Role of cytopathology in the diagnosis and management of gastrointestinal tract cancers
Introduction
The gastrointestinal tract is a term used to define the series of tube like structures and accessory organs that are involved in the process of digestion and absorption of ingested food and eventual elimination of waste products. Broadly it may be divided into an upper and lower gastrointestinal tract and the accessory organs. The upper gastrointestinal tract comprises the esophagus, stomach and duodenum (first portion of the small intestine). The lower gastrointestinal tract comprises the remainder of the small intestine (jejunum and ileum), large intestine (cecum with attached vermiform appendix, ascending, transverse, descending and sigmoid colon, and rectum) and anus. The accessory organs comprise the liver, gall bladder, pancreas, hepatobiliary and pancreatic tracts.
Any portion of the gastrointestinal tract may be affected by malignancy, however curiously the small intestine where most of the digestion takes place (with the exception of the region of the ampulla of Vater in the second portion of the duodenum) is rarely involved. The highest incidence of malignancy is in the esophagus, stomach and colorectal regions. In fact esophagogastric and colorectal malignancies are amongst the commonest cancers in humans. Numerous screening protocols have been designed for at risk patients for esophagogastric lesions, and screening for colorectal cancer is advocated for all from the age of 50, and earlier if there are known risk factors (polyposis, inflammatory bowel disease).
Carcinomas are by far the most common malignancy of the gastrointestinal tract. With the exception of the proximal and distal most portions (esophagus and anus), where squamous cell carcinomas may be common, most carcinomas are adenocarcinomas. Other common primary neoplastic lesions include lymphoproliferative, neuroendocrine and mesenchymal (gastrointestinal stromal) tumors. The gastrointestinal tract may also be secondarily involved by direct tumor spread from neighboring organs/tissues (urinary bladder, prostate, cervix, uterus and ovaries), as well as metastases from distant sites (melanoma, Merkel cell tumor). Benign lesions may clinically and radiologically mimic gastrointestinal malignancy, including hamartomas, benign ulcers and strictures (as caused by ischemia, protozoal, bacterial and viral etiologies, inflammatory bowel disease, diverticulitis), endometriosis (1) and solitary rectal ulcer syndromes.
In the past only the more proximal and distal portions of the gastrointestinal tract could be sampled by blind or direct visualization techniques, without the necessity of open surgery or external radiologic image guided methods. Currently most portions of the gastrointestinal tract may be sampled by upper and lower intestinal endoscopies with the use of available smaller fiber-optic tubes, with direct visualization of the lesions, endoscopic ultrasound guided biopsy methods as well as externally via various radiologic techniques (ultrasound, CT). The newer instruments and techniques have made it relatively easier to collect not only cytologic but also histologic specimens from most gastrointestinal sites. The cytologic sample may be an adjunct and complementary to the main specimen (2).
Cytologic sampling of the gastrointestinal tract is particularly useful for sampling of large areas of interest (for example large segment Barrett’s esophagus, ulcerative colitis) where even with more extensive biopsy sampling protocols a larger surface area is sampled with cytologic brushing techniques than the more limited visualized biopsy sites. Cytologic sampling may be the sole specimen collected in very narrow areas of the intestinal tract (ducts and strictures), in subepithelial, submucosal and mural mass lesions and in endoscopic sampling of extraintestinal tissues [adjacent organs or regional lymph nodes (Figure 1) and masses] (3,4).
Gastrointestinal malignancy may be suspected on clinical and serologic (elevated CEA, AFP) grounds and by imaging techniques (X-ray, ultrasound, computed tomography, magnetic resonance imaging and barium scans), however cytohistologic sampling with morphologic evaluation of lesional tissue is in most instances necessary to provide a definitive diagnosis before treatment is initiated.
The pathologist’s primary task is to differentiate lesional from non-lesional native tissue. Once the lesional tissue has been identified, reactive and reparative lesions need to be differentiated from infectious and neoplastic diseases. The neoplastic lesions then are classified into benign and malignant entities, with determination of tumor type. Whenever possible it is also necessary to differentiate primary from metastatic malignancies, and indicate possible cells or tissue of origin. This is accomplished by cytomorphologic criteria and with judicious use of ancillary studies (special stains, immunohistochemistry, flow cytometry, molecular analysis), as well as correlation with clinical, serologic and imaging findings. Cytologic techniques, depending on the tumor location and type may be employed for primary diagnosis, prognosis, and prediction of tumor behavior as well as secondary/recurrent diagnoses, and may also be used for staging purposes. Cancer therapies are increasingly directed toward individual molecular targets; therefore, increasing the use of ancillary techniques in cytology. FNA material embedded in formalin-fixed cell blocks can be reliably used in immunohistochemical studies. In fact, the cell block technique for immunostaining shows better results compared with cytospins and smears. However, if cell block is not feasible, then cytospins or monolayer preparations may be used (5,6). Liquid based preparations provide better results for DNA and RNA extraction testing (7,8). It is important to note that a negative molecular test does not exclude a diagnosis, especially if strong clinical and cytomorphologic evidence is present to suggest a particular diagnosis; other ancillary tests may sometimes be necessary (9).
The cytomorphologic evaluation of gastrointestinal malignancies is highly dependent on the availability of expertise in procuring, processing and evaluating the cytologic specimens as well as the availability of specialized equipment. These resources are quite variable in different parts of the world as well as regionally within each country and medical institution. Material for cytomorphologic examination may be obtained by various means, depending on the location of the tumor and tumor type. Luminal lesions may be sampled endoscopically with brushings and lavage techniques. This is particularly useful in narrow, strictured lesions where access to the tumor by the biopsy forceps is limited (10,11). These techniques are also useful for sampling broad surface areas of precancerous lesions such as Barrett’s esophagus and chronic ulcerative colitis in which dysplastic and non dysplastic mucosa does not differ endoscopically. Deeper/submucosal and mural lesions may be sampled by fine needle aspiration (lymphomas and sarcomas). The needle aspiration techniques often require the additional use of imaging modalities at the time of sampling (ultrasound or other imaging techniques). Overall, cytologic techniques are particularly useful for preoperative diagnosis of gastrointestinal lesions that may otherwise be inaccessible or pose significant risks for standard biopsy method complications (bleeding, perforation, tumor dissemination). Also, cytologic preparations have a shorter turnaround time and are potentially cheaper than biopsies.
Cytologic specimens should first be examined at low/scanning power to assess smear background, overall cellularity, cellular preservation and architectural arrangements. Next, high power systematic screening should be performed for the presence of infectious agents and cytologic abnormalities. Reporting should include a mention of the specimen adequacy and sample preservation, and diagnostic language should be similar to that used for reporting histopathologic samples, with which clinicians are familiar. Every attempt should be made to give as definitive a diagnosis as possible. In cases where a specific diagnosis cannot be rendered, a differential or broader category should be used and the reason(s) for doing so should be reported. It is extremely helpful to discuss the more ambiguous cases with the responsible clinicians before the final report is rendered. Cytology is a screening as well as a diagnostic procedure. The absence of positivity for a malignant process does not exclude malignancy, as the sensitivity of the procedure is less than 100%. As always clinical, serologic (in certain cases) and radiologic correlation is essential with repeat sampling for suboptimal/inadequate samples or for additional ancillary testing. Interdisciplinary discussions (as in tumor boards) should be performed before definitive treatment is instituted.
Cytologic reporting
Cytologic diagnoses are reported using the conventional diagnostic nomenclature for nongynecologiccytologic specimens. The five general diagnostic categories are unsatisfactory, negative for malignancy, atypical/indeterminate, suspicious for malignancy, and positive for malignancy. If clinical and radiologic findings correlate as either benign or malignant with cytologic findings, the diagnoses are considered conclusive for benign or malignant disease. No additional or confirmatory studies are usually indicated. For lesions in which the clinical, radiologic, and cytologic diagnoses differ, additional studies are indicated. Also atypical/indeterminate and suspicious for malignancy cytologic diagnoses may warrant further diagnostic studies (12).
Specimen collection methods
Lavage
Lavage of mucosal lesions with isotonic saline.
Salvage cytology
First endoscopic biopsies of suspicious lesions are performed. Next the brush, biopsy forceps or the cytology brush channel of the endoscope is rinsed with a balanced salt solution. The sample is then centrifuged or filtered to produce smears and cell blocks.
Brushings
Brushings are obtained via the biopsy channel of the endoscope with two or three smears made with a rapid rolling motion of the brush on glass slides. The slides should be rapidly fixed in 95% ethanol for Papanicolaou staining or air dried for Romanowsky staining (e.g., Diff-Quik). Cell block preparations may also be obtained by rinsing the brush in fixative solution. Advantages of brushing cytology over biopsy include sampling of larger surface mucosal areas under direct visualization. It is also useful in obtaining samples from strictures of the gastrointestinal tract, when biopsy forceps sampling is not possible. Brush samples have been shown to be both sensitive and specific in detecting high grade dysplasia and carcinoma in the gastrointestinal tract (13). It is recommended that brush cytology should normally be performed before biopsy, as cumulative results were significantly better than results obtained by brushing after biopsy (14).
Direct smears
Imprint cytology from endoscopic biopsy specimens is particularly useful as an immediate assessment of adequacy of the biopsy sample and may also be helpful for triaging purposes. In addition, ancillary testing for example KRAS mutation detection in colon cancer may also be performed (15).
Transmucosal fine needle aspiration biopsy
Fine needle aspiration is useful in the diagnosis of deeper submucosal, mural and extrinsic mass lesions via direct endoscopy or visualization by radiologic means (endoscopic ultrasonography, ultrasound, CT guided methods). This method may also be used for preoperative staging as it permits sampling of adjacent lymph nodes and masses, as well as more distant metastases (16). The material obtained is processed for smears and cell block preparations, and can provide adequate material for ancillary studies.
Blind abrasive techniques
Balloon-like sampling devices have been used mainly in the esophagus. Cells are obtained from abrasion of the epithelial surface by inflation of the device, and then deflation for removal. Tissue from the balloon surface may be directly smeared onto glass slides, or rinsed into fixative solutions for smears and cell block preparations.
Abrasive balloon devices are inexpensive (costing one sixth that of endoscopy and biopsy), easy to use, and provide rapid results. They may be used for screening populations at high risk for esophageal carcinoma in the field by trained nonphysician medical professionals. There was a significant rate of detection of early squamous lesions when this technique was used in China, Iran, and South Africa, where rates of disease are sufficiently high to render screening cost effective (17-22). This technique has also recently been advocated for use in screening patients with long-segment Barrett’s esophagus in the United States.
Sample procurement and processing
The specimen sample must be processed optimally to maximize the diagnostic yield of the procedure. Air dried smears, alcohol fixed smears, and needle rinses in transport media for cytospin preparations and/or cell block preparations may be performed. The presence of a pathologist or cytotechnologist at the time of the procedure reduces the frequency of nondiagnostic specimens. Chang and colleagues showed that the presence of a pathologist in the endoscopy suite to perform immediate assessment resulted in an adequate specimen in 100 percent of cases, as compared with only 71 percent when a pathologist was not present (22).
The presence of a pathologist at the time of the procedure also permits appropriate triage of the aspiration material for ancillary studies, such as cultures, immunohistochemistry, and flow cytometry studies.
Upper gastrointestinal tract
Esophagus
The normal esophagus is lined by non-keratinized stratified squamous epithelium. Mucosal injuries, ulceration and infections evoke reactive and reparative changes which may be mistaken for dysplasia and carcinoma. Certain infectious agents have characteristic cytomorphology (yeast and pseudohyphal forms of Candida species, characteristic viral inclusions of Herpes simplex and CMV infections).
Reactive/reparative changes
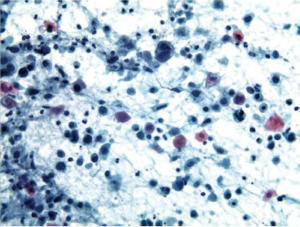
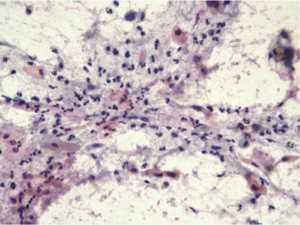
Cells are present in cohesive two dimensional/flat sheets. There is uniform nucleomegaly with vesicular chromatin, nucleoli and smooth thin nuclear borders. Mitotic figures may be present. There is an inflammatory background (Figure 2).
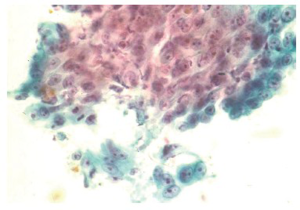
Radiation induced changes produce proportionate cellular and nucleomegaly, multinucleation, cytoplasmic metachromasia, nuclear and cytoplasmic vacuolation.
Chemotherapy induced changes are similar, but are more problematic as there is often increase in the nuclear to cytoplasmic ratio and nuclear irregularity.
The most reliable criteria to differentiate severe reactive atypia from malignancy are the lack of three dimensional groupings, cell dishesion, single cells, pleomorphism, coarse irregular chromatin and thick irregular nuclear membranes.
Squamous carcinoma
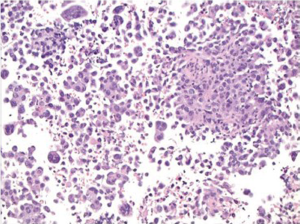
Squamous cell carcinoma is the most common esophageal malignancy in Black males and females in the United States. Cytologic smears are characterized by isolated tumor cells with increased nuclear to cytoplasmic ratios, nuclear hyperchromasia, dense cytoplasm with sharply defined borders are seen. There is a prominent “dirty” background tumor diathesis (Figure 3). The differential includes reactive changes and dysplasia (which lacks the tumor diathesis). The cytomorphologic features depend on the degree of differentiation. Some poorly differentiated carcinomas may be difficult to differentiate from adenocarcinomas without ancillary stains.
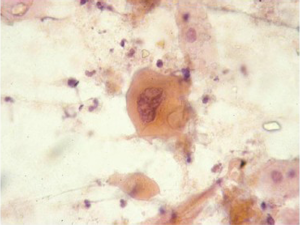
Barrett esophagus and dysplasia
Specialized intestinal epithelium with the characteristic goblet cells can be recognized on brush cytology (Figure 4). Owing to the inherent advantage of sampling a wider and circumferential area, brushing procedure is likely to be more representative and superior than multiple endoscopic biopsies (23). However, low grade dysplasia is difficult to differentiate from reactive changes. Adjunct use of new genetic markers, such as fluorescence in situ hybridization (FISH), may aid in differentiation (24). High grade dysplasia resembles adenocarcinoma, but lacks the tumor diathesis and cellular dispersion with discohesive single cells. It is clinically important to grade dysplasia as the management for high grade dysplasia differs with either more frequent surveillance intervals or resection (25,26). Multiple biomarkers, including p16 and p53 and nuclear DNA content abnormalities, have been proposed for predicting cancer risk; p53 and p21 protein accumulation has been found to correlate with increased grade/severity of dysplasia and risk of progression to carcinoma (27,28).
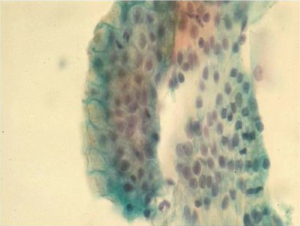
Adenocarcinoma
This is the most frequent esophageal malignancy in Whites males in the United States. Its incidence has risen in epidemic proportions (more than 350% in the past few decades) in this population group. Incidence rates have also increased in Black males, but still remain at much lower levels. These tumors are mostly located in the mid and distal third of the esophagus, and are presumed to arise in the setting of Barrett’s esophagus (Figure 5).
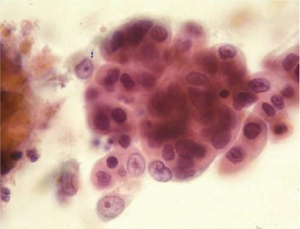
Adenocarcinoma cells are seen as numerous small clusters and glandular groups with overlapping and loss of polarity. Loosely cohesive cells and scattered single cells may be seen in a necrotic background. The cytoplasm is variable in amount, delicate, finely granular and may show vacuolation. The tumor cell nuclei are enlarged, pleomorphic, have irregular nuclear membranes and show prominent nucleoli. A background of Barrett’s intestinal metaplasia may be present. The differential includes severe repair, high grade dysplasia in Barrett’s epithelium and poorly differentiated squamous cell carcinoma.
Other neoplasms
Primary neuroendocrine tumors
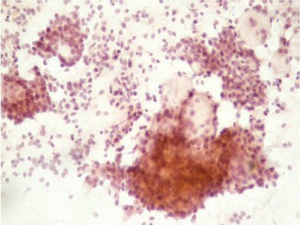
Primary malignant melanoma
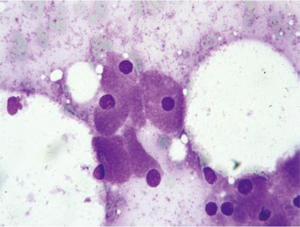
Lymphoma
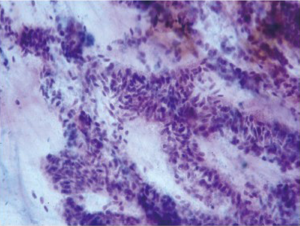
Stomach
Mostly mucus secreting columnar cells are seen in large cohesive sheets with a honeycomb pattern. The nuclei are basally situated, and have a fine chromatin pattern. The background is clean. Parietal, chief and neuroendocrine cells are rarely seen in brush specimens.
Epithelial repair, infection
Changes may be secondary to gastritis and ulceration. Morphologic changes are similar to changes described in the esophagus. Brushings should be taken from the center of the ulcer and the edges. Helicobacter pylori infection may be asymptomatic, present with chronic gastritis or ulceration. H.pylori infection may be a cofactor in the development of gastric carcinoma and lymphoma. Helicobacter organisms are short curved or spiral shaped rods that inhabit the mucus covering the epithelial surface of the gastric mucosa (Figure 9). The organisms are readily demonstrated by imprint cytology of gastric biopsy specimens and by brush cytology; the diagnostic sensitivity is 97% compared with approximately 76% in biopsies. Imprint cytology should be performed with care so as to not adversely affect the quality of the biopsy specimen (29-31).
Gastric dysplasia and adenomas
Gastric dysplasia is associated with atrophic gastritis. Dysplastic cells are present in flat sheets and show uniform nucleomegaly. Adenoma cells are seen in three-dimensional clusters. Dysplasia and adenomas are precursor lesions to carcinoma, and show similar cytologic features. Low grade dysplasia cannot be reliably differentiated from reactive changes and should not be diagnosed definitively. High grade dysplasia is similar to carcinoma but is less cellular, and lacks tumor diathesis, cell dispersion and marked pleomorphism.
Adenocarcinoma
Gastric adenocarcinomas are commonly divided into intestinal and diffuse (signet ring) cell types, and account for 90-95% of gastric malignancies.
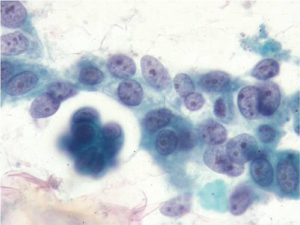
Intestinal type is usually associated with intestinal metaplasia of the gastric epithelium and resembles typical esophageal and colorectal carcinomas. There is a necrotic/inflammatory background, and numerous single malignant cells are present. Helpful criteria to diagnose well-differentiated adenocarcinoma include loosely cohesive three-dimensional groups of cells with loss of polarity and similar single cells in the background (Figure 10).
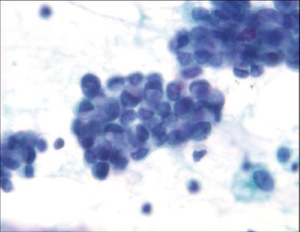
The diffuse type tends to be more infiltrative with less mucosal involvement and a higher rate of false-negative diagnosis by surface brushing techniques unless ulceration is present. The background is usually clean and lacks a tumor diathesis. The specimen is less cellular, with a majority of single cells. The tumor cells are round and smaller than intestinal type. Typical signet ring cells with hyperchromatic, eccentric sharply pointed (crescentic) nuclei and large cytoplasmic mucin vacuoles are present (Figure 11). Some signet ring cells may have bland nuclei and be confused with histiocytes. Signet ring carcinoma can be very difficult to detect on both cytologic and histologic specimens. High power examination, attention to detail and a high degree of suspicion is the best safeguard against failure to detect this carcinoma. If necessary, keratin, epithelial membrane antigen (EMA) and mucin stains are helpful in differentiating the single tumor cells from histiocytes. Histiocytes will express CD68 and KP-1 antibody.
Endocrine tumor
This is the second most common epithelial tumor of stomach. Usually presents as polypoid lesions. The tumor cells are dyshesive and monomorphic, with eccentric, stippled “salt and pepper” nuclei. Tumor cells have a moderate amount of granular cytoplasm, and may have a spindle cell appearance. Many stripped, bare nuclei may be present. Composite adenocarcinoma-neuroendocrine (carcinoid) tumors may occur.
Gastrointestinal endocrine tumors are classified into three categories: (I) Well-differentiated endocrine tumors; (II) Well-differentiated endocrine carcinomas; (III) Poorly differentiated endocrine (small cell) carcinoma.
Cytologicatypia, mitotic index, proliferative rate (MIB-1 staining) are important parameters of this classification.
The differential diagnosis includes adenocarcinoma and lymphoma. Endocrine differentiation can be confirmed by immunocytochemical stains for chromogranin, synaptophysin and CD 56. Adenocarcinoma cells will be both keratin and EMA positive. Lymphoma cells are positive for Leukocyte common antigen (LCA/CD45).
Lymphoma
Non-Hodgkin lymphoma is the second most common malignancy of the stomach. It accounts for around 5% of gastric malignancies, and its incidence is increasing. The stomach is the most common site for extranodal non-Hodgkin lymphomas. They are classified into low grade and high grade and have specific appearances. Lesions may be polypoid, fungating, ulcerative or infiltrative.
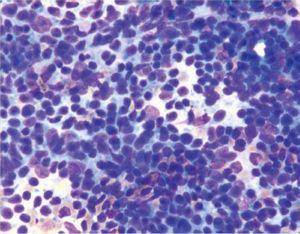
Cytologically there is a single cell population with dispersed monotonous cells and scant cytoplasm and many lymphoglandular bodies (Figure 12). The nuclei have a lymphoid chromatin character. In cellular specimens the lymphocytes may appear clumped, resembling epithelial cell groups. The differential diagnosis may include chronic inflammation, endocrine tumor and poorly differentiated carcinoma. Marker studies are required to confirm the diagnosis.
Gastrointestinal stromal tumor
GI stromal tumors (GIST) arise from the pacemaker cells of the GI tract, the interstitial cells of Cajal. Predictors of malignant behavior include tumor size, mitotic activity and necrosis, and are best evaluated on resected tumor specimens.
Aspirates show numerous spindle cells with delicate wispy cytoplasm. Rounded epithelioid cells (Figure 13) with vacuolated cytoplasm may also be present (Figure 14). Cells may resemble mesenchymal elements of normal stomach. CD117 and CD 34, as well as Ki-67 are useful immunocytochemical markers.
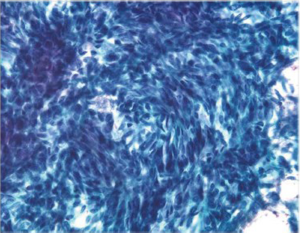
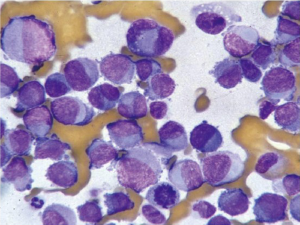
EUS-FNA is highly accurate for diagnosing GISTs and has a sensitivity of 82%, a specificity of 100%, and an overall accuracy of 86% (32). It is feasible to perform molecular analysis CKIT and PDGFRA (platelet derived growth factor receptor) genes in cytologic material obtained by EUS-FNA. Recently the use of discovered on GIST-1 (DOG-1) in cytology cell blocks was more sensitive and specific than CKIT in the diagnosis of GIST (33). The detection of specific mutations in cytologic samples allows the prediction of therapeutic response, enabling greater efficiency in the use of neoadjuvant therapy (34).
Duodenum
Normal duodenal mucosal cells are tall columnar cells with basal nuclei and “striated” apical cell borders. They form large, flat honeycomb sheets with interspersed mucin secreting goblet cells (Figure 15). The more proximal portions of the duodenum are evaluated by cytologic methods. The major pathologic disorders involve the mucosa, frequently near the ampulla of Vater.
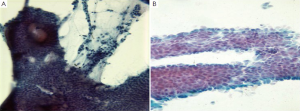
Cytologic techniques including brushings, washings and aspirates from the terminal common duct, extrahepatic biliary system and cannulated pancreatic duct are being increasingly utilized in the diagnosis of periampullary tumors. These diagnostic techniques provide greater access to these structures than the larger biopsy forceps at ERCP. The aspirates should be processed rapidly to prevent digestion of cells by the high enzyme contents. Transporting the specimens on ice and using a refrigerated centrifuge have been recommended.
Epithelial reparative changes may be seen in inflammatory disease, calculous disease, with stents and in benign tumors. Duodenal adenomas are often associated with an adenocarcinoma. Adenomatous lesions show small sheets and clusters of elongated columnar cells with granular chromatin and one or more nucleoli (35). High grade dysplastic change with nuclear overlapping, loss of polarity, hyperchromatic coarse clumped chromatin and dishesion may be identified (Figure 15). Single cells are more frequently seen with adenocarcinoma in comparison to dysplasia; however, brush cytology cannot always differentiate between high grade dysplasia and adenocarcinoma. Therefore cytologic sampling does not provide any significant improvement over biopsy diagnosis (36).
Multiple biopsies of diffuse lesions and surgical resection of the entire well defined lesion is indicated when a diagnosis of a premalignant lesion of the small intestine is suspected or rendered on cytologic examination. Adenocarcinoma, neuroendocrine tumors, lymphoma and GI stromal tumors may be seen, and have features similar to lesions in the stomach.
The majority of tumors in the duodenum and periampullary region are well differentiated adenocarcinomas. The difficulty of separating these well differentiated tumors from reactive changes makes the sensitivity of diagnosis relatively low and false negatives frequent. False negative diagnoses may also be due to desmoplasia, or poor sampling. False positive diagnoses are rare in experienced hands (37). The less common moderate to poorly differentiated tumors do not pose major diagnostic problems.
Lower gastrointestinal tract
Small intestine
The distal duodenum, jejunum and ileum are usually not sampled by cytologic means.
Large intestine
Cytologic examination of the large intestine is less frequently used than cytology of the upper GI tract. Cytologic differentiation of adenomas from well differentiated colonic adenocarcinomas and reactive/inflammatory changes is difficult. Therefore cytologic examination is of limited value in the work-up of the more common colonic lesions.
It may be of use to sample larger areas than tissue biopsy, assess large polyps, and evaluate patients with numerous polyps. It is often used as an adjunct to tissue biopsy in some centers, rendering the highest detection rate for malignancy. Surveillance cytology brush specimens from patients with Idiopathic Inflammatory bowel disease in the nonulcerated inactive phase of the disease may be used to screen for the presence of high grade dysplasia, which occurs without a visible colonic lesion. Oral lavage solutions may be used in the future to screen asymptomatic high-risk individuals for colonic malignancy (38). Imprint cytology of the peritoneum overlying a primary colonic tumor has been proposed as an adjunct to routine histology for more precise staging of serosal involvement (39).
Colonic adenocarcinomas show discohesive three dimensional aggregates of tumor cells (Figure 16). Branching papillary fragments and microacinar areas may be present. Cell groups show loss of polarity, with crowded disorderly arrangement. Tumor cells have round, oval or cigar shaped nuclei, and many single cells. There is a prominent “dirty” tumor diathesis.
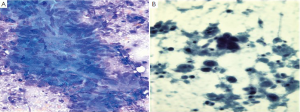
The cytologic diagnosis of neuroendocrine tumors, lymphoma and GI stromal tumors are as described for the stomach and small intestine above.
Anal canal
The incidence of anal HPV related squamous cell carcinoma is on the rise, especially in HIV positive men who have sex with men (MSM). Women who are HIV positive and women with cervical intraepithelial lesions (CIN) have an increased risk of HPV infections of the anal canal and anal intraepithelial lesions (AIN). Like cervical cancer, anal cancer is also associated with precursor lesions (AIN) detectable on exfoliative cytology. Anal-rectal cytology screening programs have been developed in an effort to detect and to eradicate precursor lesions prior to progression to invasive squamous cell carcinoma and are recommended for these population groups. Either conventional or liquid based anal-rectal cytology specimens are acceptable, but liquid based specimens are preferred, as apart from better morphologic details, residual liquid can be used for ancillary studies, such as testing for high-risk HPV DNA. Anal cytologic specimens may be collected by health care professionals or by patients using a gloved finger or by direct scraping/brushing (by means of an endocervical brush, wooden spatula, moistened cotton or Dacron swabs).
A minimum of 2,000-3,000 nucleated squamous cells comprise adequate specimens. Some glandular/columnar cells from the anal transition zone should ideally be present to indicate that the anorectal transition zone has been sampled (Figure 17). Many anal squamous dysplasias and carcinomas arise in this transition zone. Proper training and experience in obtaining these specimens yields satisfactory specimens. The evaluation of anal Pap slides is reported in a manner similar to that of gynecologic Pap test slides. Anal intraepithelial neoplasia (AIN) is divided into low and high grade by criteria similar to those used for cervical squamous dysplasias. Diagnostic terminology as defined by the Bethesda System for Reporting Cervical Cytology (TBS 2001) (40) should be used. Cytologic interpretations on anal specimens do not always correlate with severity of lesions identified on subsequent biopsy; thus, patients with atypical squamous cells of undetermined significance (ASC-US) or worse should be referred for anoscopy (41,42).
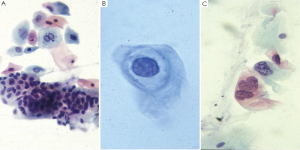
Neoplasms
Neuroendocrine tumors
Lymphoma
Melanoma
GI stromal tumors
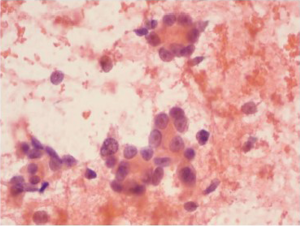
Interpretive pitfalls in gastrointestinal tract cytology
It is important to be aware of which structures the brush or needle is passing through in obtaining the cytological samples, to be aware of differences and avoid potential pitfalls, because normal tissues as well as lesional tissue may be sampled. Potential pitfalls are as follows: Lymph nodes. Contamination of a lymph node FNA by normal gastrointestinal mucosa is an important diagnostic pitfall. For example, EUS-FNA of a perigastric lymph node might produce a specimen containing sheets of normal gastric mucosa.
EUS-FNA is increasingly being used for the diagnosis of gastrointestinal stromal tumors (GISTs), and other spindle cell tumors (for example, leiomyomas), as the technique permits sampling of deep-seated mural lesions. Pathologists need to be careful to avoid over-interpreting normal gastrointestinal smooth muscle as a neoplastic process. Differentiation of GIST from other primary spindle cell tumors has important therapeutic implications; and immunohistochemical (CD117, CD34, smooth muscle actin, muscle specific actin, S-100 protein) stains are useful for the differential diagnosis. Finally, the pathologist should remain aware that some spindle cell neoplasms of the gastrointestinal tract may be metastatic lesions; spindle cell melanoma is a classic example of a metastatic lesion that may be misinterpreted as GIST.
Summary
Interest in gastrointestinal cytology has mirrored technical advances in this field over the last few decades. These advances allow the visualization of and simultaneous brushing of abnormal mucosa, obtaining needle aspirates and excising mucosal biopsy samples for pathologic evaluation. The use of EUS-FNA now helps in the diagnosis of submucosal and deeper seated lesions, preoperative staging of gastrointestinal tract malignancies, and determining further management of patients. EUS-FNA has thus revolutionalized the practice of gastrointestinal medicine and is rapidly becoming the technique of choice for sampling deep-seated lesions that were previously accessible only by laparotomy. Such advances have brought pathologists to the forefront for the management of gastrointestinal tract lesions.
These newer techniques have also presented challenges for pathologists. They can be time-consuming and therefore require an organized endoscopy service with good communication between endoscopist and pathologist so that the demands on the pathology laboratory and the pathologist’s time are minimized. There are also important issues related to reimbursement. Finally, the interpretations of GI tract cytology are fraught with pitfalls, most of which are the result of contamination of the specimen by normal enteric mucosal elements. Awareness of these pitfalls can improve diagnostic accuracy and prevent false-positive diagnoses.
Cytologic evaluation provides rapid interpretation, is a less invasive technique than open biopsy, and provides a cost-effective modality for the diagnosis and management of gastrointestinal lesions. Requisite patient information, on site evaluation and effective communication are important to improve diagnostic accuracy.
Acknowledgements
Disclosure: The authors declare no conflict of interest.
References
- Ohsaki H, Nakamura M, Arie K, et al. Endometriosis of sigmoid colon mimicking malignant tumor diagnosed by intraoperative imprint cytology. Diagn Cytopathol 2012;40:159-62. [PubMed]
- Geisinger KR. Endoscopic biopsies and cytologic brushings of the esophagus are diagnostically complementary. Am J Cln Pathol 1995;103:295-9. [PubMed]
- Logroño R, Waxman I. Interactive role of the cytopathologist in EUS-guided fine needle aspiration: an efficient approach. Gastrointest Endosc 2001;54:485-90. [PubMed]
- Vilmann P, Jacobsen GK, Henriksen FW, et al. Endoscopic ultrasonography with guided fine needle aspiration biopsy in pancreatic disease. Gastrointest Endosc 1992;38:172-3. [PubMed]
- Fetsch PA, Simsir A, Brosky K, et al. Comparison of three commonly used cytologic preparations in effusion immunocytochemistry. Diagn Cytopathol 2002;26:61-6. [PubMed]
- Bibbo M. How technology is reshaping the practice of nongynecologic cytology: frontiers of cytology symposium. Acta Cytol 2007;51:123-52. [PubMed]
- Filho AL, Gonçalves AE, Martinho O, et al. Liquid-based cytology in DNA-based molecular research: viability and potential application. Anal Quant Cytol Histol 2009;31:395-400. [PubMed]
- Wohlschlaeger J, Worm K, Schmitt F, et al. Assessment of DNA, small NCRNA and MRNA detection over time in liquid-based cytology specimens [abstract]. Cytopathology. 2009;20, Issue Supplement s1:67.
- Schmitt F, Barroca H. Role of ancillary studies in fine-needle aspiration from selected tumors. Cancer Cytopathol 2012;120:145-60. [PubMed]
- Cohen J. Successful Training in Gastrointestinal Endoscopy. Boston: Wiley-Blackwell; 2011.
- Xing GS, Geng JC, Han XW, et al. Endobiliary brush cytology during percutaneous transhepaticcholangiodrainage in patients with obstructive jaundice. HepatobiliaryPancreat Dis Int 2005;4:98-103.
- Guidelines of the Papanicolaou Society of Cytopathology for fine-needle aspiration procedure and reporting. The Papanicolaou Society of Cytopathology Task Force on Standards of Practice. Mod Pathol 1997;10:739-47. [PubMed]
- Alexander JA, Jones SM, Smith CJ, et al. Usefulness of cytopathology and histology in the evaluation of Barrett’s esophagus in a community hospital. Gastrointest Endosc 1997;46:318-20. [PubMed]
- Keighley MR, Thompson H, Moore J, et al. Comparison of brush cytology before or after biopsy for diagnosis of gastric carcinoma. Br J Surg 1979;66:246-7. [PubMed]
- Malapelle U, Bellevicine C, Russo A, et al. KRAS testing on colo-rectal carcinoma cytological imprints. Diagn Cytopathol 2011;39:274-7. [PubMed]
- Knight CS, Eloubeidi MA, Crowe R, et al. Utility of endoscopic ultrasound-guided fine-needle aspiration in the diagnosis and staging of colorectal carcinoma. DiagnCytopathol 2011; [Epub ahead of print].
- The Coordinating Group for the Research of Esophageal carcinoma. Chinese Academy of Medical Sciences and Honan Province.The early detection of carcinoma of the esophagus. ScientiaSinica 1973;16:457-63.
- The use of exfoliative cytology in clinical diagnosis and mass surveys for cancer of the esophagus. Chinas Med 1967;6:479-84. [PubMed]
- Dowlatshahi K, Daneshbod A, Mobarhan S. Early detection of cancer of oesophagus along Caspian Littoral.Report of a pilot project. Lancet 1978;1:125-6. [PubMed]
- Berry AV, Baskind AF, Hamilton DG. Cytologic screening for esophageal cancer. Acta Cytol 1981;25:135-41. [PubMed]
- Huang GJ, Shao LF, Zhang DW, et al. Diagnosis and surgical treatment of early esophageal carcinoma. Chin Med J (Engl) 1981;94:229-32. [PubMed]
- Chang KJ, Katz KD, Durbin TE, et al. Endoscopic ultrasound-guided fine-needle aspiration. Gastrointest Endosc 1994;40:694-9. [PubMed]
- Padmavathy F, Siddaraju N, Sistla SC. Role of brush cytology in the diagnosis of Barrett’s esophagus: an analysis of eight cases. Diagn Cytopathol 2011;39:60-4. [PubMed]
- Brankley SM, Wang KK, Harwood AR, et al. The development of a fluorescence in situ hybridization assay for the detection of dysplasia and adenocarcinoma in Barrett’s esophagus. J Mol Diagn 2006;8:260-7. [PubMed]
- Reid BJ, Blount PL, Feng Z, et al. Optimizing endoscopic biopsy detection of early cancers in Barrett’s high-grade dysplasia. Am J Gastroenterol 2000;95:3089-96. [PubMed]
- Buttar NS, Wang KK, Sebo TJ, et al. Extent of high-grade dysplasia in Barrett’s esophagus correlates with risk of adenocarcinoma. Gastroenterology 2001;120:1630-9. [PubMed]
- Wang KK, Sampliner REPractice Parameters Committee of the American College of Gastroenterology. Updated guidelines 2008 for the diagnosis, surveillance and therapy of Barrett’s esophagus. Am J Gastroenterol 2008;103:788-97. [PubMed]
- Chatelain D, Fléjou JF. High-grade dysplasia and superficial adenocarcinoma in Barrett’s esophagus: histological mapping and expression of p53, p21 and Bcl-2 oncoproteins. Virchows Arch 2003;442:18-24. [PubMed]
- Mendoza ML, Martín-Rabadán P, Carrión I, et al. Helicobacter pylori infection. Rapid diagnosis with brush cytology. Acta Cytol 1993;37:181-5. [PubMed]
- Kaur G, Madhavan M, Basri AH, et al. Rapid diagnosis of Helicobacter pylori infection in gastric imprint smears. Southeast Asian J Trop Med Public Health 2004;35:676-80. [PubMed]
- KnezevićStromar I. Jakić-Razumović J, Knezević-Obad A. Imprint cytology of gastric mucosa biopsy--fast, simple and reliable method for detection of Helicobacter pylori infection. CollAntropol 2008;32:171-5.
- Watson RR, Binmoeller KF, Hamerski CM, et al. Yield and performance characteristics of endoscopic ultrasound-guided fine needle aspiration for diagnosing upper GI tract stromal tumors. Dig Dis Sci 2011;56:1757-62. [PubMed]
- Hwang DG, Qian X, Hornick JL. DOG1 antibody is a highly sensitive and specific marker for gastrointestinal stromal tumors in cytology cell blocks. Am J Clin Pathol 2011;135:448-53. [PubMed]
- Gomes AL, Bardales RH, Milanezi F, et al. Molecular analysis of c-Kit and PDGFRA in GISTs diagnosed by EUS. Am J Clin Pathol 2007;127:89-96. [PubMed]
- Veronezi-Gurwell A, Wittchow RJ, Bottles K, et al. Cytologic features of villous adenoma of the ampullary region. Diagn Cytopathol 1996;14:145-9. [PubMed]
- Yu GH, Nayar R, Furth EE. Adenocarcinoma in colonic brushing cytology: High-grade dysplasia as a diagnostic pitfall. Diagn Cytopathol 2001;24:364-8. [PubMed]
- Logrono R, Kurtycz DF, Molina CP, et al. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: the experience at 2 university hospitals. Arch Pathol Lab Med 2000;124:387-92. [PubMed]
- Rosman AS, Federman Q, Feinman L. Diagnosis of colon cancer by lavage cytology with an orally administered balanced electrolyte solution. Am J Gastroenterol 1994;89:51-6. [PubMed]
- Murphy PD, Hoffman J, Karczenski C, et al. Serosal imprint cytology in colonic cancer: a simple staging technique. Int J Colorectal Dis 1994;9:96-9. [PubMed]
- Nayar R, Solomon D. Second edition of ‘The Bethesda System for reporting cervical cytology’ - atlas, website, and Bethesda interobserver reproducibility project. Cytojournal 2004;1:4.
- Chin-Hong PV, Palefsky JM. Natural history and clinical management of anal human papillomavirus disease in men and women infected with human immunodeficiency virus. Clin Infect Dis 2002;35:1127-34. [PubMed]
- Campion MB, Kipp BR, Humphrey SK, et al. Improving cellularity and quality of liquid-based cytology slides processed from pancreatobiliary tract brushings. Diagn Cytopathol 2010;38:627-32. [PubMed]