Diagnosis and differential diagnosis of hepatic graft versus host disease (GVHD)
Introduction
Allogeneic hematopoietic cell transplantation (HCT) is a widely used therapy for a variety of malignant and nonmalignant hematologic diseases. Graft versus host disease (GVHD) is a common complication that (in its acute form) manifests as injury to the skin, gastrointestinal (GI) mucosa, and liver. Although GVHD is associated with increased overall morbidity and mortality (1-7), early recognition and prompt management can prevent the development of permanent organ damage. Diagnosis of hepatic GVHD, in particular, can be challenging due to non-specific clinical signs and symptoms and the fact that histopathologic features demonstrate significant overlap with those of other entities seen in the post-HCT setting.
Molecular biology of GVHD
On a cellular level, acute GVHD represents an exaggerated but otherwise normal response of donor immune cells to “foreign” (host) antigens. GVHD is thought to be initiated by tissue injury (either directly as a result of the conditioning regimen or indirectly by microorganisms that cross a compromised mucosal/skin barrier as a result of conditioning). This initial insult leads to upregulation of key surface receptors on host antigen-presenting cells and increased presentation of “foreign” antigens. Activation and proliferation of donor T-cells follows and culminates in destruction of susceptible host tissues by donor cytotoxic T and NK cells. Interestingly, while similar mechanisms are thought to occur in all GVHD target organs, some studies suggest that the downstream molecular signals in each organ may be distinct. For example, hepatic GVHD may be mediated by Fas ligands whereas skin and gut GVHD may be mediated by perforin and granzyme pathways (8,9). It is thought that organs such as skin, gut, and liver are specifically targeted due to their higher basal expression of MHCII antigens, and/or proximity to the initial insult (e.g., microorganisms on skin surface or GI mucosa).
In contrast to acute GVHD, the underlying mechanisms of chronic GVHD are not fully understood. In the liver, there is some evidence that donor T follicular helper cells play a role by causing aberrant B-cell function in germinal centers and alloantibody deposition (10). However, much more research is needed to understand the cellular events leading to chronic GVHD.
GVHD: definition
GVHD was historically defined as acute or chronic based on the onset of clinical findings. In this system, acute GVHD was defined as that which occurs within the first 100 days post-transplantation and chronic GVHD as that which occurs after 100 days post-transplantation. However, due to advances in therapy and in our understanding of GVHD, there has been a recognition that the 100-day criterion does not accurately describe the spectrum of findings representing GVHD. This recognition has led to the establishment of more specific criteria distinguishing acute from chronic GVHD, and are based on specific clinical, laboratory, and histopathologic findings, rather than time of onset. These changes are summarized in the 2005 (11) and 2006 (12) reports of the NIH working group on Criteria for Clinical Trials in Chronic Graft-verus-host disease and have been revised in 2014 (13,14).
According to NIH criteria, acute GVHD is defined by specific abnormalities of the skin, GI tract, and liver that occur in two general scenarios: (I) classic—occurring within the first 100 days post-transplantation; or (II) persistent, recurrent, or late onset—occurring after 100 days but with identical features as those of classic acute GVHD (usually associated with steroid taper or withdrawal of immunosuppression). The diagnosis of acute GVHD is based on a combination of clinical and (specifically defined) biopsy findings (12).
According to the most recently published National Institutes of Health (NIH) consensus development project on criteria for clinical trials in chronic GVHD, it is also recognized as having two forms: (I) classic—which demonstrates specific clinical findings defined as chronic GVHD and lacks the (histologic) findings of acute GVHD in skin, GI tract, and liver; and (II) overlap—which demonstrates specific clinical findings defined as chronic GVHD and additionally demonstrates at least some features of acute GVHD (e.g., specific skin changes, GI mucosal injury, intrahepatic bile duct damage) (13).
In contrast to acute GVHD in which tissue biopsy may play an important role, chronic GVHD is defined by specific clinical criteria, and these changes may involve a much wider range of tissues, such as lung, genital tract, muscles and joints (in addition to skin, GI tract, and liver). By design, these criteria are based on clinical signs and symptoms that can be easily assessed in an office setting by physical examination, with the exception of lung function and liver function. No tissue biopsy is required (although tissue biopsy may be useful in cases lacking diagnostic clinical findings). Thus, chronic GVHD is typically a clinical diagnosis.
Clinical presentation of hepatic GVHD
Hepatic GVHD can present clinically in three different ways: (I) with marked elevation in alkaline phosphatase and total bilirubin and milder elevations in aspartate transaminase (AST) and alanine transaminase (ALT); (II) with sharp elevations in AST and ALT with or without jaundice; and (III) with slowly progressive cholestasis.
The first presentation, manifested by elevated alkaline phosphatase (usually two or more times the upper limit of normal), increased total bilirubin, and milder elevations in AST and ALT is the typical picture of acute GVHD and usually occurs in the first two weeks post-HCT. Patients may present with jaundice and typically have a history of preceding skin rash and diarrhea (e.g., other sequelae of acute GVHD).
The second presentation, known as the hepatitic variant, is characterized by sharp elevation in aminotransferases (greater than ten times the upper limit of normal) with relatively milder increases in alkaline phosphatase. This presentation is almost always associated with tapering of immunosuppression or donor lymphocyte infusion. Whereas in the typical acute GVHD presentation in which skin and gut findings usually precede liver abnormalities, the hepatitic variant may present without evidence of skin or gut disease.
In the third presentation, a slow but progressive increase in alkaline phosphatase and gamma-glutamyl transpeptidase (GGT) levels is seen, followed by jaundice. Although by NIH criteria, no laboratory or histopathologic features are recognized as diagnostic of chronic GVHD in the liver, indolent cholestatic disease is the classic clinical presentation of liver involvement in the setting of chronic GVHD and is usually associated with skin, mouth, and eye changes specific to chronic GVHD. Given this pattern of organ involvement, the indolent cholestatic presentation of GVHD can clinically resemble autoimmune disorders, such as Sjogren syndrome and autoimmune hepatitis (11,13).
Indications for liver biopsy in HCT patients
Due to the risk of complications such as bleeding, liver biopsy is infrequently performed in the evaluation of post-HCT liver dysfunction. However, it may be valuable when findings in other organ systems are equivocal, when there are multiple possibilities in the clinical differential diagnosis, and when clinical and/or laboratory findings show no improvement after presumed adequate empiric therapy.
Histopathologic findings in hepatic GVHD
Recommended stains for histopathologic evaluation include: H&E, connective tissue stain (e.g., Masson trichrome), PAS, PAS with diastase (PASD), reticulin, iron, and CK7 (or CK19). PAS and PASD stains allow for evaluation of phagocytic activity (an indicator of hepatic damage) and highlight the basement membrane of the biliary epithelium; reticulin stain allows for evaluation of parenchymal collapse; iron stain aids in the evaluation of iron overload; and CK7 (or CK19) highlights biliary epithelium, facilitating assessment of bile duct loss (15).
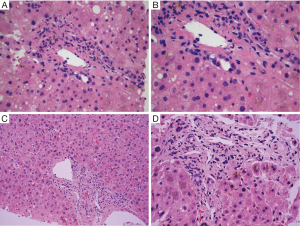
The characteristic finding in hepatic GVHD is damage to biliary epithelium. Biliary epithelial changes include nuclear pleomorphism, loss of nuclear polarity, nuclear overlap, cytoplasmic vacuolization, eosinophilic change of the cytoplasm, and rarely apoptosis. Infiltration of lymphocytes into biliary epithelium may be seen, and there is generally a relatively sparse portal lymphocytic infiltrate (Figure 1A,B). Changes are typically found in small (interlobular) bile ducts and may not affect all portal areas (e.g., patchy involvement), depending on the timing of the biopsy. Evidence of cholestasis is generally present, which may include hepatocellular bile accumulation and canalicular bile plugs. In contrast to pure downstream duct obstruction and some drug reactions, bile ductular proliferation is usually not prominent, and other features of downstream duct obstruction (e.g., portal edema, intraepithelial neutrophils) are not typically seen. Lobular inflammation is typically mild and predominantly composed of lymphocytes. Endothelialitis is variably present but is thought to be a relatively specific finding for GVHD in the context of HCT (16).
In the hepatitic pattern, prominent necroinflammatory foci with scattered acidophil bodies and lobular disarray are characteristic. Portal areas may demonstrate mixed inflammation including lymphocytes, macrophages, plasma cells, and eosinophils (17). Bile duct damage may be present, although it may be less pronounced than in classic GVHD.
Although by NIH guidline, there are no specific histopathologic criteria for the diagnosis for chronic GVHD in the liver, longstanding steroid refratory GVHD is associated with ductopenia and fibrosis (e.g., sequelae of chronic bile duct injury) and may be associated with the clinical picture of slowly progressive cholestasis (Figure 1C,D). These findings are also defined as “late phase” changes (Table 1).
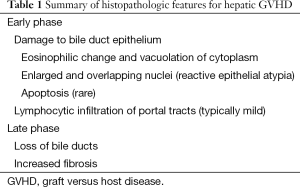
Full table
Diagnosis of hepatic GVHD
The forthcoming updated NIH guidelines on the Histopathologic Diagnosis of Chronic GVHD to be published this year endorses three diagnostic categories for GVHD: (I) no GVHD; (II) possible GVHD; and (III) likely GVHD (includes consistent with and diagnostic of GVHD) (personal communication from H. M. Shulman). The distinctions between these categories are based on histopathologic findings and the availability of relevant clinical history.
Key considerations include the following:
- There are no absolute histopathologic criteria for the diagnosis of hepatic GVHD. As with most cellular processes, there is a spectrum of changes, and no one feature is pathognomonic. Thus, it is necessary to integrate clinical history, clinical findings, and laboratory results in order to arrive at the diagnosis. Furthermore, hepatic GVHD may be considered a diagnosis of exclusion, as other etiologies with overlapping histologic features must be excluded.
- It is unusual for GVHD to present with liver only involvement. As such, the pathologist should inquire about additional clinical findings (e.g., skin rash, diarrhea), if these are not provided by the clinical team. In addition, biopsies of skin and/or GI tract mucosa have often been performed prior to liver biopsy, and those findings should be taken into account when considering the diagnosis of hepatic GVHD. For example, if skin and/or colon biopsies reveal no features suggestive of GVHD but liver biopsy reveals some of the histopathologic features of GVHD, it is advisable to refrain from rendering a diagnosis of unequivocal hepatic GVHD and instead consider “possible GVHD”, provide a comment in which GVHD is in the differential diagnosis, and suggest exclusion of other etiologies with overlapping histology (see discussion of differential diagnoses below).
- If the patient has received prior immunosuppression (e.g., GVHD prophylaxis or treatment for GVHD prior to liver biopsy), the liver biopsy is likely to have less necroinflammatory activity and portal inflammation than is typically seen in acute GVHD (17,18). In addition, if a biopsy is taken soon after onset of symptoms, histologic features of GVHD may not yet be fully developed.
- The prevalence of GVHD in the post-HCT setting is high; thus, the positive predictive value of a diagnosis of GVHD is relatively high and the negative predictive value is relatively low. Particularly if the biopsy material is scant or contains few portal tracts, the absence of features supportive of GVHD does not exclude the possibility of GVHD, and these limitations should be conveyed in the biopsy report.
- Because it is unclear if the degree of biliary damage predicts outcome or response to therapy, grading of hepatic GVHD (using Lerner or other criteria) is not recommended. Furthermore, whether ductopenia is reversible is unknown. At least anecdotally, there is some evidence that portal bile ducts have the capacity to regenerate.
Differential diagnosis of hepatic GVHD
The differential diagnosis of liver dysfunction in the post-HCT setting is long and includes infection (particularly viral), drug-induced liver injury (DILI), immunotherapy (IT)-related hepatotoxicity, sepsis-associated cholestasis, sinusoidal obstructive syndrome (SOS), and malignancy. For some of these etiologies the clinical picture will be different from that of hepatic graft-versus-host disease, and this will help narrow the differential diagnosis. However, for others close attention to histopathologic features in the liver biopsy is absolutely necessary.
Viral hepatitis
Although less common in the era of viral screening and viral prophylaxis, acute hepatitis due to hepatitis B virus (HBV), herpes simplex virus (HSV), varicella zoster virus (VZV), and adenovirus can rarely lead to fulminant hepatitis in the transplant setting. In contrast, liver abnormalities due to hepatitis C virus (HCV) and cytomegalovirus (CMV) infection are more common but are typically mild and generally not the cause of severe liver dysfunction in HCT patients.
The histopathologic features of acute viral hepatitis are variable, but scattered foci of necrosis with or without viral inclusions in adjacent hepatocytes is a classic finding. Bile duct injury is rare, except in chronic HCV (which is well known to cause damage to portal bile ducts). In contrast to GVHD, however, biliary changes due to HCV are usually focal and associated with a well circumscribed lymphoid aggregate (Figure 2A,B).
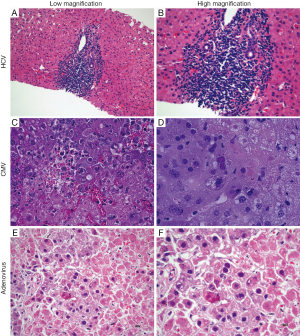
Most commonly exclusion of viral etiologies will rely on laboratory testing; however, two viruses, adenovirus and CMV infection may be identified by the presence of characteristic nuclear inclusions. The characteristic finding in CMV hepatitis (Figure 2C,D) is scattered neutrophilic foci (e.g., microabscesses) centered on degenerating virus-infected hepatocytes with large eosinophilic nuclear inclusions with or without amphophilic granular cytoplasmic inclusions. Scattered small macrophage aggregates (e.g., microgranulomas) also may be seen. Immunohistochemical staining may be helpful for confirmation.
In adenoviral hepatitis (Figure 2E,F), two patterns of injury have been described. In the first, scattered foci of hepatocyte necrosis are seen with minimal associated inflammation. Nuclear viral inclusions, highlighted by “smudgy” chromatin, may be visible at the interface of necrosis and viable hepatocytes. A second pattern (which may be an earlier phase of the first) demonstrates scattered loose aggregates of macrophages in hepatic parenchyma. Within the aggregates, degenerating hepatocytes may be seen; viral inclusions may be present at the interface of the macrophage aggregates and uninvolved parenchyma. An adenovirus immunostain may be useful for confirmation and to exclude the possibility of CMV infection.
Drug-induced liver injury (DILI)
DILI is one of the most frequent causes of severe liver dysfunction after HCT (7,19). Common medications causing DILI are those used for myeloablative conditioning (e.g., cyclophosphamide, bis-chloroethylnitrosourea, busulfan), other chemotherapy agents (e.g., cytarabine, carmustine, mitomycin, 6-mercaptopurine, dacarbazine), drugs for GVHD prophylaxis (e.g., cyclosporine, methotrexate), and antimicrobials (e.g., amphotericin and azole antifungal agents, trimethoprim-sulfamethoxazole, ribavirin) (5,20). Given the number of medications used and their sometimes unpredictable effect on liver function, it is often difficult to define the contribution of any single drug.
The most common histopathologic patterns of DILI are (I) necroinflammatory; (II) and cholestatic. Necroinflammatory injury can demonstrate a range of appearances but classically demonstrates zonal necrosis and minimal lobular inflammation. Other features may include ballooning degeneration of hepatocytes, hepatic rosette formation, and ductular reaction. Increased numbers of eosinophils may be seen. This pattern of injury may mimic the hepatitic pattern of GVHD; however, histologic findings that favor drug injury over GVHD include the presence of eosinophils in portal areas and significant bile ductular proliferation.
Cholestatic injury also has a range of appearances, and two patterns in particular can mimic GVHD: (I) cholangitic and (II) chronic. With the cholangitic cholestasis pattern (most often seen with macrolide antibiotics), hepatocellular and canalicular cholestasis is seen along with active bile duct injury. Histologic features include lymphocytic infiltration, reactive changes, and apoptosis of bile duct epithelium. The chronic cholestasis pattern is characterized by loss of bile ducts and relatively sparse portal lymphocytic infiltrates. Lobular cholestasis may or may not be present. This pattern mimics the late phase of GVHD. Again, the presence of portal eosinophils and bile ductular reaction favor DILI over GVHD.
Immunotherapy (IT)-induced hepatotoxicity
IT in the treatment of cancer has recently demonstrated significant clinical responses and is being increasingly applied (21,22). IT therapeutic strategies, including vaccines, adoptive IT, cytokines, and antibodies, can induce immunity against tumor antigens. However, these immune responses can also cause damage to a variety of organ systems including the liver (23). Studies suggest that IT regimens may result in a cytokine storm that contributes to systemic toxicities and immune alterations, rendering patients more susceptible to complications (22,24). Clinical signs and symptoms of IT-induced hepatotoxicity are usually nonspecific. Histopathologic features are equally non-specific and include parenchymal lymphocytic infiltrates, portal lymphocytic aggregates, and liver necrosis (24). Because this type of hepatoxicity often requires intensive care management, when an IT-treated patient presents with acute hepatitis shortly after HCT, this differential diagnosis of IT induced damage should always be considered. However, because bile duct injury is not a specific feature, it may not be straightforward to distinguish it form GVHD.
Sepsis-associated cholestasis
In the first few weeks post-transplant, HCT patients may present with severe jaundice, fevers, and abnormal liver function tests. Such patients should be evaluated for evidence of sepsis. Endotoxins and cytokines released as a result of septicemia inhibit secretion of intrahepatic bile and can lead to cholestasis. Histopathologic findings include hepatocellular cholestasis, ductular reaction with acute cholangiolitis, and cholangiolar bile plugs. In contrast, hepatic GVHD typically shows neither significant bile ductular proliferation nor cholangiolar bile plugs.
Sinusoidal obstructive syndrome (SOS)
SOS, formerly known as veno-occlusive disease (VOD), is a serious and potentially fatal complication of HCT caused by some conditioning regimens (e.g., total body irradiation and high dose chemotherapy) (7,19,25,26). Toxins released as a result of the conditioning regimen cause injury to the endothelium of sinusoids and central venules and activation of the coagulation cascade. This in turn leads to deposition of fibrinogen and other proteins in the venular walls and perisinusoidal space and may cause fibrous obliteration of central venules (20). The end result is a marked elevation in hepatic venous pressure.
Although the overall incidence of SOS has declined due to changes in conditioning regimens, it is still an important cause of liver dysfunction in the post-HCT setting. Typically occurring 3 to 4 weeks post-HCT, its onset is signaled by jaundice, tender hepatomegaly, thrombocytopenia, ascites, and weight gain (5,7,19). SOS may be diagnosed clinically using the modified Seattle diagnostic criteria which rely on total bilirubin, weight gain, and the presence of right upper quadrant pain or hepatomegaly. However, if clinical findings are equivocal, transjugular liver biopsy can be extremely useful, particularly because this approach allows measurement of the hepatic venous pressure gradient (27).
Histopathologic features of SOS are distinct from those of graft-versus-host disease and include perisinusoidal and perivenular edema and hemorrhage, sinusoidal congestion, and hemorrhagic centrilobular necrosis. Late findings include sinusoidal collagen deposits, central vein occlusion, and sclerosis of venular walls (5,7,19).
Lymphoma
Allogeneic HCT has become the standard of care for patients with life-threatening hematologic malignancies such as high-risk leukemias and aggressive lymphomas (28-30). However, hepatic GVHD is a serious complication and can be extremely challenging to distinguish from liver involvement by primary (31-33) or relapsed T-cell lymphoma based on radiographic and routine histologic analysis. Fortunately, primary hepatic lymphoma (PHL) is rare, constituting only 0.016% of all non-Hodgkin lymphoma (34). The predominant type of PHL is B-cell lymphoma and is most commonly diffuse large B cell type (DLBCL) (35).
Morphologic features of PHL are relatively non-specific, but typically show much more prominent atypical lymphocytic infiltration than GVHD (34,36). Both nodular and diffuse growth patterns are observed. The nodular variant is associated with destructive growth, causing obliteration of adjacent portal tracts. In the diffuse variant, the neoplastic lymphoid cells extend along the sinusoids and infiltrate portal tracts, leaving the cellular architecture of liver intact. Immunophenotypical abnormalities by flow cytometry or immunohistochemistry are required for diagnosis of lymphoma. Cytogenetics and molecular studies to demonstrate clonality by immunoglobulin heavy chain/light chains or T-cell receptor gene rearrangements can also support a diagnosis of lymphoma.
Post-transplant lymphoproliferative disorder (PTLD)
PTLD, also known as EBV-positive lymphoid infiltration, consists of a group of diverse diseases ranging from benign self-limited polyclonal lymphoid hyperplasias to clonal malignancies (37,38) (Figure 3). Although the pathophysiology of PTLD is only partially understood, prior Epstein-Barr virus (EBV) infection and transplant-related immunosuppression are key elements in the establishment of PTLD. While most PTLD cases arise in the setting of solid organ transplantation (35), it has also been demonstrated following allogeneic HCT (39). The reported incidence ranges between 0.6% and 10%. HLA mismatch, splenectomy, and recipient EBV seronegativity are risk factors for PTLD following allogenic HCT (40). PTLD is a heterogenous group of disease, ranging from reactive B-cell hyperplasia to immunoblastic lymphoma, the latter portending a more grim prognosis The pathological diagnosis of PTLD is based on the 2008 WHO classification and includes four main categories: (I) early lesions; (II) polymorphic PTLD; (III) monomorphic PTLD; and (IV) classic Hodgkin lymphoma type PTLD (35).
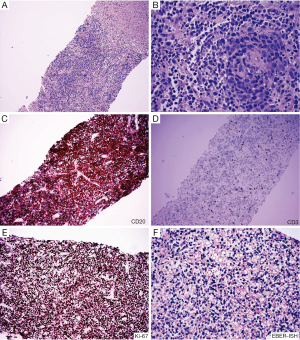
Patients typically present during the first year after HCT with non-specific clinical signs and symptoms. PTLD may form liver masses and present with biliary obstruction. Alternatively, diffuse infiltration of the liver may be seen, with neoplastic lymphoid cells concentrating in portal areas and a mononucleosis-like pattern of infiltration in the sinusoids. Ancillary studies demonstrating a marked prominence of B-cells, EBV positivity, presence of light or heavy chain restriction, and presence of gene rearrangement support the diagnosis of monomorphic PTLD. As with the usually type lymphomas, the infiltrate of lymphocytes is typically much intense in monomorphic type PTLD than in GVHD. However, as the following case demonstrates GVHD may rarely present with a prominent lymphoid infiltrate which can mimic lymphoma.
A rare case of hepatic GVHD
A 63-year-old male with a clinical history of angioimmunoblastic T-cell lymphoma underwent allogeneic HCT from a female donor after effective salvage chemotherapy. The HCT was uneventful; however, on day 108 post-transplantation multiple skin rashes were noted on the patient’s palms and neck. CT-PET scan identified a focus of activity in the liver, and laboratory studies revealed a mild increase of liver enzymes and bilirubin. As recurrent lymphoma was of primary concern, ultrasound-guided liver biopsy was performed.
Biopsy findings demonstrated a prominent lymphocytic infiltrate in portal areas, concerning for recurrent disease (Figure 4). Liver biopsy revealed lymphocytic infiltration of the portal tracts associated with ductulitis and endothelialitis. Additionally, fluorescence in situ hybridization (FISH) for sex chromosomes was performed which identified the T-cell infiltrate as donor-derived (XX), whereas background host hepatocytes were XY.
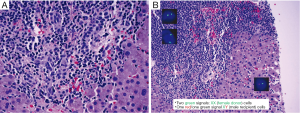
High-dose immunosuppressive therapy was instituted soon after, resulting in progressive improvement of skin lesions and eventual normalization of liver enzymes and bilirubin levels.
As demonstrated here, hepatic GVHD can rarely present as a prominent lymphocytic infiltrate, and with a prior history of lymphoma, relapse may be a primary concern. In such cases, careful attention to concomitant findings (e.g., skin rash) and a complete lymphoma work-up by immunohistochemical and other molecular tests may be needed to establish the diagnosis.
Summary
GVHD is a common complication following allogeneic hematopoietic stem cell transplantation and usually presents as injury to the skin, GI mucosa, and liver. In some cases, hepatic GVHD may be difficult to distinguish from other disorders observed in the post-HCT setting. Additionally, clinical signs and symptoms are frequently confounded by the superimposed effects of pretransplant chemoradiotherapy, IT, GVHD prophylaxis, and infection. Thus, (I) careful attention to and correlation with clinical findings and laboratory values is essential for diagnosis of hepatic GVHD; (II) GVHD should be included in the differential diagnosis for any patient with increased aminotransferases after allogeneic HCT; and (III) although clinical decisions may need to be made in real time and may ultimately rest on the clinical findings, a second opinion from an expert liver pathologist should be sought in challenging cases.
Acknowledgements
Funding: This work was supported by Collaborative Institutional Research Grant Pilot Funding for Investigators in Oncology UC Davis (MC), National Natural Science Foundation of China (No. 81273259, No. 81471589, KS). DW was sponsored by Edwin Everest Foundation Fellowship.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Wysocki CA, Panoskaltsis-Mortari A, Blazar BR, et al. Leukocyte migration and graft-versus-host disease. Blood 2005;105:4191-9. [PubMed]
- Kim BK, Chung KW, Sun HS, et al. Liver disease during the first post-transplant year in bone marrow transplantation recipients: retrospective study. Bone Marrow Transplant 2000;26:193-7. [PubMed]
- Levitsky J, Sorrell MF. Hepatic complications of hematopoietic cell transplantation. Curr Gastroenterol Rep 2007;9:60-5. [PubMed]
- Kambham N, Higgins JP, Sundram U, et al. Hematopoietic stem cell transplantation: graft versus host disease and pathology of gastrointestinal tract, liver, and lung. Adv Anat Pathol 2014;21:301-20. [PubMed]
- McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450-60. [PubMed]
- Carnevale-Schianca F, Leisenring W, Martin PJ, et al. Longitudinal assessment of morbidity and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation: retrospective analysis of a multicenter phase III study. Biol Blood Marrow Transplant 2009;15:749-56. [PubMed]
- Arai S, Lee LA, Vogelsang GB. A systematic approach to hepatic complications in hematopoietic stem cell transplantation. J Hematother Stem Cell Res 2002;11:215-29. [PubMed]
- Welniak LA, Blazar BR, Murphy WJ. Immunobiology of allogeneic hematopoietic stem cell transplantation. Annu Rev Immunol 2007;25:139-70. [PubMed]
- van den Brink MR, Burakoff SJ. Cytolytic pathways in haematopoietic stem-cell transplantation. Nat Rev Immunol 2002;2:273-81. [PubMed]
- Srinivasan M, Flynn R, Price A, et al. Donor B-cell alloantibody deposition and germinal center formation are required for the development of murine chronic GVHD and bronchiolitis obliterans. Blood 2012;119:1570-80. [PubMed]
- Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biol Blood Marrow Transplant 2005;11:945-56. [PubMed]
- Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: II. Pathology Working Group Report. Biol Blood Marrow Transplant 2006;12:31-47. [PubMed]
- Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease: I. The 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant 2015;21:389-401.e1.
- Pai CC, Chen M, Mirsoian A, et al. Treatment of chronic graft-versus-host disease with bortezomib. Blood 2014;124:1677-88. [PubMed]
- Stift J, Baba HA, Huber E, et al. Consensus on the histopathological evaluation of liver biopsies from patients following allogeneic hematopoietic cell transplantation. Virchows Arch 2014;464:175-90. [PubMed]
- Quaglia A, Duarte R, Patch D, et al. Histopathology of graft versus host disease of the liver. Histopathology 2007;50:727-38. [PubMed]
- Strasser SI, Shulman HM, Flowers ME, et al. Chronic graft-versus-host disease of the liver: presentation as an acute hepatitis. Hepatology 2000;32:1265-71. [PubMed]
- Akpek G, Boitnott JK, Lee LA, et al. Hepatitic variant of graft-versus-host disease after donor lymphocyte infusion. Blood 2002;100:3903-7. [PubMed]
- Norvell JP. Liver disease after hematopoietic cell transplantation in adults. Transplant Rev (Orlando) 2015;29:8-15. [PubMed]
- Tuncer HH, Rana N, Milani C, et al. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J Gastroenterol 2012;18:1851-60. [PubMed]
- Dougan M, Dranoff G. Immune therapy for cancer. Annu Rev Immunol 2009;27:83-117. [PubMed]
- Mirsoian A, Bouchlaka MN, Sckisel GD, et al. Adiposity induces lethal cytokine storm after systemic administration of stimulatory immunotherapy regimens in aged mice. J Exp Med 2014;211:2373-83. [PubMed]
- Amos SM, Duong CP, Westwood JA, et al. Autoimmunity associated with immunotherapy of cancer. Blood 2011;118:499-509. [PubMed]
- Bouchlaka MN, Sckisel GD, Chen M, et al. Aging predisposes to acute inflammatory induced pathology after tumor immunotherapy. J Exp Med 2013;210:2223-37. [PubMed]
- Carreras E, Bertz H, Arcese W, et al. Incidence and outcome of hepatic veno-occlusive disease after blood or marrow transplantation: a prospective cohort study of the European Group for Blood and Marrow Transplantation. European Group for Blood and Marrow Transplantation Chronic Leukemia Working Party. Blood 1998;92:3599-604. [PubMed]
- Carreras E, Díaz-Beyá M, Rosiñol L, et al. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol Blood Marrow Transplant 2011;17:1713-20. [PubMed]
- Shulman HM, Gooley T, Dudley MD, et al. Utility of transvenous liver biopsies and wedged hepatic venous pressure measurements in sixty marrow transplant recipients. Transplantation 1995;59:1015-22. [PubMed]
- Kharfan-Dabaja MA, Hamadani M, Sibai H, et al. Managing Hodgkin lymphoma relapsing after autologous hematopoietic cell transplantation: a not-so-good cancer after all! Bone Marrow Transplant 2014;49:599-606. [PubMed]
- Hamadani M, Abu Kar SM, Usmani SZ, et al. Management of relapses after hematopoietic cell transplantation in T-cell non-Hodgkin lymphomas. Semin Hematol 2014;51:73-86. [PubMed]
- Rezvani AR, Sandmaier BM. Allogeneic hematopoietic cell transplantation for indolent non-Hodgkin lymphoma: indications and outcomes. Curr Opin Hematol 2013;20:509-14. [PubMed]
- Emile JF, Azoulay D, Gornet JM, et al. Primary non-Hodgkin's lymphomas of the liver with nodular and diffuse infiltration patterns have different prognoses. Ann Oncol 2001;12:1005-10. [PubMed]
- Salmon JS, Thompson MA, Arildsen RC, et al. Non-Hodgkin's lymphoma involving the liver: clinical and therapeutic considerations. Clin Lymphoma Myeloma 2006;6:273-80. [PubMed]
- Avlonitis VS, Linos D. Primary hepatic lymphoma: a review. Eur J Surg 1999;165:725-9. [PubMed]
- Noronha V, Shafi NQ, Obando JA, et al. Primary non-Hodgkin's lymphoma of the liver. Crit Rev Oncol Hematol 2005;53:199-207. [PubMed]
- Végso G, Hajdu M, Sebestyén A. Lymphoproliferative disorders after solid organ transplantation-classification, incidence, risk factors, early detection and treatment options. Pathol Oncol Res 2011;17:443-54. [PubMed]
- Loddenkemper C, Longerich T, Hummel M, et al. Frequency and diagnostic patterns of lymphomas in liver biopsies with respect to the WHO classification. Virchows Arch 2007;450:493-502. [PubMed]
- Orazi A, Hromas RA, Neiman RS, et al. Posttransplantation lymphoproliferative disorders in bone marrow transplant recipients are aggressive diseases with a high incidence of adverse histologic and immunobiologic features. Am J Clin Pathol 1997;107:419-29. [PubMed]
- Liebowitz D. Epstein-Barr virus and a cellular signaling pathway in lymphomas from immunosuppressed patients. N Engl J Med 1998;338:1413-21. [PubMed]
- Lowe T, Bhatia S, Somlo G. Second malignancies after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant 2007;13:1121-34. [PubMed]
- Sundin M, Le Blanc K, Ringdén O, et al. The role of HLA mismatch, splenectomy and recipient Epstein-Barr virus seronegativity as risk factors in post-transplant lymphoproliferative disorder following allogeneic hematopoietic stem cell transplantation. Haematologica 2006;91:1059-67. [PubMed]


