
Clinicopathological and prognostic significance of programmed death ligant-1 expression in gastric cancer: a meta-analysis
Introduction
Gastric cancer (GC) is a common and life-threatening malignant tumor of the digestive system with a high incidence in China. Most patients are in the middle or advanced stage of the disease when they commence treatment and the 5-year survival rate is only 20–50% (1), placing GC as the second highest mortality cancer in the world. The molecular mechanisms underpinning the occurrence and development of GC are complex and the role played by immune escape and the design of therapies to address it are of increasing interest (2). The use of immune checkpoint inhibitors in particular has become the focus of tumor immunotherapy in recent years.
Programmed death 1 (PD-1) is an important immunosuppressive molecule that regulates tumor growth, prevents excessive inflammatory responses, and maintains the survival of transplanted donors. The ligand of PD-1, programmed death ligand-1 (PD-L1/B7-H1), is widely expressed in various types of tumor and immune cells (3), and has the effect of immune surveillance (4). PD-L1 is expressed in the anti-tumor T cell response of various solid tumors including renal cell carcinoma and breast, pancreatic, colorectal, and esophageal cancer (5,6). It inhibits the proliferation and activity of T cells and plays a significant role in the negative regulation of the immune response (7). In addition, PD-L1 expression is associated with poor prognosis in patients with various malignant solid tumors including GC (8,9) and is valuable in predicting prognosis and clinicopathological characteristics and as a biomarker in the tumor microenvironment (10-12). Its targeted immunoregulation is of great significance in the treatment of infection, tumors and autoimmune diseases, and the corresponding antibodies have the same effect (13). At present, there are many studies on the clinicopathological and prognostic significance of PD-L1 expression in GC, but the results are not consistent. We performed a meta-analysis to clarify the correlation of PD-L1 expression with clinicopathology and prognosis in GC.
We present the following article in accordance with the PRISMA reporting checklist (available at http://dx.doi.org/10.21037/jgo-20-568).
Methods
Retrieval strategy
We searched the PubMed, ScienceNet, EMbase, CNKI, and Wanfang databases for publications appearing between January 2010 and April 2020 using the key terms PD-L1, PD-1, programmed death cell 1, programmed death 1 ligand, and gastric cancer or GC. The literature was collected by the combination of subject words and free words according to the characteristics of different databases.
Inclusion and exclusion criteria
The following inclusion criteria were used: (I) all patients were pathologically diagnosed with GC; (II) PD-L1 expression was obtained by immunohistochemical (IHC) method; (III) the relationship between PD-L1 expression and the prognosis of GC was clarified; (IV) all GC patients had been followed up and the results were reported; (V) treatises, not reviews, case reports, conference abstract, and others were included; (VI) articles contained complete research data; (VII) the full text was in English; (VIII) the Newcastle-Ottawa Quality Assessment Scale (NOS) score was ≥6 points.
Data extraction and quality evaluation
The following data were extracted: name of the first author, year of publication, country, total cases, survival rate, number of PD-L1 positive cases, number of PD-L1 negative cases, tumor stage, and presence of lymph node metastasis. If a clear survival report was not available, the Kaplan-Meier curve was read for survival information through Engauge Digitizer 4.1.
The quality of the articles was evaluated by NOS, with the highest score being 9. A score ≥6 was rated as high quality.
Statistical analysis
Meta-analysis was performed by Stata15.0 software (StataCorp., College Station, TX). The data were analyzed by hazard ratio (HR), odds ratio (OR), and 95% confidence interval (CI). Heterogeneity between studies was judged with I2 and Q tests. If there was no heterogeneity (P>0.10, I2<50%), the fixed effect model (FEM) was used for analysis and the random effect model (REM) then used for analysis. Sensitivity analysis was performed to evaluate the stability of the results by eliminating the study results one by one, that is, removing the effect of each study on the overall results to explore the source of heterogeneity. Publication bias was analyzed by funnel plot.
Results
Results of literature screening
A total of 761 related studies were obtained and after reading the title, abstract and full text, 704 articles were excluded. A further 42 articles with other intervention measures and low quality but without a control group were also excluded. Finally, 15 articles (14-28) were included in the meta-analysis, with a total of 3,218 patients. The screening procedure is shown in Figure 1.
Research characteristics
Basic research characteristics of the 15 included studies are shown in Table 1. The NOS score ranged from 7 to 8, indicating that all were high-quality studies.
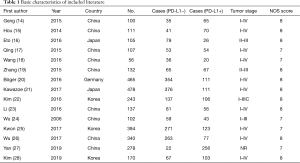
Full table
The total sample size was 3,218 cases, with individual sample sizes ranging from 56 to 478. The studies were from four countries, including nine from China (14,15,17-19,23,24,26,27), three from South Korea (22,25,28), two from Japan (16,21), and one from Germany (20). The relationship between PD-L1 and 3-year survival was provided in 15 studies (14-28) and 12 (14,16,19-28) reported the relationship between PD-L1 and 5-year survival. The correlation between PD-L1 and lymphatic metastasis was reported in 12 studies (14,16,19-28) and among these, 10 studies recruited patients in stage I–IV in GC (14,15,17,18,20,21,23,25,26,28), and four recruited GC patients in stage I–III (16,19,22,24).
Relationship between PD-L1 expression and 3-year survival rate in GC patients
The 3-year survival rate was reported in all 15 studies. There was significant heterogeneity between the studies (I2=78.1%, P<0.001), so the REM was used. The results show that positive expression of PD-L1 was related to a decrease in the 3-year survival rate (HR =1.23, 95% CI: 1.02–1.49, P=0.028) and in the subgroup analysis of ethnicity, the positive expression of PD-L1 in Asians was also related to a decrease in the 3-year survival rate (HR =1.29, 95% CI: 1.06–1.57, P=0.010) (Figure 2).
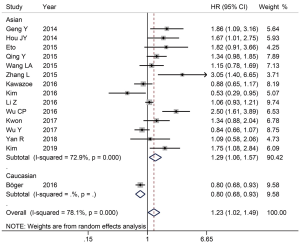
Relationship between PD-L1 expression and 5-year survival rate in GC patients
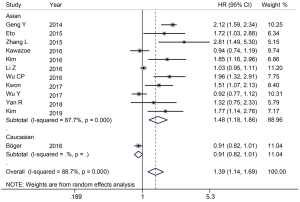
The 5-year survival rate was reported in 12 studies (14,16,19-28). There was significant heterogeneity between the studies (I2=88.7%, P<0.001) (Figure 3), so the REM was used. The result showed that the positive expression of PD-L1 was related to a decrease in the 5-year survival rate (HR =1.39, 95% CI: 1.14–1.69, P=0.001). In the subgroup analysis of ethnicity, the positive expression of PD-L1 in Asians was also related to a decrease in the 5-year survival rate (HR =1.48, 95% CI: 1.18–1.86, P=0.001) (Figure 3).
Relationship between PD-L1 expression and clinicopathological characteristics in GC patients
To further understand the role of PD-L1 as a biomarker, we investigated the relationship between PD-L1 expression and the clinicopathological features of GC using REM. The correlation between PD-L1 expression and lymphatic metastasis was reported in 12 studies (14,16,19-28) and there was significant heterogeneity among them (I2=73.9%, P<0.001). The positive expression of PD-L1 in the group with lymph node metastasis was 1.73 times higher than that in the group without lymph node metastasis, and the difference was statistically significant (OR =1.73, 95% CI: 1.18–2.54, P<0.01) (Figure 4A). The result showed that patients with higher expressions of PD-L1 are more prone to lymph node metastasis.
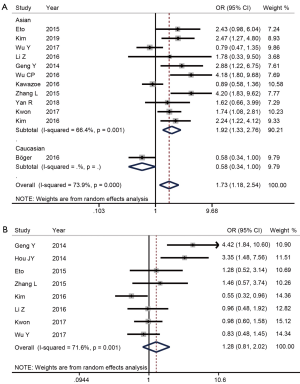
The correlation between PD-L1 expression and tumor stage was reported in eight (14-16,19,22,23,25,26) studies and there was significant heterogeneity among them (I2=71.6%, P<0.001). There was no significant difference in PD-L1 expression among different tumor stages (OR =1.28, 95% CI: 0.81–2.02, P=0.292) (Figure 4B).
Sensitivity analysis and publication bias
The heterogeneity of 3-year (Figure 5A) and 5-year (Figure 5B) survival rates was high among the studies. After eliminating the study results one by one, sensitivity analysis revealed no significant change in overall heterogeneity, which indicated that the results were robust. Publication bias was analyzed by funnel plot. The 3-year (Figure 6A) and 5-year (Figure 6B) survival rates of the included literature were mostly in the funnel plot, indicating that there was a small publication bias.


Discussion
An increasing number of studies show that PD-L1 is highly expressed in a variety of malignant tumors and can be used as a marker in tumor prognosis. However, its relationship with survival in GC patients remains controversial. Statistical analysis of the 15 articles and 3,218 patients assessed in this study showed that the 3- and 5-year overall survival rates of patients with positive expression of PD-L1 were significantly lower than those in whom it was negatively expressed. The positive expression of PD-L1 was more common in patients with positive lymphatic metastasis but had no correlation with tumor stage. Therefore, PD-L1 expression can be used as a reliable indicator for monitoring the clinical prognosis of GC patients.
Studies have shown that PD-L1 is barely expressed in normal gastric tissues, while its expression level is significantly up-regulated in GC tissues (29). In the tumor microenvironment, overexpression of PD-L1 can produce immunosuppressive effects (30) and promote the immune escape of tumor cells. Blocking this signal pathway can induce T cells to activate and kill tumor cells, enhancing the endogenous anti-tumor effect to provide a new approach to the treatment of GC (31). Consistent with the results of this study, many others have shown that patients with high PD-L1 expression have a poor 5-year overall survival rate, indicating PD-L1 is closely related to prognosis (32-34). While a variety of PD-L1 monoclonal antibodies have been applied in the clinical treatment of cancer patients with good clinical efficacy (35,36) there remain few clinical studies on PD-L1 monoclonal antibodies in GC patients. The present study shows that PD-L1 is more common in GC patients with lymph node metastasis, which may provide a theoretical basis for its use in evaluating the prognosis of GC.
There are several limitations to this study. Firstly, the number of publications in the meta-analysis is small. Secondly, although subgroup analysis was performed, only one publication concerned Caucasian groups. Finally, the heterogeneity of the results is large, which is caused by many factors including technical deviation due to test methods and operators. Larger, multicenter prospective cohort studies of the predictive role of PD-L1 expression in GC are needed to verify our results.
Conclusions
In summary, we found that the high expression of PD-L1 decreases 3- and 5-year survival rates and promotes lymph node metastasis in GC patients although this was not related to tumor stage. PD-L1 is a negative factor in GC prognosis, and whether it can be used as prognostic factor in GC requires verification through high-quality prospective studies with uniform criteria. Further studies on the mechanism of PD-L1 overexpression in GC will also provide more reliable evidence for individualized treatment of GC.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the PRISMA reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-568
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-568). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Meyer HJ, Wilke H. Treatment strategies in gastric cancer. Dtsch Arztebl Int 2011;108:698-705, 706. [PubMed]
- Wang X, Teng F, Kong L, et al. PD-L1 expression in human cancers and its association with clinical outcomes. Onco Targets Ther 2016;9:5023-39. [Crossref] [PubMed]
- Sunshine J, Taube JM. PD-1/PD-L1 inhibitors. Curr Opin Pharmacol 2015;23:32-8. [Crossref] [PubMed]
- Ling Q, Jing H. Clinical research progress of PD-1/PD-L1 checkpoint inhibitors in advanced gastric cancer. Chinese Journal of Biochemical Pharmaceutics 2016;36:4-7.
- Strome SE, Dong H, Tamura H, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res 2003;63:6501-5. [PubMed]
- Hirano F, Kaneko K, Tamura H, et al. Blockade of B7-H1 and PD-1 by monoclonal antibodies potentiates cancer therapeutic immunity. Cancer Res 2005;65:1089-96. [PubMed]
- Keir ME, Butte MJ, Freeman GJ, et al. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol 2008;26:677-704. [Crossref] [PubMed]
- Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A 2004;101:17174-9. [Crossref] [PubMed]
- Huang B, Chen L, Bao C, et al. The expression status and prognostic significance of programmed cell death 1 ligand 1 in gastrointestinal tract cancer: a systematic review and meta-analysis. Onco Targets Ther 2015;8:2617-25. [PubMed]
- Boussiotis VA. Molecular and Biochemical Aspects of the PD-1 Checkpoint Pathway. N Engl J Med 2016;375:1767-78. [Crossref] [PubMed]
- Zhang T, Xie J, Arai S, et al. The efficacy and safety of anti-PD-1/PD-L1 antibodies for treatment of advanced or refractory cancers: a meta-analysis. Oncotarget 2016;7:73068-79. [Crossref] [PubMed]
- Haiming F, Ye Z, Tao J, et al. Efficacy and safety of anti PD-1/PD-L1 antibodies in the treatment of advanced nonsmall cell lung cancer: A meta-analysis. Chinese Journal of Evidence-Based Medicine 2017;17:144-51.
- Zhang F, Qiao J, Wang Y, et al. Expression and clinical significance of PD-1/PD-L1 in gastric cancer tissues. Chinese Journal of Cancer Biotherapy 2018;25:170-6.
- Geng Y, Wang H, Lu C, et al. Expression of costimulatory molecules B7-H1, B7-H4 and Foxp3+ Tregs in gastric cancer and its clinical significance. Int J Clin Oncol 2015;20:273-81. [Crossref] [PubMed]
- Hou J, Yu Z, Xiang R, et al. Correlation between infiltration of FOXP3+ regulatory T cells and expression of B7-H1 in the tumor tissues of gastric cancer. Exp Mol Pathol 2014;96:284-91. [Crossref] [PubMed]
- Eto S, Yoshikawa K, Nishi M, et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 2016;19:466-71. [Crossref] [PubMed]
- Qing Y, Li Q, Ren T, et al. Upregulation of PD-L1 and APE1 is associated with tumorigenesis and poor prognosis of gastric cancer. Drug Des Devel Ther 2015;9:901-9. [Crossref] [PubMed]
- Wang LA, Wei X, Li Q, et al. The prediction of survival of patients with gastric cancer with PD-L1 expression using contrast-enhanced ultrasonography. Tumour Biol 2016;37:7327-32. [Crossref] [PubMed]
- Zhang L, Qiu M, Jin Y, et al. Programmed cell death ligand 1 (PD-L1) expression on gastric cancer and its relationship with clinicopathologic factors. Int J Clin Exp Pathol 2015;8:11084-91. [PubMed]
- Böger C, Behrens HM, Mathiak M, et al. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 2016;7:24269-83. [Crossref] [PubMed]
- Kawazoe A, Kuwata T, Kuboki Y, et al. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer 2017;20:407-15. [Crossref] [PubMed]
- Kim JW, Nam KH, Ahn SH, et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 2016;19:42-52. [Crossref] [PubMed]
- Li Z, Lai Y, Sun L, et al. PD-L1 expression is associated with massive lymphocyte infiltration and histology in gastric cancer. Hum Pathol 2016;55:182-9. [Crossref] [PubMed]
- Wu C, Zhu Y, Jiang J, et al. Immunohistochemical localization of programmed death-1 ligand-1 (PD-L1) in gastric carcinoma and its clinical significance. Acta Histochem 2006;108:19-24. [Crossref] [PubMed]
- Kwon MJ, Kim KC, Nam ES, et al. Programmed death ligand-1 and MET co-expression is a poor prognostic factor in gastric cancers after resection. Oncotarget 2017;8:82399-414. [Crossref] [PubMed]
- Wu Y, Cao D, Qu L, et al. PD-1 and PD-L1 co-expression predicts favorable prognosis in gastric cancer. Oncotarget 2017;8:64066-82. [Crossref] [PubMed]
- Yan R, Yang X, Wang X, et al. Association Between Intra-Tumoral Immune Response and Programmed Death Ligand 1 (PD-L1) in Gastric Cancer. Med Sci Monit 2019;25:6916-21. [Crossref] [PubMed]
- Kim JH, Kim SY, Shin EY, et al. Expression patterns of programmed death-1 and programmed death-1 ligand-1 on T cells in gastric cancer. Oncol Lett 2019;18:2661-9. [PubMed]
- Sheng W, Jingyi S, Fang W, et al. Expression and clinical significance of PD-L1 and PD-1 in gastric carcinoma. Acta Universitatis Medicinalis Anhui 2015;50:821-5.
- Gani F, Nagarajan N, Kim Y, et al. Program Death 1 Immune Checkpoint and Tumor Microenvironment: Implications for Patients With Intrahepatic Cholangiocarcinoma. Ann Surg Oncol 2016;23:2610-17. [Crossref] [PubMed]
- Minn AJ, Wherry EJ. Combination Cancer Therapies with Immune Checkpoint Blockade: Convergence on Interferon Signaling. Cell 2016;165:272-75. [Crossref] [PubMed]
- Kantekure K, Yang Y, Raghunath P, et al. Expression patterns of the immunosuppressive proteins PD-1/CD279 and PD-L1/CD274 at different stages of cutaneous T-cell lymphoma/mycosis fungoides. Am J Dermatopathol 2012;34:126-8. [Crossref] [PubMed]
- Mahoney KM, Rennert PD, Freeman GJ. Combination cancer immunotherapy and new immunomodulatory targets. Nat Rev Drug Discov 2015;14:561-84. [Crossref] [PubMed]
- Gadiot J, Hooijkaas AI, Kaiser AD, et al. Overall survival and PD-L1 expression in metastasized malignant melanoma. Cancer 2011;117:2192-201. [Crossref] [PubMed]
- Tumeh PC, Harview CL, Yearley JH, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014;515:568-71. [Crossref] [PubMed]
- Dai C, Lin F, Geng R, et al. Implication of combined PD-L1/PD-1 blockade with cytokine-induced killer cells as a synergistic immunotherapy for gastrointestinal cancer. Oncotarget 2016;7:10332-44. [Crossref] [PubMed]
(English Language Editor: B. Draper)



