Self-expanding metal stents (SEMS) provide superior outcomes compared to plastic stents for pancreatic cancer patients undergoing neoadjuvant therapy
Introduction
With a 5-year survival rate of only 5%, pancreatic cancer is the fourth leading cause of cancer-related death in the United States (1). Neoadjuvant therapy is increasingly utilized for patients with pancreatic cancer with the goal of decreasing tumor burden in anticipation of later surgical resection (2,3). The intent is that, by local control and/or tumor down-staging with therapy, there will be a resultant survival benefit, which recent data has confirmed (4). The majority of patients are treated with a combination of gemcitabine, 5-FU and platinum compounds along with radiation therapy (5). Although the pool of patients who are candidates for neoadjuvant therapy has been estimated to be only 4.5% of the overall number diagnosed with pancreatic cancer (3), this represents an important population for whom there is an opportunity to prolong survival and increase quality of life. Chemotherapy in patients with obstructing pancreatic cancers requires stenting to relieve the biliary obstruction, as many chemotherapeutic agents require functioning bilirubin transport mechanisms and bile excretion to avoid toxicity (6). Stent occlusion in these high-risk patients can lead to life-threatening complications. Metal stents have larger diameters than plastic stents, and therefore are less susceptible to occlusion. Although it was once thought that metal stents would interfere with surgical margins, such that they were only placed in patients whose cancers were so advanced as to preclude surgical resection, it is now accepted that metal stents can be successfully removed at the time of definitive surgery (7-9).
While there are a number of studies comparing use of plastic versus metal stents in the pancreatic cancer population, there is little data specifically evaluating that subset of patients who undergo neoadjuvant therapy in anticipation of later pancreaticoduodenectomy. This unique population may be different for a number of reasons. First, this population is more susceptible to chemotherapy-induced neutropenia, and thus may be more prone to infection (2). Patients undergoing neoadjuvant chemotherapy may be at increased risk for biliary sludge due to sloughing of cellular material generated as a result of chemotherapy, increased bacterial colonization of the stent due to immune compromise, as well as hemobilia due to chemotherapy-induced thrombocytopenia, all increasing the risk of stent obstruction and subsequent cholangitis. Our study aims to expand current knowledge by undertaking a head-to-head analysis of patients with plastic and metal stents among this neoadjuvant therapy cohort, which has not been evaluated in prior studies. We hypothesized that placement of metal rather than plastic stents in patients undergoing neoadjuvant chemotherapy results in lower rates of stent-related complications, leading to improved stent-related outcomes.
Methods
The study was approved by the Institutional Review Board of the University of Michigan Health System. We undertook a retrospective review of pancreatic cancer patients treated by the University of Michigan Multidisciplinary Pancreatic Cancer Destination Program between January 1, 2005 and June 31, 2010. Using an electronic database, a list of patients who were seen as part of the Destination Program during this time period and later underwent neoadjuvant therapy was generated. The records of each of these patients were individually examined, and only patients who had one or more biliary stents placed for malignant obstruction were included in the study. For example, patients with pancreatic tail cancers, with no need for stenting, were excluded. Procedural and treatment records were reviewed. Data including patient demographics, procedural details and complications were collected. Demographic information collected included age at diagnosis, gender, and race. Procedural details included tumor location, resectability (unresectable, borderline resectable, resectable), TNM stage (if documented), stent type (plastic vs. metal), stent diameter, and time from stent placement to stent occlusion or surgery/attempted surgery. Furthermore, data regarding complications, whether they were stent-related, and whether they required patient hospitalization, were collected. In terms of complications, stent obstruction was defined as biochemical evidence of cholestasis, along with evidence of biliary dilation on imaging, including ERCP. Cholangitis was defined as fever with biochemical evidence of cholestasis. Cholecystitis was defined as characteristic pain, fever or leukocytosis, in combination with supportive evidence on imaging. Pancreatitis was defined as a three-fold elevation in amylase or lipase or evidence of pancreatic inflammation on imaging. We also collected data regarding whether a given patient actually underwent surgical resection or attempted surgical resection after undergoing neoadjuvant therapy. The (n) number of stent exchanges in a single patient was also noted, as was time from initial stent placement to surgery and total survival time from initial stent placement. If a patient was lost to follow-up (receiving local care), the date of the last clinical contact at the referral center was used as the end-date for purposes of calculating stent survival time.
Statistical methods
Continuous data were summarized using means and standard deviations (SD) or ranges. Categorical variables were summarized by counts and percentages. Time to stent complication was compared between metal and plastic stents using Kaplan-Meier estimation and log-rank testing with all stents assumed to be independent. Stent complications were assumed to follow a Poisson process. The complication rate was estimated as the ratio of complications to total stent exposure time and 95% confidence intervals were calculated. A probability (P) value of 0.05 or smaller was considered significant for all hypothesis tests. The above procedures were done in SAS 9.2 (SAS Institute Inc., Cary, NC).
Results
52 patients met inclusion criteria, with a mean age of 65 years (SD 9.58). 54% were male, and 85% were borderline resectable (15% resectable) at initial diagnosis. All received gemcitabine-based neoadjuvant regimens. A majority (71%) ultimately underwent surgery, whether an aborted operation (23%) or successful resection (48%). In patients eventually undergoing surgery, the mean time from initial stent placement to surgery was 134.1 days (range, 26-420 days). Only 21% of patients (11 of 52) made it to surgery with their initial stent in place. Of these eleven patients, 7 had a plastic stent and 4 had a metal stent. A total of 113 stents were placed in these 52 patients (70 plastic, 43 metal). Plastic stents were the initial stent placed in 43 patients. There were 9 complications in 276 months with metal stents in place, compared with 27 complications in 129 months with plastic stents in place. The complication rate was almost 7 times higher with plastic stents, 0.21 (95% CI, 0.14-0.30), than with metal stents, 0.03 (95% CI, 0.01-0.06). Of the stent complications, nearly 70% involved stents 10 French or larger. Furthermore 67% of complications occurred in patients who ultimately underwent surgery.
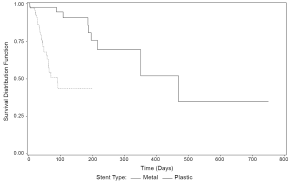
All 9 metal stent complications were due to stent occlusion, 3 with cholangitis and 1 involving migration. For plastic stents, there were 23 cases of stent occlusion, 15 with cholangitis, 7 stent migrations, and 1 episode of cholecystitis. A total of 15 patients were hospitalized for plastic stent complications, while 5 patients were hospitalized for metal stent complications. The first quartile estimate of time to stent complication (Figure 1) was almost 5 times longer for metal than for plastic stents (44 vs. 200 days) (P<0.0001).
Discussion
The superior patency of metal biliary stents over their plastic counterparts among the spectrum pancreatic cancer cohorts with biliary obstruction has been firmly established in a number of prior studies. A recent retrospective study by Decker et al. examined the rate of repeat endoscopic intervention in 29 pancreatic cancer patients who underwent biliary stent placement prior to pancreaticoduodenectomy (10). This study was not limited to the neoadjuvant treatment population, but found that 39% (7 of 18) of patients in the plastic stent group required pre-operative stent intervention, while no patients in the metal stent group (11 patients) required re-intervention. However, there is a paucity of information available regarding the rates of re-intervention in the specific subset of pancreatic cancer patients who are candidates for neoadjuvant therapy in anticipation of later surgical resection.
A recent retrospective study by Boulay et al. evaluated 49 patients with resectable or locally advanced pancreatic cancer who had plastic stents placed for malignant biliary obstruction, and then underwent neoadjuvant therapy (11). The majority of patients (55%) underwent repeat endoscopic intervention with stent exchange due to plastic stent complications including, most commonly, stent occlusion and cholangitis. The study concluded that plastic stents were not advisable in this subset of patients because they do not remain patent for the amount of time necessary for most patients to complete neoadjuvant therapy, which often lasts 2 to 4 months. While their report did include 7 metal stent patients, showing a 14% rate of repeat intervention, it represented too small a sample population to allow statistical comparison (11). The expanded cohort size in our study has facilitated meaningful comparisons, allowing conclusions that may guide clinical decision making. No published randomized controlled trials exist currently to examine this issue.
While, in theory, patients undergoing chemotherapy may be more susceptible to stent complications for reasons set forth earlier, at least some studies refute this conclusion. In one retrospective analysis of 80 patients with plastic stents, the rate of stent occlusion was not found to be significantly different between those exposed to chemotherapy (37%) and those unexposed (39%), and mean duration of patency was not shortened by chemotherapy (12). A later Japanese study of 147 patients, also retrospective, showed that the rate of biliary infectious complications in metal stents was unchanged by administration of chemotherapy (13). However, the treatments may not be directly comparable. The key consideration is that for patients undergoing neoadjuvant therapy, a stent complication may render disease unresectable due to local complications or delay surgery to the point that disease progression renders the patient inoperable.
It is also important to recognize, as demonstrated by our data, that neoadjuvant therapy is not a complete solution to the challenge of treating pancreatic cancer, which has an extremely poor 5-year survival rate. Of the patients in our study, over a quarter either had progression of disease or no improvement in tumor burden after neoadjuvant therapy, such that they were not ultimately operative candidates despite the neoadjuvant therapy. Furthermore, of those patients who underwent surgery, roughly one third were not successfully resected due to progression of disease discovered during surgical exploration. This confirms earlier estimates that neoadjuvant therapy is able to convert approximately 33% of borderline resectable patients to resectable candidates, but may not improve overall outcome (11). We were unable to accurately estimate overall survival outcome in our study, due to the high number of patients who were lost to follow-up (local care), either prior to or following surgical resection.
One argument against routine use of metal stents has been their increased cost as compared to their plastic counterparts. However, our data supports the conclusion that it is actually more economically sound to use metal stents for two reasons. First, since metal stents remain in place substantially longer without complication, they do not need to be exchanged like plastic stents, which must be routinely exchanged roughly every 3 months based on the known median time to occlusion. Our data shows that the mean time from initial stent placement to surgery is roughly 4.5 months, and up to 7.5 months, such that a plastic stent would have to be exchanged at least once prior to surgery. This overall mean duration of stent patency is consistent with that elucidated in prior published studies (14). One meta-analysis concluded that a metal stent would be cost-effective if future re-interventions cost greater than $1,820, representative of a patient expected to have at least a 4 to 6 month survival following initial stent placement (14). Furthermore, our data shows that patients who receive plastic stents have a roughly 3-fold greater rate of hospitalization for stent-related complications than patients receiving metal stents. The extra cost of a metal stent pales in comparison to the economic cost of even a short hospital stay.
Our data expands the literature in this unique and growing patient population by including a formal metal stent comparison group, and demonstrating a statistically significant difference in stent patency and complication rate in the metal stent group. Metal stents not only have a 7-fold lower absolute complication rate, they also remain in place approximately 5 times longer without complication as indicated by our Kaplan-Meier analysis. Recent data have shown that metal stents neither interfere with surgical margins, nor obscure tumor imaging pre-operatively. The importance of successful neoadjuvant therapy has been recently emphasized by evidence of its association with improved outcomes for this lethal malignancy (4).
In terms of our study’s practical application for the interventional endoscopist, our group reserves ERCP for palliation of jaundice after a pancreatic protocol CT provides staging information. A tissue diagnosis may be confirmed by EUS-FNA and/or on-site review of ERCP brushings followed by metal stent placement. Many of the patients in our study cohort had stenting performed at initial presentation, often with plastic stents of small caliber and typically prior to referral. Therefore, the choice of plastic versus metal stent at initial presentation depended in large part on the level of suspicion and/or confirmation of malignancy versus benign causes of biliary obstruction. For cases of confirmed malignant obstruction, our data supports the clear improved efficacy of metal stents due to their longevity without complications both in patients who are destined for surgical resection, as well as those who are ultimately poor candidates for resection due to the extent of their disease. The presence of a metal stent is no longer considered the barrier to surgery it once was.
We acknowledge several important limitations to our study. First, the comparatively small number of patients in our metal stent group limits the power of the study. Second, for purposes of statistical analysis, we chose to look at stents independently, rather than individual patients, in order to account for the fact that an individual patient may have multiple stents placed during their course of treatment. While this made some elements of our analysis easier, it may have obscured other factors. Finally, given the retrospective nature of our study, factors other than stent choice may have impacted the clinical outcomes of each cohort.
In summary, our compelling evidence indicates that self-expanding metal, not plastic stents should be used for malignant biliary obstruction in patients undergoing neoadjuvant therapy for pancreatic cancer, due to lower rates of complication, hospitalizations, and longer stent patency.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin 2008;58:71-96. [PubMed]
- Evans DB, Varadhachary GR, Crane CH, et al. Preoperative gemcitabine-based chemoradiation for patients with resectable adenocarcinoma of the pancreatic head. J Clin Oncol 2008;26:3496-502. [PubMed]
- Gillen S, Schuster T, Meyer Zum Büschenfelde C, et al. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med 2010;7:e1000267. [PubMed]
- Artinyan A, Anaya DA, McKenzie S, et al. Neoadjuvant therapy is associated with improved survival in resectable pancreatic adenocarcinoma. Cancer 2011;117:2044-9. [PubMed]
- Sharma C, Eltawil KM, Renfrew PD, et al. Advances in diagnosis, treatment and palliation of pancreatic carcinoma: 1990-2010. World J Gastroenterol 2011;17:867-97. [PubMed]
- Weston BR, Ross WA, Wolff RA, et al. Rate of bilirubin regression after stenting in malignant biliary obstruction for the initiation of chemotherapy: how soon should we repeat endoscopic retrograde cholangiopancreatography? Cancer 2008;112:2417-23. [PubMed]
- Wasan SM, Ross WA, Staerkel GA, et al. Use of expandable metallic biliary stents in resectable pancreatic cancer. Am J Gastroenterol 2005;100:2056-61. [PubMed]
- Lawrence C, Howell DA, Conklin DE, et al. Delayed pancreaticoduodenectomy for cancer patients with prior ERCP-placed, nonforeshortening, self-expanding metal stents: a positive outcome. Gastrointest Endosc 2006;63:804-7. [PubMed]
- Mullen JT, Lee JH, Gomez HF, et al. Pancreaticoduodenectomy after placement of endobiliary metal stents. J Gastrointest Surg 2005;9:1094-104; discussion 1104-5. [PubMed]
- Decker C, Christein JD, Phadnis MA, et al. Biliary metal stents are superior to plastic stents for preoperative biliary decompression in pancreatic cancer. Surg Endosc 2011;25:2364-7. [PubMed]
- Boulay BR, Gardner TB, Gordon SR. Occlusion rate and complications of plastic biliary stent placement in patients undergoing neoadjuvant chemoradiotherapy for pancreatic cancer with malignant biliary obstruction. J Clin Gastroenterol 2010;44:452-5. [PubMed]
- Lofts FJ, Evans TR, Mansi JL, et al. Bile duct stents: is there an increased rate of complications in patients receiving chemotherapy? Eur J Cancer 1997;33:209-13. [PubMed]
- Nakai Y, Isayama H, Kawabe T, et al. Efficacy and safety of metallic stents in patients with unresectable pancreatic cancer receiving gemcitabine. Pancreas 2008;37:405-10. [PubMed]
- Moss AC, Morris E, Leyden J, et al. Do the benefits of metal stents justify the costs? A systematic review and meta-analysis of trials comparing endoscopic stents for malignant biliary obstruction. Eur J Gastroenterol Hepatol 2007;19:1119-24. [PubMed]