
Proton stereotactic body radiation therapy for liver metastases—results of 5-year experience for 81 hepatic lesions
Introduction
The liver is a common organ for metastatic disease involvement in many solid malignancies including gastrointestinal, breast, and lung cancer. Among patients with colorectal cancer (CRC), approximately 50% will develop metastatic disease to the liver (1,2). Aggressive local treatment to these lesions can increase survival in certain scenarios. In patients with oligometastatic disease to the liver, there is even a possibility of long-term disease control. Hepatic resection is the standard of care in these situations with five-year survival rates generally around 25–45% (3-7). There is also data on surgical resection of liver metastases in patients with non-CRC primaries (8-10). Unfortunately, a majority of patients are not candidate for resection (11). Therefore, safe and effective treatments for metastatic hepatic disease are an important clinical need.
Other local therapy options for patients that develop liver metastases include radiofrequency ablation (RFA), cryotherapy, transarterial chemoembolization (TACE), yttrium-90 (Y90), and stereotactic body radiation therapy (SBRT). High rates of local control (LC) of hepatic disease appears to be a key determinant of overall survival (OS) (3,5,10,12,13). For example, Aloia et al. showed RFA was associated with a seven-fold increased risk of local failure and three-fold increased risk of death compared to resection despite similar rates of intrahepatic and extrahepatic failure among patients with solitary hepatic disease from CRC (13). A recent meta-analysis demonstrated superior LC for SBRT compared to RFA in the treatment of liver metastases (14). Along with good LC, the ideal treatment would also be associated with minimal morbidity. SBRT has been shown to be safe in dose escalation studies (15-18) with excellent control rates above 90% with biologically effective doses (BED) >100 Gy10 (19). Rusthoven et al. reported a 2-year LC rate of 94% for 38 lesions treated with 60 Gy in 3 fractions, with a 2-year LC rate of 100% for lesions 3 cm or less (16). SBRT also has the advantage over other local therapies of being non-invasive.
Proton therapy’s unique characteristics of the Bragg peak and a lack of exit dose can potentially provide additional benefits for Proton SBRT treatment of liver metastases. Multiple studies illustrate a dosimetric advantage of proton therapy compared to photon-based therapy in liver radiotherapy resulting in higher volumes of liver irradiation with photon therapy (20-22). Subsequent courses of treatment may be limited by prior liver radiation exposure with photon therapy. Proton SBRT limits the integral dose to the liver making subsequent courses of treatment more feasible. Joo et al. reported a 59% out-of-field recurrence rate in the liver for these patients showing the high probability of additional courses of liver directed therapy (23). Proton-based radiation treatment to the liver has also been shown to be safe in patients with hepatocellular carcinoma (HCC), who most often have underlying cirrhosis (24-26). Only one phase II Proton SBRT liver metastases study is published to date showing lower rates of LC compared to contemporary series (27). However, the authors note that this is likely related to a lower BED. A phase I dose escalation trial showed Proton SBRT to be safe and well tolerated with the maximal tolerated dose not reached at a dose of 60 GyE (Gray equivalent) (28).
In this study we analyzed all patients that were treated with Proton SBRT for liver metastases at our institution in the initial five years. The primary objective was to identify the LC rate of Proton SBRT in patients with metastatic liver disease. Secondary objectives include evaluation of OS and toxicity. We present the following article in accordance with the Strengthening the Reporting of Observational studies in Epidemiology (STROBE) reporting checklist. (available at: http://dx.doi.org/10.21037/jgo-20-424).
Methods
A retrospective analysis was completed of all 46 patients with 81 liver metastases that were treated at our institution between September 2012 and December 2017. The fractionation regimens, LC rates, OS, and toxicity were evaluated. Potential bias was reduced by analyzing every patient that was treated for liver metastases over this time period.
Fractionation schemes and dose constraints
Some patients were treated on our institutional phase I/II clinical trial (NCT01697371) with a 3-fraction regimen with dose escalation of 36, 48, and 60 GyE. The dose in proton therapy is defined in terms of effective dose, which is the physical dose in Gray multiplied by a relative biological effectiveness (RBE) of 1.1, which has been validated in the clinic (29). The 60 GyE regimen had no dose limiting toxicity and is used for the phase II trial with dose constraints specified by protocol (28). Other patients who did not meet eligibility criteria for the phase II trial were treated in 3 or 5 fractions. For the five-fraction regimen, common dose prescriptions included 30 and 50 GyE (range, 30–50 GyE). Dose constraints were adapted from the NRG BR001 protocol (NCT02206334). The liver constraint for three fractions is V17 <700 cc and for five fractions is V21 <700 cc. The stomach constraint for three fractions is V30 <0.03 cc and V22.5 <10 cc and for five fractions is V35 <0.5 cc and V26.5 <5 cc. The small bowel constraint for three fractions is V34.5 <0.03 cc and V24 <20 cc and for five fractions is V40 <0.03 cc and V28.5 <20 cc.
Immobilization and tumor volume definition
Patients underwent computed tomography (CT) simulation with intravenous contrast with a full body pod for immobilization, as previously described (30). Tumor motion was accounted for by either four-dimensional CT (4DCT) or deep inspiration breath hold (DIBH) (SDX, Qfix, Avondale, PA).
For patients using 4DCT, an internal target volume (ITV) was defined to encompass the enhancing or hypoattenuating lesion on the maximum intensity projection (MIP) scan (31). For patients using SDX, gross tumor volume (GTV) was defined to encompass the enhancing or hypoattenuating lesion on the CT scan with contrast. The GTV was then expanded by a 5 mm radial and 10 mm craniocaudal margin to create the ITV (16). Additionally, each passively scattered proton field was optimized to account for proton beam modulation, Bragg peak, depth dose, uncertainty, and energy optimization. A multi-beam, computer-generated treatment plan was created to deliver the prescription dose in 3 or 5 equal fractions.
Statistical analysis
LC was defined according to Response Evaluation Criteria in Solid Tumors (RECIST) guidelines (version 1.1) (32). OS was assessed as the time period between completion of the last treatment and either death by any cause, or censored at last known follow up. The Kaplan-Meier method was used to estimate LC and OS. Statistical analysis was completed using Python (33).
Toxicity
Acute toxicity was graded according to Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. Specific factors of interest included evaluation for radiation-induced liver disease (RILD). RILD is a clinical syndrome of anicteric ascites, hepatomegaly, and elevation of alkaline phosphatase (ALP) relative to other transaminases that may occur 2 weeks to 3 months following radiation to the liver. Grade 3 ALP (>5 times the upper limit of normal) with ascites not due to cancer progression was considered RILD. Other evaluated toxicity included toxicity to any related organ system within 90 days from the start of treatment.
Follow up
Patients were planned to be evaluated once during the treatment course, at 4 weeks after treatment, and at 3 months after treatment. Evaluation included physical examination, laboratory values, follow-up imaging (starting at 3 months), and a detailed account of possible symptoms. Any observed toxicity within 90 days of treatment was recorded as acute toxicity. Regular follow-up was planned to continue at 3-month intervals to evaluate treatment response. In cases of missing follow-up data, patients were censored when last known to be alive.
Ethical statement
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of Loma Linda University Health (IRB#5200383) and informed consent was taken from all the patients.
Results
Patient population
Forty-six patients with 81 total lesions were treated with Proton SBRT. Patient characteristics are listed in Table 1. The median age was 65.5 years old (range, 33–86 years). The median follow up was 15 months (range, 1–54 months). Half of the patients were male (n=23) and half were female (n=23) and 87% of patients had an Eastern Cooperative Oncology Group (ECOG) score of 0. The primary site of disease was colorectal in 50.0% of cases. Extrahepatic disease was present in 43.5% of patients with 19.6% receiving some sort of prior local therapy to the liver before Proton SBRT. Two or more lesions were treated in 56.5% of patients, with one patient receiving treatment to a total of five lesions. The median size of the GTV was 2.5 cm (range, 0.7–8.9 cm) with a median volume of 4.07 cc (range, 0.23–125.58 cc). A majority of patients (89.1%) underwent 4DCT for immobilization.
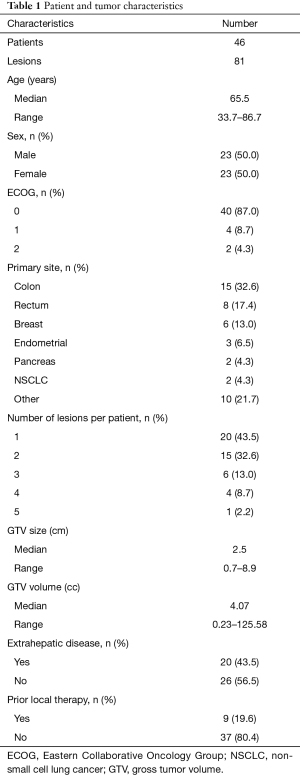
Full table
Fractionation schemes and BED
For the three-fraction treatment, doses included 27 GyE (27.8%), 30 GyE (16.7%), 36 GyE (19.4%), 48 Gy (19.4%), and 60 GyE (16.7%). For the five-fraction treatment, common doses included 30 GyE (46.7%) and 50 GyE (40%). There was a large range of BEDs that were used for the 81 lesions that were treated. The BED was calculated assuming an alpha/beta ratio of 10. There were 37 lesions treated with a BED ≤60, 9 lesions with a BED of 61–80, 22 lesions with a BED of 81–100, and 13 lesions with a BED >100. A common prescription in the low BED group was 30 GyE in 5 fractions, which was often used to meet the dose constraint for small bowel or stomach when it was in close proximity to the target volume.
Clinical outcomes
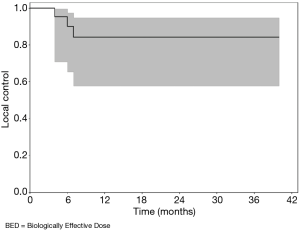
LC at 6 months was 97.3% (95% CI, 89.8% to 99.3%) for the 81 lesions evaluated. LC at 1 year and 2 years was 92.5% (95% CI, 82.7% to 96.8%). LC at 3 years was 71.9% (95% CI, 53.2% to 84.2%) (see Figure 1). The longest duration of LC was in a patient who had 54 months follow-up, but there were also 10 other patients that had continued LC >45 months after treatment.
LC for patients that had a BED of ≥100 is illustrated in Figure 2. In these 13 lesions, the LC at 6 months was 90.0% (95% CI, 65.3% to 97.4%). The LC at 1 year was 84.0% (95% CI, 57.8% to 94.6%). Three of the 13 patients experienced local failure after the 7-month mark, after which the curve remains flat. Two of these lesions were large measuring 5.4 and 6.9 cm. The third lesion was a 1.5 cm lesion from a primary rectal cancer that received 48 GyE in 3 fractions. This patient also had a 1.3 cm lesion that was treated with 36 GyE in 3 fractions, which also experienced treatment failure at the same time point.
LC was also analyzed for 37 lesions that received a BED of ≤60. The median tumor size among these 37 lesions was 1.8 cm. The LC at 6 months was 97.1% (95% CI, 81.4% to 99.6%). The 1 year and 2-year LC rates were 90.4% (95% CI, 73.1% to 96.8%). The 3-year LC rate was 64.7% (95% CI, 33.7% to 84.1%). This data is not presented in graphical form.
The median survival for the 46 patients was 30.0 months. The 1-year OS was 68.1% (95% CI, 52.1% to 79.8%), 2-year OS was 58.1% (95% CI, 41.8% to 72.1%), 3-year OS was 43.8% (95% CI, 27.3% to 58.1%), and 4-year OS was 20.1% (95% CI, 6.6% to 40.2%) (see Figure 3). At the time of analysis eight patients were still alive.
Toxicity
Grade 1 toxicity was experienced in 17 of 46 (37.0%) patients and grade 2 toxicity was experienced in 3 of 46 patients (6.5%). No grade 3 or higher toxicities were reported and no cases of RILD were detected. The median percentage of liver that received no radiation was 59.9% (range, 25.0–91.7%). Among the 17 patients that experienced a grade 1 toxicity, 10 patients (58.8%) experienced elevated liver enzymes, four patients (23.5%) experienced fatigue, one patient (5.9%) experienced skin hyperpigmentation, and two patients (11.8%) experienced both elevated liver enzymes and skin hyperpigmentation. Among the three patients that experienced grade 2 toxicity, two patients experienced fatigue that was not relieved by rest, which limited instrumental activities of daily living (ADL) and the third patient experienced a greater increase in liver enzymes (see Figure 4).
Discussion
In this study, we report the results of our 5-year institutional experience treating liver metastases with Proton SBRT. The 2-year LC for all 81 lesions treated was 92.5%. There was no grade 3 or higher toxicity and no incidences of RILD.
The LC was high despite a large number of patients treated with a low BED. A 2-year LC of 90.4% for 37 lesions treated with a BED ≤60 was unexpected. Smaller tumor size in this cohort (median 1.8 cm) is a possible contributor. It is also possible that a lower BED can delay the disease progression, but may not lead to a durable response, as the 3-year LC rate dropped to 64.7%. The 2-year LC rate was 84.0% for 13 lesions treated with a BED ≥100. The lower LC compared to the lower BED cohort may be explained by two large lesions that had early local failure. Liver metastases greater than 5 cm are typically excluded from most SBRT protocols. The curve remains flat after these early failures suggesting a durable response for the other lesions treated with a high BED.
LC, the primary end point in this study, was similar to photon-based SBRT as expected (16,19). However, the main advantage of Proton SBRT is the reduced integral liver dose providing the option of safely delivering multiple courses of treatment. When patients are considered for hepatic resection, the limit for safe resection is generally 20–30% of remnant liver (34). The median percentage of liver that received no radiation dose from our data was 59.9%, despite a majority of patients receiving two or more courses of treatment. This is significant as the out-of-field recurrence rate in the liver can be high (23). Therefore, preserving the possibility for additional courses of SBRT in the future is a major benefit of Proton SBRT.
The MS was 30 months, which is high considering almost half of patients had extrahepatic disease. This partially reflects the efficacy of systemic treatment. In general, patients were considered for Proton SBRT to new or progressing liver metastases if the extrahepatic disease was stable on systemic treatment. The 4-year OS was 20.1% and eight patients were still alive at the time of this analysis. Since LC of liver metastases appears to be associated with OS (3,5,10,12,13), SBRT offers the possibility of long-term survival in some patients with oligometastatic disease even if they are not candidates for hepatic resection.
Proton SBRT appears to be safe with no patients experiencing grade 3 or higher toxicity or RILD. This is significant considering 56.5% of patients completed treatment to two or more liver metastases. Routine use of DIBH for those who can tolerate it, as opposed to 4DCT, can further reduce integral liver dose, potentially increasing the benefit of Proton SBRT even further.
Our phase I dose escalation study established the safety of the 60 GyE in 3 fraction dose regimen (28). This is the preferred regimen for treatment now as long as there are no dose-limiting adjacent structures. For lesions adjacent to chest wall or rib, we use 50 GyE in 5 fractions to reduce the risk of chest wall pain or rib fracture (35). We use 30 GyE in 5 fractions in patients that have liver metastasis near stomach or bowel. However, an alternative local treatment modality should be considered, if possible, as durable LC is expected to be low in these situations.
The limitations of this study include the common limitations that apply to any observational study. All patients treated with Proton SBRT for liver metastases over this time period were analyzed to reduce selection bias. However, patients that received Proton SBRT for liver metastases were likely carefully selected by the referring provider based on performance status and ability to tolerate treatment. These results are likely not generalizable to all patients with liver metastases, especially patients with poor performance status or inadequate hepatic reserve.
In conclusion, Proton SBRT has high LC rates with an excellent toxicity profile for treatment of liver metastases even when a majority of patients received multiple courses of treatment. We continue to accrue patients for our Phase II study treating liver metastases with Proton SBRT to 60 GyE in 3 fractions.
Acknowledgments
Sandra C. Teichman for her assistance in the IRB approval process.
Funding: None.
Footnote
Reporting Checklist: The authors have completed the STROBE reporting checklist. Available at: http://dx.doi.org/10.21037/jgo-20-424
Data Sharing Statement: Available at: http://dx.doi.org/10.21037/jgo-20-424
Peer Review File: Available at: http://dx.doi.org/10.21037/jgo-20-424
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at: http://dx.doi.org/10.21037/jgo-20-424). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Institutional Review Board (IRB) of Loma Linda University Health (IRB#5200383) and informed consent was taken from all the patients.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Welch JP, Donaldson GA. The clinical correlation of an autopsy study of recurrent colorectal cancer. Ann Surg 1979;189:496-502. [PubMed]
- Geoghegan JG, Scheele J. Treatment of colorectal liver metastases. Br J Surg 1999;86:158-69. [Crossref] [PubMed]
- Rees M, Tekkis PP, Welsh FK, et al. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-35. [Crossref] [PubMed]
- Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-18; discussion 318-21. [Crossref] [PubMed]
- Wei AC, Greig PD, Grant D, et al. Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 2006;13:668-76. [Crossref] [PubMed]
- Robertson DJ, Stukel TA, Gottlieb DJ, et al. Survival after hepatic resection of colorectal cancer metastases: a national experience. Cancer 2009;115:752-9. [Crossref] [PubMed]
- Morris EJ, Forman D, Thomas JD, et al. Surgical management and outcomes of colorectal cancer liver metastases. Br J Surg 2010;97:1110-8. [Crossref] [PubMed]
- Takemura N, Saiura A. Role of surgical resection for non-colorectal non-neuroendocrine liver metastases. World J Hepatol 2017;9:242-51. [Crossref] [PubMed]
- Lendoire J, Moro M, Andriani O, et al. Liver resection for non-colorectal, non-neuroendocrine metastases: analysis of a multicenter study from Argentina. HPB (Oxford) 2007;9:435-9. [Crossref] [PubMed]
- Chua TC, Saxena A, Liauw W, et al. Hepatic resection for metastatic breast cancer: a systematic review. Eur J Cancer 2011;47:2282-90. [Crossref] [PubMed]
- Scheele J, Stangl R, Altendorf-Hofmann A. Hepatic metastases from colorectal carcinoma: impact of surgical resection on the natural history. Br J Surg 1990;77:1241-6. [Crossref] [PubMed]
- Chang DT, Swaminath A, Kozak M, et al. Stereotactic body radiotherapy for colorectal liver metastases: a pooled analysis. Cancer 2011;117:4060-9. [Crossref] [PubMed]
- Aloia TA, Vauthey JN, Loyer EM, et al. Solitary colorectal liver metastasis: resection determines outcome. Arch Surg 2006;141:460-6; discussion 466-7. [Crossref] [PubMed]
- Lee J, Shin IS, Yoon WS, et al. Comparisons between radiofrequency ablation and stereotactic body radiotherapy for liver malignancies: Meta-analyses and a systematic review. Radiother Oncol 2020;145:63-70. [Crossref] [PubMed]
- Schefter TE, Kavanagh BD, Timmerman RD, et al. A phase I trial of stereotactic body radiation therapy (SBRT) for liver metastases. Int J Radiat Oncol Biol Phys 2005;62:1371-8. [Crossref] [PubMed]
- Rusthoven KE, Kavanagh BD, Cardenes H, et al. Multi-institutional phase I/II trial of stereotactic body radiation therapy for liver metastases. J Clin Oncol 2009;27:1572-8. [Crossref] [PubMed]
- Goodman KA, Wiegner EA, Maturen KE, et al. Dose-escalation study of single-fraction stereotactic body radiotherapy for liver malignancies. Int J Radiat Oncol Biol Phys 2010;78:486-93. [Crossref] [PubMed]
- Rule W, Timmerman R, Tong L, et al. Phase I dose-escalation study of stereotactic body radiotherapy in patients with hepatic metastases. Ann Surg Oncol 2011;18:1081-7. [Crossref] [PubMed]
- Ohri N, Tomé WA, Méndez Romero A, et al. Local Control After Stereotactic Body Radiation Therapy for Liver Tumors. Int J Radiat Oncol Biol Phys 2021;110:188-95. [Crossref] [PubMed]
- Wang X, Krishnan S, Zhang X, et al. Proton radiotherapy for liver tumors: dosimetric advantages over photon plans. Med Dosim 2008;33:259-67. [Crossref] [PubMed]
- Petersen JB, Lassen Y, Hansen AT, et al. Normal liver tissue sparing by intensity-modulated proton stereotactic body radiotherapy for solitary liver tumours. Acta Oncol 2011;50:823-8. [Crossref] [PubMed]
- Chuong M, Kaiser A, Molitoris J, et al. Proton beam therapy for liver cancers. J Gastrointest Oncol 2020;11:157-65. [Crossref] [PubMed]
- Joo JH, Park JH, Kim JC, et al. Local Control Outcomes Using Stereotactic Body Radiation Therapy for Liver Metastases From Colorectal Cancer. Int J Radiat Oncol Biol Phys 2017;99:876-83. [Crossref] [PubMed]
- Nakayama H, Sugahara S, Tokita M, et al. Proton beam therapy for hepatocellular carcinoma: the University of Tsukuba experience. Cancer 2009;115:5499-506. [Crossref] [PubMed]
- Fukumitsu N, Sugahara S, Nakayama H, et al. A prospective study of hypofractionated proton beam therapy for patients with hepatocellular carcinoma. Int J Radiat Oncol Biol Phys 2009;74:831-6. [Crossref] [PubMed]
- Bush DA, Kayali Z, Grove R, et al. The safety and efficacy of high-dose proton beam radiotherapy for hepatocellular carcinoma: a phase 2 prospective trial. Cancer 2011;117:3053-9. [Crossref] [PubMed]
- Hong TS, Wo JY, Borger DR, et al. Phase II Study of Proton-Based Stereotactic Body Radiation Therapy for Liver Metastases: Importance of Tumor Genotype. J Natl Cancer Inst 2017; [Crossref] [PubMed]
- Kang JI, Sufficool DC, Hsueh CT, et al. A phase I trial of Proton stereotactic body radiation therapy for liver metastases. J Gastrointest Oncol 2019;10:112-7. [Crossref] [PubMed]
- Paganetti H, Niemierko A, Ancukiewicz M, et al. Relative biological effectiveness (RBE) values for proton beam therapy. Int J Radiat Oncol Biol Phys 2002;53:407-21. [Crossref] [PubMed]
- Wroe AJ, Bush DA, Schulte RW, et al. Clinical immobilization techniques for proton therapy. Technol Cancer Res Treat 2015;14:71-9. [Crossref] [PubMed]
- Knecht ML, Wang N, Vassantachart A, et al. Individualized 4-dimensional computed tomography proton treatment for pancreatic tumors. J Gastrointest Oncol 2017;8:675-82. [Crossref] [PubMed]
- Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228-47. [Crossref] [PubMed]
- Rossum GV, Drake FL. Python 3 Reference Manual: CreateSpace; 2009.
- Guglielmi A, Ruzzenente A, Conci S, et al. How much remnant is enough in liver resection? Dig Surg 2012;29:6-17. [Crossref] [PubMed]
- Stephans KL, Djemil T, Reddy CA, et al. A comparison of two stereotactic body radiation fractionation schedules for medically inoperable stage I non-small cell lung cancer: the Cleveland Clinic experience. J Thorac Oncol 2009;4:976-82. [Crossref] [PubMed]





