
The diagnosis and outcome of Krukenberg tumors
Introduction
Krukenberg tumors are ovarian metastases from non-gynecological, mainly gastrointestinal cancers, such as gastric and colorectal cancers (1). These tumors are bilateral at approximately 80% of cases affecting women, especially young women with significant morbidity and symptomatology (2). Previous retrospective studies suggested that Krukenberg tumors were associated with poor prognosis compared to metastatic disease at other sites arising from the same primary (3). They are disproportionally unresponsive to systemic chemotherapy, growing into large size, even when the other sites of metastatic disease do respond (4,5). In contrast to at least a subset of primary epithelial ovarian cancers where neoadjuvant chemotherapy followed by surgical debulking provides survival benefit (6), identification of the primary followed by palliative chemotherapy is often the initial management for patients with metastatic gastrointestinal cancers to achieve systemic disease control. But given limited benefit from systemic therapy, metastasectomy appeared to be the only effective treatment option when complete surgical debulking and resection of the primary tumor can be performed (7,8). In a series of 216 patients with stage IV gastric cancer, metastasectomy plus chemotherapy offered superior overall survival (OS) compared to palliative chemotherapy alone for patients with Krukenberg tumors (9). Similarly, a retrospective cohort of patients with colorectal cancer metastatic to the ovary derived a significant survival benefit from palliative oophorectomy (7).
Therefore, it is crucial to distinguish patients with Krukenberg tumors from those with primary ovarian cancers before decision on initial management. However, clinical parameters at disease presentation and even modern diagnostic imaging techniques cannot reliably distinguish Krukenberg tumors from primary ovarian cancers (10,11). In recent years, deep learning algorithms such as convolutional neural networks (CNNs) have demonstrated their ability in image recognition of histopathological slides (12), dermatoscopic images (13), and radiographic images (14), often with accuracy approaching or superior to human performance.
To address the literature gap and the clear need of accurate tools to distinguish Krukenberg tumors from primary ovarian cancers preoperatively, we identified patients with Krukenberg tumors, confirmed the role of palliative surgical management. We then developed a diagnostic model from preoperative computed tomography (CT) images using deep learning algorithms to distinguish patients with Krukenberg tumors from primary ovarian cancers. We also developed a diagnostic model incorporating common clinical, biochemical, and radiographic parameters for preoperative diagnosis of Krukenberg tumors. We present the following article in accordance with the STROBE (Strengthening the Reporting of Observational studies in Epidemiology) reporting checklist (available at http://dx.doi.org/10.21037/jgo-20-364).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Mayo Clinic (18-006391) and individual consent for this retrospective analysis was waived.
Patients
We retrospectively identified eligible patients from institutional patient registry between 1997 and 2018 by searching keywords “Krukenberg”, “ovarian metastasis”, “ovarian metastases” from “clinical note” section. We included women age 18 or older, histologically confirmed diagnosis of Krukenberg tumors and excluded those without histological confirmation of Krukenberg tumors. We retrospectively reviewed clinical information and treatment history of these patients. The clinical information included patient’s baseline characteristics, pathological and molecular features of the tumor, tumor marker levels, and clinical outcome. Treatment history included the types of surgery, neoadjuvant therapy, adjuvant therapy, or palliative therapy and response to therapy. We also identified patients with primary malignant epithelial ovarian cancers from institutional cancer center registry between 1997 and 2018 by searching topography code (ICD-O-3) C569, morphology code (ICD-O-3) 80503, 84413, 84603, and 84613. We only included patients with primary malignant epithelial ovarian cancers of grade 3 or 4 histology and clinical stage 3 or 4 (based on AJCC 5th to 7th editions) disease. We excluded patients younger than 18 years of age and those without available preoperative abdominal/pelvic CT scans and radiology reports before surgical resection of the Krukenberg tumors or primary ovarian cancers.
Radiographic evaluations
We then identified available preoperative abdominal/pelvic CT images and the corresponding radiology reports. From the radiology reports, we extracted features including size of left ovarian mass, size of right ovarian mass, peritoneal metastasis, metastasis at other distant sites, lymph node metastasis, the presence of ascites, and cystic or solid component. An independent staff diagnostic radiologist (SP Sheedy) assessed the quality of CT scans, reviewed and confirmed these radiographic features of Krukenberg tumors.
We retrieved CT images with intravenous contrast from institutional diagnostic radiology database with QREADS, an image reviewer developed in-house for routine clinical use (15). Axial images in soft tissue window with the largest cross-sectional area of Krukenberg tumors or primary ovarian cancers were selected for tumor segmentation with rectangle shape regions of interest identified. These images in Portable Network Graphics format were processed with R package “magick” version 2.3 (16) to set the size of all images to 256×256 pixels with the same pixel size and extra space filled with black pixels. We used the FastAI 1.0.61 deep learning library (17) with Google’s Colab Notebooks to train a multiscale neural network model to classify Krukenberg tumors and primary ovarian cancers (18). A randomizer with FastAI was used to select 80% of the images for training and the rest 20% for testing. We studied three different residual deep neural network (ResNet) architectures (19) with initial weights from ImageNet (20) and unfroze all layers, with area under the receiver operating characteristic curve (AUC) as the metrics for training. We tuned the hyperparameters of the model including and data augmentation parameters of image rotation, flipping and contrast changes. The performance of the neural network model was summarized using sensitivity, specificity, accuracy, and AUC with 95% confidence interval (95% CI) derived from 100 bootstrap datasets.
Statistical analysis
Sample size calculation was not performed due to retrospective nature of this feasibility study. We summarized categorical data as frequency counts and percentages and continuous measures as means, standard deviations, medians, and interquartile ranges (IQRs). Continuous variables were compared with Student’s t-test or Wilcoxon-Mann-Whitney test. Categorical variables were compared with chi-square test or Fisher’s exact test. OS was calculated from the date of Krukenberg tumor diagnosis to the date of death. Disease-free or progression-free (PFS) survival was calculated from the date of treatment start or tumor resection to the date of either disease progression/recurrence or death. Time-to-event data were summarized using Kaplan-Meier method with surviving patients censored at the date of last follow-up. Survival of different groups of patients was compared using log-rank tests. Cox proportional hazards regression model was used for multivariable survival analyses of potential prognostic indicators. The results were summarized as hazard ratio (HR) and 95% CI. Univariate and multivariable logistic regressions were used to evaluate various clinical, biochemical, and radiographic factors as predictors of Krukenberg tumor versus primary ovarian cancer diagnoses. The results were summarized as odds ratio (OR) and 95% CI. Eighty percent of the dataset was used for training and 20% was used for testing. Factors with P value less than 0.05 in the univariate logistic regression were moved forward to be included in the multivariable logistic regression. We evaluated the goodness of fit of logistic regression models using sensitivity, specificity, accuracy, and AUC and 95% CI derived from 500 bootstrap datasets. All statistical tests were two-sided. P value less than 0.05 was considered statistically significant.
Results
Clinical, histopathological, biochemical, and radiographic features of patients with Krukenberg tumors
A total of 214 patients with histologically confirmed Krukenberg tumor were included in the study with median age of 52 years (IQR, 44–62 years). As shown in Table 1, 104 (48.6%) patients had colorectal cancer, followed by 45 (21.0%) patients with gastric and gastroesophageal junction cancers. Tumors of 57 (29.8%) patients had signet ring histology. Ninety-eight (45.8%) patients had Krukenberg tumors diagnosed at the time of primary tumor diagnosis, whereas 116 (54.2%) patients developed metachronous ovarian metastases. At the time of initial cancer diagnosis, 150 (72.5%) patients had stage IV disease. One hundred and sixty-four (77.4%) patients had surgical resection of their Krukenberg tumor at some point during their treatment course with or without systemic therapy. Seventy-seven (49.0%) patients also had other peritoneal metastatic disease; 73 (46.8%) patients also had metastatic disease at distant sites other than peritoneum at the time of Krukenberg tumor diagnosis. The response rate of patients with Krukenberg tumors to systemic therapy was 38.5%. One hundred and nineteen (55.6%) patients had died at last follow-up. Median follow-up duration since primary tumor diagnosis was 32.9 months (IQR, 18.7–64.2 months). Median follow-up duration from Krukenberg tumor diagnosis was 19.7 months (IQR, 5.5–39.0 months).
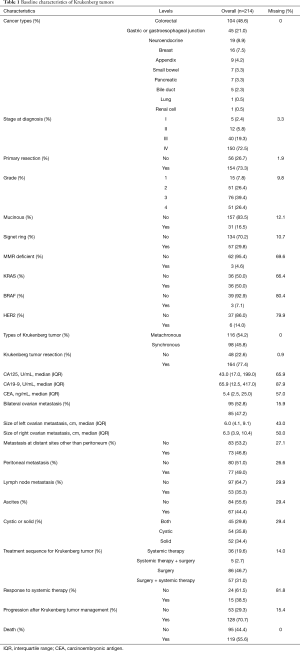
Full table
We also compared the clinical and radiographic features of Krukenberg tumors from colorectal and gastric cancers (Table S1). Compared to patients with Krukenberg tumors from colorectal cancer, those with Krukenberg tumors from gastric cancer had higher incidence of ascites (P=0.008), higher proportion of solid ovarian masses (P<0.001) and a trend of higher incidence of synchronous Krukenberg tumors (P=0.052).
Treatment outcomes of Krukenberg tumors in patients with colorectal cancer and gastric cancer
Seventy-six patients with colorectal cancer received surgical resection of their Krukenberg tumor whereas 23 patients received medical management only. Median PFS of patients who received surgical resection was 22.2 months (95% CI: 15.9–35.0 months), significantly higher than 6.7 months (95% CI: 3.0–12.7 months) of those who received medical management only (P=0.0002) (Figure 1A). Univariate survival analysis of patients with Krukenberg tumors from colorectal cancer identified poor prognostic factors for PFS including bilateral ovarian metastases (HR 1.75, P=0.032), metastasis at other distant sites (HR 1.92, P=0.019), peritoneal metastasis (HR 1.97, P=0.013), high carcinoembryonic antigen (CEA) (HR 1.02, P=0.013), and medical management of Krukenberg tumor (HR 2.63, P<0.001). Medical management of Krukenberg tumor was a poor prognostic factor for PFS (HR 2.42, P=0.035) after adjusting for other prognostic factors (Table S2). Median OS of patients who received surgical resection was 48.1 months (95% CI: 34.4–73.3 months), significantly higher than 30.6 months (95% CI: 23.2–37.1 months) of those who received medical management only (P=0.015) (Figure 1B). Univariate survival analysis of patients with Krukenberg tumors from colorectal cancer identified poor prognostic factors for OS including bilateral ovarian metastases (HR 2.64, P=0.001), metastasis at other distant sites (HR 1.86, P=0.033), peritoneal metastasis (HR 2.08, P=0.012), lymph node metastasis (HR 2.32, P=0.012), high CEA (HR 1.01, P=0.002), and medical management of Krukenberg tumor (HR 2.04, P=0.017); whereas surgical resection of primary tumor was a good prognostic factor (HR 0.40, P=0.015). However, none of these were statistically significant in multivariable analysis after adjusting for other prognostic factors (Table S3).
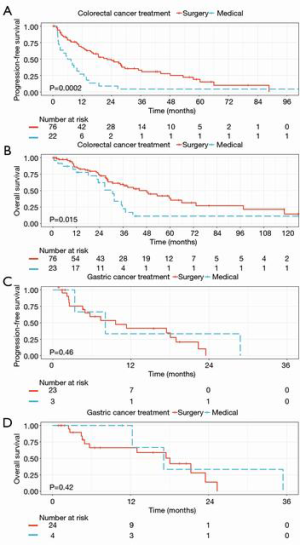
Twenty-four patients with gastric cancer received surgical resection of their Krukenberg tumors whereas 4 patients received medical management only. Median PFS of patients who received surgical resection was 9.6 months (95% CI: 4.9 months–not reached), comparable to 8.0 months (95% CI: 3.4–not reached months) of those who received medical management only (P=0.46) (Figure 1C). Median OS of patients who received surgical resection was 18.0 months (95% CI: 5.7–not reached months), comparable to 17.1 months (95% CI: 12.3–not reached months) of those who received medical management only (P=0.42) (Figure 1D).
Models that distinguish Krukenberg tumors from primary ovarian cancers before surgical resection
Among 331 patients with primary ovarian cancers included in this study, 126 (38.1%) patients had grade 4 disease and 99 (29.9%) patients had stage IV disease. Among 214 patients with histopathological confirmed Krukenberg tumors, preoperative radiology reports diagnosed 60 (40.0%) patients with Krukenberg tumors, 42 (28.0%) patients with primary ovarian cancers, 33 (22.0%) patients with either diagnosis, and 15 (10%) patients with neither diagnosis. Among 331 patients with primary ovarian cancers, preoperative radiology reports diagnosed 223 (70.6%) patients with primary ovarian cancers, 8 (2.5%) patients with Krukenberg tumors, and 85 (26.9%) patients with either diagnosis. The accuracy of radiology reports to make either diagnosis of Krukenberg tumor or primary ovarian cancer was 60.7%.
Univariate logistic regression model identified the following clinical and radiographic features significantly associated with primary ovarian cancers versus Krukenberg tumor: older age (OR 2.15, P<0.001), elevated CA125 (OR 1.51, P<0.001), presence of peritoneal metastasis (OR 6.30, P<0.001), presence of ascites (OR 2.37, P<0.001), lower CEA (OR 0.11, P<0.001), presence of bilateral ovarian masses (OR 0.59, P=0.005), lack of metastasis at other distant sites (OR 0.43, P<0.001). Multivariable logistic regression confirmed that older age (OR 2.98, P<0.001), elevated CA125 (OR 1.57, P=0.004) and lower CEA (OR 0.03, P=0.031) were significantly associated with primary ovarian cancers versus Krukenberg tumor after adjusting for other features (bilateral ovarian masses, metastasis at other distant sites, peritoneal metastasis, and ascites) (Table 2). Multivariable logistic regression model distinguished primary ovarian cancers from Krukenberg tumors with 87.5% (95% CI: 75.0–100.0%) accuracy and AUC of 0.96 (95% CI: 0.87–1.00) (Table 3).
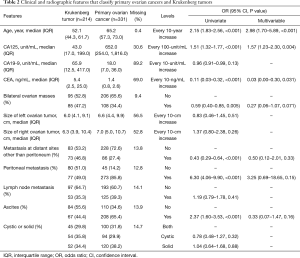
Full table

Full table
The final neural network model with optimized hyperparameters used ResNet50 architecture. We included 165 CT images of Krukenberg tumors and 247 CT images of primary ovarian cancers for model training and testing. Data augmentation parameters used in the final model are left-right flipping, no vertical flipping, maximal rotation of 10 degrees, no zoom, maximal lighting and contrast change 0.2, and no warping. The model was trained with learning rate of 1e−5 to 1e−3 and 50 epochs in each cycle. The neural network model distinguished primary ovarian cancers from Krukenberg tumors with 62.8% (95% CI: 51.8–74.5%) accuracy and AUC of 0.61 (95% CI: 0.53–0.72) (Table 3).
Discussion
In this study, we systematically evaluated common clinical, biochemical, and radiographic features of Krukenberg tumors as preoperative diagnostic predictors to distinguish them from primary ovarian cancers. Our patient population with Krukenberg tumors was representative in terms of the distribution of the primary tumors with colorectal cancer as the most common primary (21). In contrast, Krukenberg tumors of patients from Asian countries most commonly came from gastric primary (22). We observed similar OS and prognostic factors to those reported in the literature (23-25). In addition, palliative surgical management with or without perioperative systemic therapy in patients with colorectal cancer was associated with significantly longer PFS and OS in line with previous reports (7,8,26,27). However, the association with OS was not statistically significant after adjusting for other prognostic factors. This discrepancy was likely due to the use of palliative surgery in our study instead of cytoreductive resection of limited disease or oligometastatic disease with curative intent reported before (8,26). As to our patients with Krukenberg tumors from gastric cancer, we did not observe prolonged survival from palliative surgical resection of the Krukenberg tumors, different from previous reports (9,28-30), likely due to small sample size in our study. To compare the median PFS between patients with colorectal and gastric cancers, those with colorectal cancer treated with surgical resection of their Krukenberg tumor had significantly longer PFS than their gastric cancer counterparts. Potential explanations include the prognosis of advanced gastric cancer is worse compared with that of advanced colorectal cancer; and patients with Krukenberg tumor of gastric origin usually have a lower performance status score and severe anemia.
In contrast to studies on the prognosis of Krukenberg tumors, there are few studies on clinical and radiographic diagnosis of Krukenberg tumors versus primary ovarian cancers. Early studies compared CT or magnetic resonance imaging (MRI) image features of Krukenberg tumors to primary ovarian cancers. The former often has bilateral solid ovarian tumors containing well demarcated intratumoral cystic spaces with wall enhancement (10,31-33). Our study evaluated not only these radiographic features reported in the literature but also the diagnostic values of clinical and biochemical factors in addition to radiographic features representing the extent of disease. We utilized the technical advantage of neural network algorithm to evaluate radiographic features of CT images that may not be easily identified by radiologists in routine clinical practice. However, the neural network model from CT images of ovarian masses only had modest performance in distinguishing Krukenberg tumors from primary ovarian cancers. This finding was consistent with those from our multivariable logistic regression model where radiographic features extracted from radiology reports did not significantly contribute to the model performance. On the other hand, the logistic regression model with simple clinical and biochemical factors including age, CA125 and CEA levels can distinguish these two diagnoses with very promising accuracy, which could potentially be clinically useful. In addition, we also found that the models derived from clinical, biochemical and radiographic features were either comparable with or outperformed radiology reports, which further reinforced previous findings in the literature. For example, the CA125 and CEA ratio (34) and the combination of a complex ovarian mass with papillary projections presenting on the ultrasound and serum CA125 level above 170 U/mL can distinguish primary ovarian cancer with high performance (35).
Our feasibility study has several limitations. First, this is a retrospective cohort study with patient population from a single tertiary referral center. Interpretation of survival outcome and prognostic factors should be cautious due to selection and referral biases despite multivariable analysis adjusting for other prognostic factors. Second, we extracted radiographic features solely from radiology reports without further confirmation with all CT images used. These reports could contain very heterogenous information due to many radiologists involved in a time span of two decades and the lack of standardized reporting that could lead to errors of omission. However, we aimed to utilize the real-world information for our study which could be an advantage over other studies. Third, Krukenberg tumors are uncommon metastatic sites from non-gynecologic cancers (36). The size of our patient population, although comparable to previous studies, is still not ideal for model training especially neural network models with available CT images. As a matter of fact, to increase the number of available CT images, we had to include patients with stage III primary ovarian cancers in the comparison group despite the lack of distant metastatic disease other than peritoneal metastasis. Relatively small sample size leads to another limitation: Neural network models have high susceptibility to overfitting due to the large number of network parameters relative to the number of features from CT scans and the number of different sets of CT scan images available. To reduce overfitting, we used data augmentation strategies consisting of generating new images from random combinations of translations, rotations and flipping of existing images. However, due to the rarity of Krukenberg tumors, we were not able to obtain another dataset to externally validate our models. Lastly, we do not have molecular profiling data from circulating tumor DNA available for analysis, which may aid differential diagnosis between primary ovarian and Krukenberg tumors.
Our present study, for the first time, systematically evaluated the diagnostic utility of clinical, biochemical and radiographic features in distinguishing Krukenberg tumors from primary ovarian cancers. We developed a simple diagnostic model composed of age, CA125, and CEA levels to distinguish these two diagnoses with very promising accuracy. Despite some limitations, these findings could potentially provide complementary information for decisions on the initial management of ovarian masses.
Acknowledgments
Funding: This work was supported by Mayo Clinic CCaTS grant number UL1TR002377.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-364
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jgo-20-364
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-364). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by institutional/regional/national ethics/committee/ethics board of Mayo Clinic (18-006391) and individual consent for this retrospective analysis was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Agnes A, Biondi A, Ricci R, et al. Krukenberg tumors: Seed, route and soil. Surg Oncol 2017;26:438-45. [Crossref] [PubMed]
- Lewis MR, Deavers MT, Silva EG, et al. Ovarian involvement by metastatic colorectal adenocarcinoma: still a diagnostic challenge. Am J Surg Pathol 2006;30:177-84. [Crossref] [PubMed]
- Jeung YJ, Ok HJ, Kim WG, et al. Krukenberg tumors of gastric origin versus colorectal origin. Obstet Gynecol Sci 2015;58:32-9. [Crossref] [PubMed]
- Goéré D, Daveau C, Elias D, et al. The differential response to chemotherapy of ovarian metastases from colorectal carcinoma. Eur J Surg Oncol 2008;34:1335-9. [Crossref] [PubMed]
- Taylor AE, Nicolson VM, Cunningham D. Ovarian metastases from primary gastrointestinal malignancies: The Royal Marsden Hospital experience and implications for adjuvant treatment. Br J Cancer 1995;71:92-6. [Crossref] [PubMed]
- Moschetta M, Boussios S, Rassy E, et al. Neoadjuvant treatment for newly diagnosed advanced ovarian cancer: where do we stand and where are we going? Ann Transl Med 2020;8:1710. [Crossref] [PubMed]
- Garrett CR, George B, Viswanathan C, et al. Survival benefit associated with surgical oophorectomy in patients with colorectal cancer metastatic to the ovary. Clin Colorectal Cancer 2012;11:191-4. [Crossref] [PubMed]
- Ganesh K, Shah RH, Vakiani E, et al. Clinical and genetic determinants of ovarian metastases from colorectal cancer. Cancer 2017;123:1134-43. [Crossref] [PubMed]
- Cho JH, Lim JY, Choi AR, et al. Comparison of Surgery Plus Chemotherapy and Palliative Chemotherapy Alone for Advanced Gastric Cancer with Krukenberg Tumor. Cancer Res Treat 2015;47:697-705. [Crossref] [PubMed]
- Koyama T, Mikami Y, Saga T, et al. Secondary ovarian tumors: Spectrum of CT and MR features with pathologic correlation. Abdom Imaging 2007;32:784-95. [Crossref] [PubMed]
- La Fianza A, Alberici E, Pistorio A, et al. Differential diagnosis of Krukenberg tumors using multivariate analysis. Tumori 2002;88:284-7. [Crossref] [PubMed]
- Ehteshami Bejnordi B, Veta M, van Johannes Diest P, et al. Diagnostic Assessment of Deep Learning Algorithms for Detection of Lymph Node Metastases in Women With Breast Cancer. JAMA 2017;318:2199-210. [Crossref] [PubMed]
- Esteva A, Kuprel B, Novoa RA, et al. Dermatologist-level classification of skin cancer with deep neural networks. Nature 2017;542:115-8. [Crossref] [PubMed]
- Erickson BJ, Korfiatis P, Kline TL, et al. Deep Learning in Radiology: Does One Size Fit All? J Am Coll Radiol 2018;15:521-6. [Crossref] [PubMed]
- Erickson BJ, Ryan WJ, Gehring DG. Functional requirements of a desktop clinical image display application. J Digit Imaging 2001;14:149-52. [Crossref] [PubMed]
- Ooms J. Package ‘magick’. 2020. Available online: https://cran.r-project.org/web/packages/magick/magick.pdf. Accessed 15 May 2020.
- . Howard J, Gugger S. Fastai: A Layered API for Deep Learning. Information 2020;11:108. [Crossref]
- Erickson BJ. Magician’s Corner: How to Start Learning about Deep Learning. Radiol Artif Intell 2019;1:e190072 [Crossref]
- He K, Zhang X, Ren S, et al. Deep Residual Learning for Image Recognition. arXiv:1512.03385 [cs.CV], 2015.
- Russakovsky O, Deng J, Su H, et al. ImageNet Large Scale Visual Recognition Challenge. arXiv:1409.0575 [cs.CV], 2014.
- Bruls J, Simons M, Overbeek LI, et al. A national population-based study provides insight in the origin of malignancies metastatic to the ovary. Virchows Arch 2015;467:79-86. [Crossref] [PubMed]
- Kiyokawa T, Young RH, Scully RE. Krukenberg tumors of the ovary: A clinicopathologic analysis of 120 cases with emphasis on their variable pathologic manifestations. Am J Surg Pathol 2006;30:277-99. [Crossref] [PubMed]
- Lionetti R, Luca M, de , Travaglino A, et al. Prognostic factors in Krukenberg tumor. Arch Gynecol Obstet 2019;300:1155-65. [Crossref] [PubMed]
- Wu F, Zhao X, Mi B, et al. Clinical characteristics and prognostic analysis of Krukenberg tumor. Mol Clin Oncol 2015;3:1323-8. [Crossref] [PubMed]
- Zhang C, Hou W, Huang J, et al. Effects of metastasectomy and other factors on survival of patients with ovarian metastases from gastric cancer: a systematic review and meta-analysis. J Cell Biochem 2019;120:14486-98. [Crossref] [PubMed]
- Ursem C, Zhou M, Paciorek A, et al. Clinicopathologic Characteristics and Impact of Oophorectomy for Ovarian Metastases from Colorectal Cancer. Oncologist 2020;25:564-71. [Crossref] [PubMed]
- Lee SJ, Lee J, Lim HY, et al. Survival benefit from ovarian metastatectomy in colorectal cancer patients with ovarian metastasis: a retrospective analysis. Cancer Chemother Pharmacol 2010;66:229-35. [Crossref] [PubMed]
- Aurello P, Berardi G, Antolino L, et al. Is a Surgical Approach Justified in Metachronous Krukenberg Tumor from Gastric Cancer? A Systematic Review. Oncol Res Treat 2018;41:644-9. [Crossref] [PubMed]
- Cheong JH, Hyung WJ, Chen J, et al. Surgical management and outcome of metachronous Krukenberg tumors from gastric cancer. J Surg Oncol 2004;87:39-45. [Crossref] [PubMed]
- Ma F, Li Y, Li W, et al. Metastasectomy Improves the Survival of Gastric Cancer Patients with Krukenberg Tumors: A Retrospective Analysis of 182 patients. Cancer Manag Res 2019;11:10573-80. [Crossref] [PubMed]
- Kim SH, Kim WH, Park KJ, et al. CT and MR findings of Krukenberg tumors: Comparison with primary ovarian tumors. J Comput Assist Tomogr 1996;20:393-8. [Crossref] [PubMed]
- Karaosmanoglu AD, Onur MR, Salman MC, et al. Imaging in secondary tumors of the ovary. Abdom Radiol (NY) 2019;44:1493-505. [Crossref] [PubMed]
- Ha HK, Baek SY, Kim SH, et al. Krukenberg's tumor of the ovary: MR imaging features. AJR Am J Roentgenol 1995;164:1435-9. [Crossref] [PubMed]
- Yedema CA, Kenemans P, Wobbes T, et al. Use of serum tumor markers in the differential diagnosis between ovarian and colorectal adenocarcinomas. Tumour Biol 1992;13:18-26. [Crossref] [PubMed]
- Bruchim I, Ben-Harim Z, Piura E, et al. Preoperative clinical and radiological features of metastatic ovarian tumors. Arch Gynecol Obstet 2013;288:615-9. [Crossref] [PubMed]
- Omranipour R, Abasahl A. Ovarian metastases in colorectal cancer. Int J Gynecol Cancer 2009;19:1524-8. [Crossref] [PubMed]


