarian cancer with paclitaxel- or carboplatin-based intraperitoneal hyperthermic chemotherapy during secondary surgery. Gynecol Oncol 2007;106:193-200. [PubMed]
- Argenta PA, Sueblinvong T, Geller MA, et al. Hyperthermic intraperitoneal chemotherapy with carboplatin for optimally cyto-reduced, recurrent, platinum sensitive ovarian carcinoma: A pilot study. Gynecol Oncol 2013;129:81-5. [PubMed]