
Crocin reverses 1-methyl-3-nitroso-1-nitroguanidine (MNNG)-induced malignant transformation in GES-1 cells through the Nrf2/Hippo signaling pathway
Introduction
Gastric cancer (GC) is a common and potentially life-threatening malignant tumor. Globally, GC still ranks fourth and second of all malignancies for morbidity and mortality, respectively, despite considerable advances in anesthesia, surgery, post-operative care, and interventional radiology (1). East Asian countries, especially China, have high incidences of GC (2). In 2019, the National Cancer Center of China reported GC to have morbidity and mortality rates of 10.26% and 12.45%, respectively. The occurrence and development of GC are recognized as being extremely complex processes, the underlying mechanisms of which are still unclear. Due to poor diet (3), especially excessive intake of foods containing N-nitroso compounds, and patients often being at the advanced stage at the time of diagnosis, the prognosis for GC is often poor (4). Therefore, the study of malignant transformation of gastric cancer is extremely important to prevent the occurrence of GC.
Salt foods contain high levels of N-nitro compounds (NOC), which are strong carcinogens and key factors for gastric cancer (5). 1-methyl-3-nitroso-1-nitroguanidine (MNNG) is an active oncogenic NOCs, that has been successfully used to establish animal models of gastric cancer. The human gastric mucosal epithelial cells can be induced by MNNG into precancerous cell model (6), which is widely used to study the mechanism of gastric carcinogenesis (7). Currently, no effective treatment for malignant transformation of gastric mucosal epithelial cells exists in Western medicine; therefore, the beneficial role of traditional Chinese medicine (TCM) in disease has attracted a wide interest.
In recent years, TCM has been widely used in the study of gastric carcinogenesis (8,9). Saffron (Crocus sativus L.) is a valuable TCM that has been recorded in the Compendium of Materia Medica. Crocin is a natural carotenoid extracted from saffron that is known to have various biological activity including renoprotective (10), anti-oxidant (11), anti-depressant (12) and anti-inflammatory (13) effects. Further, a growing number of papers have confirmed that Crocin also inhibits the progression of multiple cancers (14-16). Zhou et al. reported that Crocin inhibited migration, invasion, and epithelial-mesenchymal transition (EMT) in GC cells (17). Hoshyar et al. found that in human GC cells, Crocin induced apoptosis by increasing the ratio of B-cell lymphoma 2 (Bcl-2)/Bcl-2-associated X protein (Bax) and caspase activation (18). Nevertheless, the role of Crocin in gastric carcinogenesis has yet to be reported.
In this study, an in vitro model was induced by treating gastric mucosal epithelial GES-1 cells, called MC cells, with MNNG. This study first revealed the role of Crocin in GES-1-T and MC cells, and in vitro experiments found that Crocin inhibited the proliferation, cell cycle and metastasis ability of MC cells. Moreover, we also explored the mechanism underlying malignant transformation of gastric mucosal cells. We present the following article in accordance with the MDAR reporting checklist (available at http://dx.doi.org/10.21037/jgo-20-406).
Methods
Drug and reagents
The human gastric mucosa epithelial cell line GES-1 and the human hypodifferentiated gastric adenocarcinoma cell line BGC-823 were purchased from The Institute of Life Sciences Cell Bank (Shanghai, China). Crocin was obtained from Shaanxi University of Chinese Medicine (Xi Xian New Area, China). MNNG was purchased from J&K Scientific Ltd. (CAS: 70-25-7, Beijing, China).
Animal model
Male Sprague-Dawley rats (180–200 g, 4–6 weeks old) were used to establish rat animal models, which were provided by experimental animal center of Sichuan University. According to the previous description (19), 1 mL of MNNG solution per 100 g body weight was given by gavage, 2 days normal diet, 1 day fasting, and this process lasted 32 weeks. The treatment group was fed rats with Crocin for 6 weeks. After the model was successfully established, following by histological evaluation. All processes were approved by the Animal Ethics Committee of Shaanxi University of Chinese Medicine (No. SYXK-Shaan-20200017), in accordance with the Shaanxi University of Chinese Medicine institutional guidelines for the care and use of animals
Cell culture and treatment
GES-1 and BGC-823 cells were cultured in RPMI-1640 medium (Gibco, USA) supplemented with 10% FBS (fetal bovine serum, Gibco, USA), 100 U/mL penicillin, and 100 µg/mL streptomycin (Invitrogen, USA) in a constant environment with 5% CO2 at 37 °C. The GES-1 cells were treated with MNNG (200 µM) for 48 hours to acquire malignant cell (MC) phenotypes, as described in an earlier study (19).
Cell viability
MC cell suspension (100 µL) was plated in a 96-well plate and cultured for 24 hours 5% CO2 at 37 °C. The cells were then treated with different doses of Crocin (0–100 µM). After 48 hours, 10 µL of Cell Counting Kit-8 (CCK-8) solution (Engreen Biosystem, China) was added to each well. Next, the cells were incubated in a standard controlled incubator for 4 hours. Finally, the absorbance was measured at 450 nm using a microplate reader, and the experiments were conducted in triplicate.
In the subsequent experiments, five groups of cells were used as follows: GES-1 (control group), BGC-823 (positive group), MC (model group), MC + Crocin (3 µM group) and MC + Crocin (10 µM group). To understand the effect of Crocin on nuclear factor erythroid 2-related factor 2 (Nrf2) signaling, cells were additionally treated with the Nrf2 inhibitor ML385 (20 µM) to form the following groups: GES-1, MC, MC + Crocin (10 µM), MC + ML385 (20 µM), and MC + Crocin + ML385.
Colony forming assay
Culture medium containing approximately 1 × 104 cells was added to a petri dish. The dish was gently rotated to disperse the cells evenly, and the cells were subsequently incubated for 2 weeks at 37 °C with 5% CO2. When colonies became visible to the naked eye, the culture was terminated. Besides, the same cells were fixed with 4% paraformaldehyde for 15 minutes. Then, the fixative was removed, and the cells were stained with 0.1% crystal violet (Sigma, USA) for 30 minutes. Colonies >0.5 mm in diameter were counted using a microscope (Nikon, Japan). The experiments were conducted in triplicate.
Cell cycle and apoptosis
Briefly, the experimental cells were fixed using pre-cooled 75% ethanol at 4 °C for 24 hours, then stained using a Cell Cycle and Apoptosis Analysis Kit (Meilunbio, China), in line with the instructions of the manufacturer. Finally, flow cytometry (Beckman Coulter, USA) was used to detect red fluorescence and light scattering at an excitation wavelength of 488 nm. Cell DNA content and light scattering were analyzed with Flowjo 10 (BD, USA). The experiments were conducted in triplicate.
Transwell invasion assay
The serum-free RPMI-1640 medium was added to the upper chamber of Corning® HTS Transwell®-24 well permeable supports (Corning, USA) and then placed at room temperature for 30 minutes. After 24 hours of starvation, cell suspension was transferred to the upper chamber with a cell density of 5×105 mL per chamber. Next, 500 µL medium containing 10% FBS was added to the lower chamber, followed by incubation at 37 °C for 36 hours. The membrane and superior lumen cells were washed twice with phosphate-buffered saline (PBS), and then removed with an aseptic cotton swab. Finally, the cells were fixed with 95% ethanol for 10 minutes and stained with crystal violet for 5 minutes. Five randomly selected fields were photographed at 200× magnification using a microscope (Nikon, Japan). The experiments were conducted in triplicate.
Western blot assay
Briefly, total protein was extracted from cells using RIPA lysis buffer (Beyotime, China), and the protein concentration was determined using a BCA kit (Thermo Fisher, USA). The proteins were separated by electrophoresis and transferred to a membrane. The membrane was then sealed in 5% skim milk for 1 hour. After that, the membrane was incubated at 4 °C overnight with the following corresponding primary antibodies (Abcam, UK; CST, USA): Anti-Ki67 (ab92742, dilution: 1/600); Anti-proliferating cell nuclear antigen (PCNA) (ab92552, dilution: 1/600); Anti-Bcl-2 (ab32124, dilution: 1/1,000); Anti-Bax (ab32503, dilution: 1/600); Anti-Cyclin A2 (ab181591, dilution: 1/600); Anti-Cyclin B1 (ab32053, dilution: 1/1,000); Anti-Cyclin D1 (ab16663, dilution: 1/200); Anti-p21 (ab109520, dilution: 1/1,000); Anti-CDX2 (ab76541, dilution: 1/10,000); Anti-Claudin-4 (ab53156, dilution: 1/500); Anti-E-Cadherin (ab1416, dilution: 1/50); Anti-Vimentin (ab8978, dilution: 1/600); Anti-Fibronectin-1 (ab2413, dilution: 1/500); Anti-Transgelin (ab155272, dilution: 1/1,000); Anti-Nrf2 (ab137550, dilution: 1/600); Anti-HO1 (ab68477, dilution: 1/1,000); Anti-TAZ (ab242313, dilution: 1/500); Anti-YAP (#14074, dilution: 1/1,000); Anti-p-YAP (#13008, dilution: 1/1,000); Anti-MST (#14946, dilution: 1/1,000); Anti-p-MST (49332, dilution: 1/1,000); Anti-LATS (#3477, dilution: 1/1,000); Anti-p-LATS (#8654, dilution: 1/1,000). Next, the membrane was incubated with the corresponding secondary antibodies for 1 hour at 4 °C. Finally, the protein bands were detected with ECL reagent (Thermo Fisher, USA). The experiments were conducted in triplicate.
Statistical analysis
Data were expressed as mean ± standard deviation (SD). All experiments were conducted in triplicate. Significant differences were determined by t-test or one-way analysis of variance (ANOVA), followed by Tukey’s multiple comparison test. P<0.05 was considered to show statistical significance.
Results
Crocin improved the growth of MNNG-treated animal model and inhibited the atypical hyperplasia and MNNG-treated GES-1 cells viability
We used Crocin to treat rats for other 6 weeks after the animals be induced by MNNG. Crocin significantly promoted the growth (Figure 1A) and inhibited the atypical hyperplasia (Figure 1B) of model animals. In vitro, The cell viability of GES-1, BGC-823, and MC cells treated with different doses of Crocin (0–100 µM) for 24 hours was determined using a CCK-8 assay. As shown in Figure 1C, Crocin exerted a dose-dependent inhibitory effect on all three cell lines. BGC-823 and MC cells treated with Crocin at a concentration of 20 µM or higher exhibited significant cytotoxicity. Therefore, for the subsequent experiments, 3 and 10 µM were selected as the low and high doses, respectively.
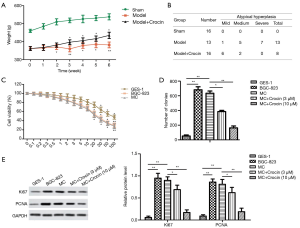
Next, the colony formation ability of these cells was investigated. As shown in Figure 1D, the number of BGC-823 cell colonies was remarkably increased compared to the number of GES-1 cell colonies, this result was similar to that of MC group. In contrast, Crocin treatment (3 or 10 µM) strongly reduced the number of MC cells. The expression levels of Ki67 and PCNA were also detected by western blot. The results revealed that, compared with the MC group, Crocin remarkably decreased the protein levels of Ki67 and PCNA (Figure 1E).
Crocin blocked MNNG-induced GES-1 cell cycle
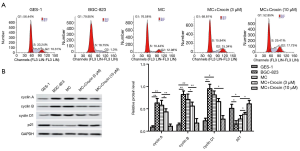
To further explore the inhibitory role of Crocin in MC cells, flow cytometry was carried out to analyze the cell cycle. As shown in Figure 2A, 56% and 10% of cells in the control group were at the G0/G1 and G2/M phases, respectively, compared with 79% and 1% in the BGC-823 group, and 76% and 12% in the MC group. After Crocin treatment, the percentage of MC cells at the G0/G1 phase dropped from 76% to 52%, and the percentage of those at the G2/M phase rose from 12% to 17%. The proteins levels of cyclin A, cyclin B, cyclin D1, and P21 were also detected. The results showed that, compared with those in the MC group, the expression levels of cyclin A, cyclin B, and cyclin D1 in cells treated with Crocin (3 or 10 µM) were significantly reduced, but the expression of P21 was increased (Figure 2B). These results suggested that Crocin mediated GES-1 cell cycle arrest induced by MNNG.
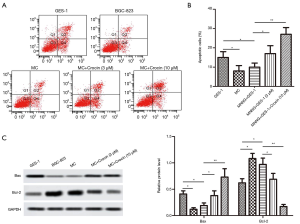
Crocin promoted MNNG-induced GES-1 cell apoptosis
To better understand the effect of Crocin on the apoptosis of GES-1 cells induced by MNNG, flow cytometry was employed to measure cell death (apoptosis or necrosis). The results showed that the apoptosis rate of MC cells obviously increased after different doses of Crocin treatment, compared with MC group (Figure 3A,B). Then, the protein expression of the apoptosis-related genes Bcl-2 and Bax was examined by western blot. As shown in Figure 3C, Crocin (3 or 10 µM) conspicuously decreased the expression level of Bcl-2 while elevating the expression level of Bax. These results suggested that Crocin possessed the ability to promote MNNG-induced apoptosis of GES-1 cells.
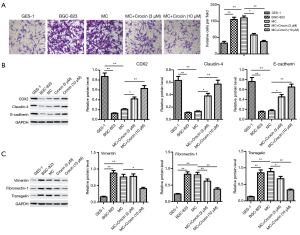
Crocin suppressed invasion and EMT in MNNG-treated GES-1 cells
Since the invasion ability of cells is associated with metastasis, we determined the effect of Crocin on invasion of MC cells. MNNG treatment increased the number of migrating cells, compared with control group. Crocin (3 or 10 µM) significantly reduced the invasive ability of MC cells (Figure 4A). Considering that EMT is involved in tumor metastasis (20), we analyzed the protein expression of epithelial genes (CDX2, Claudin-4, and E-cadherin) and mesenchymal genes (Vimentin, Fibronectin-1, and Transgelin) in these cells. As shown in Figure 4B,C, GES-1 exposed to MNNG exhibited a dramatically down-regulation of CDX2, Claudin-4, and E-cadherin, while the mesenchymal markers Vimentin, Fibronectin-1, and Transgelin were up-regulated. Compared with the MC group, Crocin (3 or 10 µM) remarkably increased the protein level of E-cadherin, but decreased the levels of N-cadherin and Vimentin. Together, we concluded that MNNG stimulated GES-1 cells not only showed invasion capacity, but also experienced EMT, which were inhibited by Crocin treatment.
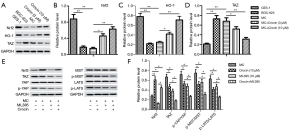
Crocin regulated the Nrf2/Hippo signaling pathway
To understand the potential molecular mechanism of Crocin inhibiting malignant transformation of GES-1 cells, the expression levels of nuclear factor erythroid 2–related factor 2 (Nrf2), heme oxygenase-1 (HO-1) and tafazzin (TAZ) were detected by western blot. As shown in Figure 5A,B,C,D, the protein levels of Nrf2 and its downstream target HO-1 were significantly decreased, and TAZ increased in the BGC-823 and MC cells compared to those in the GES-1 cells; however, Crocin treatment (3 or 10 µM) reversed these effects. We further extended these observations, Nrf2 inhibitor ML385 (20 µM) was added to MC cells, and the expression of key regulators in the Hippo pathway were detected. As a result, ML385 led to the down-regulation of Nrf2, TAZ, and p-yes-associated protein (p-YAP), also a decrease of upstream regulators of the Hippo pathway, p-mercaptopyruvate sulfurtransferase (p-MST) and p-large tumor suppressor (p-LATS). Remarkably, after treatment with Crocin (10 µM) combined with ML385, the expressions of Nrf2 were reversed, and the expressions of TAZ, p-YAP, p-MST and p-LATS were further decreased. compared with ML385 treatment alone (Figure 5E,F).
Discussion
GC is a multifactorial pathological process, and chemical carcinogenesis has become one of the most common causes of the disease. At present, MNNG is commonly used as a carcinogenic chemical to simulate normal gastric mucosal lesions (21,22), mimicking gastric nitrate intake and converting it into nitric acid and other carcinogens. In our study, we used MNNG to induce gastric mucosal GES-1 cells and to establish a rat animal model. Through the detection of GES-1 cell proliferation and atypical hyperplasia, it was found that the cell proliferation increased after MNNG treatment. Also, we found that MNNG promoted the colony formation and cycle arrest of MC cells, and also promoted their metastasis (23), with similar results seen in the BGC-823 cells. These findings indicated that GES-1 cells exhibit the biological behavior of malignant lesions, which is consistent with the conclusions of previous studies.
Recently, the role of TCM in the treatment of GC has grown more important. As reported in a previous study (13), TCM has a good anti-tumor effect and can improve patients’ quality of life. Moreover, increasing evidence has shown that TCM has a significant effect on gastric carcinogenesis (17-19). In this study, Crocin treatment obviously inhibited invasion capacity and EMT in MNNG-simulated GES-1 cells (24), as well as cell cloning and cycle arrest, indicating that Crocin could be a potential therapeutic option for GC and malignant transformation.
GC cell apoptosis is the basis of carcinogenesis (25). Caspase-3 directly induces apoptosis and stimulates other apoptosis-related factors, including Bcl-2 and Bax levels. The Bcl-2 family plays an important role the regulation of apoptosis and can be used as targets in anti-tumor treatments (26). Bcl-2 exerts its anti-apoptotic effect through interaction with and antagonism of Bax, which plays a decisive role in maintaining the stability of the internal environment of the cells (27,28). Consequently, the mechanism of expression regulation may be the key to cancer treatment strategies. In the model of MC cells, Crocin reversed the apoptosis of gastric mucous membrane cells and ameliorated the symptoms of malignant lesions (29).
Nrf2 is an important antioxidant transcription factor. It may also be a tumor suppressor, as Nrf2−/− mice have been shown to be susceptible to carcinogens (30). Tertil et al. reported that over-expression of Nrf2/HO-1 especially inhibited cell migration in lung cancer (31), while Nrf2 deletion promoted the migration of carcinoma cells (32). Kawasaki et al. found that Nrf2 expression was positively correlated with the tumor invasive behavior in GC, indicating that Nrf2 could potentially be a prognostic marker for GC (33). In this study, the expression of Nrf2 was decreased in MNNG-treated cells, and Crocin inhibited the migration and invasion, and elevated the expression of Nrf2 in MC cells, which is consistent with the results of previous studies. Moreover, under stress conditions, Nrf2 expression is up-regulated and transferred from the cytoplasm to the nucleus, which activates downstream genes such as HO-1 and NQO1, thus reducing damage to normal cells by toxic substances (34).
Up-regulation of Nrf2 has been found in many types of cancer (breast cancer, ovarian cancer and pancreatic cancer, etc.). Patients with clinically high Nrf2 level usually have poor prognosis (35). Nrf2 have been reported to induce TAZ low expression in glioblastoma cells (36), and our study found that low Nrf2 expression and high TAZ expression occur in MC cells, Nrf2 inhibitor down-regulated TAZ protein. Since TAZ is a transcriptional co-activator of the Hippo signaling pathway, this study further focused on the connection between Crocin and the Hippo signaling pathway. Hippo pathway participates in organ development, cell proliferation, metastasis, and death of various human cancers through the interaction of transcriptional enhanced associate domain family members 1-4 (TEADs1-4) and YAP/TAZ co-activator (37,38). As known, YAP and TAZ are the key effector factors downstream of this pathway, they are overexpressed in many human solid tumors (39). Once Hippo was stimulated by upstream signal molecules, MAT1/2 combined with human Salvador homolog 1 (SAV1) to activate LATS1/2, resulting in YAP/TAZ phosphorylation and stagnation in the cytoplasm (40). In contrast, YAP/TAZ dephosphorylation induces target gene expression through nuclear translocation, which promotes migration, anti-apoptosis, and metastasis of tumor cells (41). This study found that Nrf2 inhibitor could down-regulated the phosphorylation levels of MST and LATS, and further stimulate the phosphorylation of YAP/TAZ (40). After Crocin and Nrf2 inhibitor co-treatment, this change was aggravated, and the low expression of Nrf2 was improved. Based on these results, we speculated that Crocin activated Nrf2 to inhibit the Hippo signaling pathway, thereby attenuating the malignant transformation of MNNG-simulated GES-1 cells.
Conclusions
In conclusion, in a MNNG-induced cell model, Crocin inhibited the cell viability and motility induced by MNNG, and promoted apoptosis in vitro. Further investigation showed that Crocin played a beneficial role through regulating the Nrf2 signaling pathway, suggesting that Crocin may be used as a therapeutic agent for gastric carcinogenesis. However, the pathological process of intestinal metaplasia and stunting in vivo need to be further studied.
Acknowledgments
Funding: None.
Footnote
Reporting Checklist: The authors have completed the MDAR reporting checklist. Available at http://dx.doi.org/10.21037/jgo-20-406
Data Sharing Statement: Available at http://dx.doi.org/10.21037/jgo-20-406
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo-20-406). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Experiments were performed under a project license (No. SYXK-Shaan-20200017) granted by ethics board of Shaanxi University of Chinese Medicine, in compliance with Shaanxi University of Chinese Medicine institutional guidelines for the care and use of animals.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- Slavin TP, Weitzel JN, Neuhausen SL, Schrader KA, Oliveira C, Karam R. Genetics of gastric cancer: what do we know about the genetic risks? Transl Gastroenterol Hepatol 2019;4:55. [Crossref] [PubMed]
- Aggarwal BB, Gehlot P. Inflammation and cancer: how friendly is the relationship for cancer patients? Curr Opin Pharmacol 2009;9:351-69. [Crossref] [PubMed]
- Sun Z, Jia J, Du F, et al. Clinical significance of serum tumor markers for advanced gastric cancer with the first-line chemotherapy. Transl Cancer Res 2019;8:2680-90. [Crossref]
- Abe M, Yamashita S, Kuramoto T, et al. Global expression analysis of N-methyl-N'-nitro-N-nitrosoguanidine-induced rat stomach carcinomas using oligonucleotide microarrays. Carcinogenesis 2003;24:861-7. [Crossref] [PubMed]
- Cai T, Zhang C, Zhao Z, et al. The gastric mucosal protective effects of astragaloside IV in mnng-induced GPL rats. Biomed Pharmacother 2018;104:291-9. [Crossref] [PubMed]
- Luo YW, Liang M, Yao WX, et al. Functional role of lncRNA LOC101927497 in N-methyl-N′-nitro-N-nitrosoguanidine-induced malignantly transformed human gastric epithelial cells. Life Sci 2018;193:93-103. [Crossref] [PubMed]
- Zhang C, Cai T, Zeng X, et al. Astragaloside IV reverses MNNG-induced precancerous lesions of gastric carcinoma in rats: Regulation on glycolysis through miRNA-34a/LDHA pathway. Phytother Res 2018;32:1364-72. [Crossref] [PubMed]
- Zeng J, Yan R, Pan H, et al. Weipixiao attenuate early angiogenesis in rats with gastric precancerous lesions. BMC Complement Altern Med 2018;18:250. [Crossref] [PubMed]
- Hosseinzadeh H, Abootorabi A, Sadeghnia HR. Protective effect of Crocus sativus Stigma extract and crocin (trans-crocin 4) on methyl methanesulfonate-induced DNA damage in mice organs. DNA Cell Biol 2008;27:657-64. [Crossref] [PubMed]
- Yaribeygi H, Mohammadi MT, Sahebkar A. Crocin potentiates antioxidant defense system and improves oxidative damage in liver tissue in diabetic rats. Biomed Pharmacother 2018;98:333-7. [Crossref] [PubMed]
- Alavizadeh SH, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a comprehensive review. Food Chem Toxicol 2014;64:65-80. [Crossref] [PubMed]
- Zhu K, Yang C, Dai H, et al. Crocin inhibits titanium particle-induced inflammation and promotes osteogenesis by regulating macrophage polarization. Int Immunopharmacol 2019;76:105865. [Crossref] [PubMed]
- Amerizadeh F, Rezaei N, Rahmani F, et al. Crocin synergistically enhances the antiproliferative activity of 5-flurouracil through Wnt/PI3K pathway in a mouse model of colitis-associated colorectal cancer. J Cell Biochem 2018;119:10250-61. [Crossref] [PubMed]
- Mollaei H, Safaralizadeh R, Babaei E, et al. The anti-proliferative and apoptotic effects of crocin on chemosensitive and chemoresistant cervical cancer cells. Biomed Pharmacother 2017;94:307-16. [Crossref] [PubMed]
- Deng LY, Li JC, Lu SY, et al. Crocin inhibits proliferation and induces apoptosis through suppressing MYCN expression in retinoblastoma. J Biochem Mol Toxicol 2019;33:e22292. [Crossref] [PubMed]
- Zhou Y, Xu QH, Shang JJ, et al. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol 2019;234:17876-85. [Crossref] [PubMed]
- Hoshyar R, Bathaie SZ, Sadeghizadeh M. Crocin triggers the apoptosis through increasing the Bax/Bcl-2 ratio and caspase activation in human gastric adenocarcinoma, AGS, Cells. DNA Cell Biol 2013;32:50-7. [Crossref] [PubMed]
- Xu JY, Shen W, Pei B, et al. Xiao Tan He Wei Decoction reverses MNNG-induced precancerous lesions of gastric carcinoma in vivo and vitro: Regulation of apoptosis through NF-κB pathway. Biomed Pharmacother 2018;108:95-102. [Crossref] [PubMed]
- Mittal V. Epithelial mesenchymal transition in tumor metastasis. Annu Rev Pathol 2018;13:395-412. [Crossref] [PubMed]
- Ghoneum MH, Badr El-Din NK, Abdel Fattah SM, et al. Hydroferrate fluid, MRN-100, provides protection against chemical-induced gastric and esophageal cancer in Wistar rats. Int J Biol Sci 2015;11:295-303. [Crossref] [PubMed]
- Tsukamoto H, Mizoshita T, Katano T, et al. Preventive effect of rebamipide on N-methyl-N'-nitro-N-nitrosoguanidine-induced gastric carcinogenesis in rats. Exp Toxicol Pathol 2015;67:271-7. [Crossref] [PubMed]
- Bakshi HA, Hakkim FL, Sam S, et al. Dietary crocin reverses melanoma metastasis. J Biomed Res 2017;32:39-50. [PubMed]
- Zhou Y, Xu QH, Shang JJ, et al. Crocin inhibits the migration, invasion, and epithelial-mesenchymal transition of gastric cancer cells via miR-320/KLF5/HIF-1α signaling. J Cell Physiol 2019;234:17876-85. [Crossref] [PubMed]
- Bailey C. Stomach cancer. Clin Evid 2005;14:628-34. [PubMed]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell 2011;21:92-101. [Crossref] [PubMed]
- Indran IR, Tufo G, Pervaiz S, et al. Brenner, Recent advances in apoptosis, mitochondria and drug resistance in cancer cells. Biochim Biophys Acta 2011;1807:735-45. [Crossref] [PubMed]
- Shamas-Din A, Kale J, Leber B, et al. Mechanisms of action of Bcl-2 family proteins. Cold Spring Harb Perspect Biol 2013;5:a008714. [Crossref] [PubMed]
- Cai J, Wang M, Zhu M, et al. N-methyl-N-nitro-N’-nitrosoguanidine induces the expression of CCR2 in human gastric epithelial cells promoting CCL2-mediated migration. Mol Med Rep 2016;13:1083-90. [Crossref] [PubMed]
- Iida K, Itoh K, Kumagai Y, et al. Nrf2 is essential for the chemopreventive efficacy of oltipraz against urinary bladder carcinogenesis. Cancer Res 2004;64:6424-31. [Crossref] [PubMed]
- Tertil M, Golda S, Skrzypek K, et al. Nrf2-heme oxygenase-1 axis in mucoepidermoid carcinoma of the lung: Antitumoral effects associated with down-regulation of matrix metalloproteinases. Free Radic Biol Med 2015;89:147-57. [Crossref] [PubMed]
- Rachakonda G, Sekhar KR, Jowhar D, et al. Increased cell migration and plasticity in Nrf2-defcient cancer cell lines. Oncogene 2010;29:3703-14. [Crossref] [PubMed]
- Kawasaki Y, Ishigami S, Arigami T, et al. Clinicopathological significance of nuclear factor (erythroid-2)-related factor 2 (Nrf2) expression in gastric cancer. BMC Cancer 2015;15:5. [Crossref] [PubMed]
- Sajadimajd S, Khazaei M. Oxidative stress and cancer: the role of Nrf2. Current Cancer Drug Targets 2018;18:538-57. [Crossref] [PubMed]
- Lister A, Nedjadi T, Kitteringham NR, et al. Nrf2 is overexpressed in pancreatic cancer: implications for cell proliferation and therapy. Mol Cancer 2011;10:37. [Crossref] [PubMed]
- Escoll M, Lastra D, Pajares M, et al. Transcription factor NRF2 uses the Hippo pathway effector TAZ to induce tumorigenesis in glioblastomas. Redox Biol 2020;30:101425. [Crossref] [PubMed]
- Harvey KF, Zhang X, Thomas DM. The Hippo pathway and human cancer. Nat Rev Cancer 2013;13:246-57. [Crossref] [PubMed]
- Moroishi T, Hansen CG, Guan K L. The emerging roles of YAP and TAZ in cancer. Nat Rev Cancer 2015;15:73-9. [Crossref] [PubMed]
- Plouffe SW, Hong A W, Guan KL. Disease implications of the Hippo/YAP pathway. Trends Mol Med 2015;21:212-22. [Crossref] [PubMed]
- Callus BA, Verhagen AM, Vaux DL. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. FEBS J 2006;273:4264-76. [Crossref] [PubMed]
- Cordenonsi M, Zanconato F, Azzolin L, et al. The Hippo transducer TAZ confers cancer stem cell-related traits on breast cancer cells. Cell 2011;147:759-72. [Crossref] [PubMed]
(English Language Editor: J. Reynolds)


