
Surgical management of primary pancreatic neuroendocrine tumors
Introduction
Pancreatic neuroendocrine tumors (PanNETs), also known as “islet cell tumors”, are neuroendocrine tumors that arise from the embryonic endodermal ductal-acinar system. These neoplasms have a reported incidence of 1 to 1.5 per 100,000 in the world and 35 per 100,000 in the United States (US) (1). PanNETs comprise approximately 1.3–3% of pancreatic malignancies; however, their incidence has increased up to seven fold partially due to increased discovery of incidentalomas on cross-sectional imaging (2,3). The Surveillance, Epidemiology and End Results (SEER) specifically has shown that the incidence of sub-two centimeter PanNETs, in particular, have increased (4-6).
PanNETs are classified into two distinct groups, functional (F-PanNET) and nonfunctional (NF-PanNET), and their management is uniquely tied to the qualities of each tumor. Surgical resection remains the only curative treatment, and five-year survival is >90% in patients with localized disease and 15–27% for those with unresectable disease (7). Although consensus guidelines exist, surgical management requires a more in-depth assessment and a tailored approach to individual patients and their tumor type. In addition, with the greater detection of smaller, incidentally found PanNETs, there is a pressing need for development of appropriate surveillance and surgical treatment algorithms to guide management. This review describes the surgical management of localized and advanced PanNETs, as well as surveillance strategies for patients being observed.
Workup and diagnosis
Due to the predominantly indolent nature of these tumors, the diagnosis of many PanNETs can be delayed for months to years. As a result, up to 11% of cases are diagnosed with advanced metastatic disease (8). F-PanNETs often produce symptoms that raise clinical concern for a particular syndrome and work-up includes analysis of serum hormone levels (Table 1). In patients who are asymptomatic, relevant hormone studies should still be obtained if F-PanNET has not yet been ruled out.
Full table
The North American Neuroendocrine Tumor Society (NANETS) recommends the use of chromogranin A (CgA), a serum peptide secreted by 60–100% of NF-PanNETs, in the diagnosis of and the surveillance for recurrent PanNETs. However, the utility of CgA, in routine testing has been shown to be non-specific (specificity 65.5%) (9). Furthermore, CgA alone has insufficient sensitivity to diagnose small lesions and can be falsely elevated in patients with chronic kidney disease, liver failure, and the use of proton-pump inhibitors (PPIs) (10). Less often utilized serum peptides associated with NF-PanNETs include pancreastatin, ghrelin, neurotensin, motilin, pancreatic polypeptide (PPP), and enolase (10-14,29,30).
Imaging
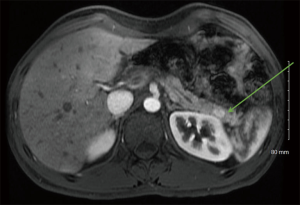
High-quality cross-sectional imaging should be performed to evaluate pancreas lesions. A multiphasic computed tomography (CT) scan or magnetic resonance imaging (MRI) is commonly utilized (14). Primary and metastatic PanNETs are most often well-circumscribed lesions that are highly vascular and demonstrate hyperenhancement during the arterial phase (Figure 1). The hepatic arterial phase of the pancreas protocol has a sensitivity of 83–88% for PanNET lesions (15). Hypoenhancement on arterial phase and the presence of calcifications within the lesion are associated with higher grade histology and the presence of lymph node metastases (31). Compared to CT, contrast enhanced MRI (Figure 2) is more sensitive and specific for identifying smaller pancreatic lesions and liver metastases (32).
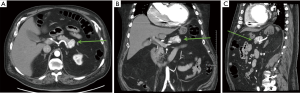
Functional imaging studies assist in staging, tumor localization, and therapeutic guidance. Such imaging techniques take advantage of the presence of somatostatin receptors—most commonly receptor subtypes 2 and 5 (SSTR-2, SSTR-5). The presence of these receptors in PanNETs is 76% and 93%, respectively (33). The most common functional imaging study used was indium-111-labeled somatostatin receptor scintigraphy (Octreoscan). More recently, positron-emission tomography (PET)/CT scan using gallium-68 (Ga-68) labeled somatostatin analogues (DOTATOC, DOTANONC and DOTATATE) is becoming more readily available and has better sensitivity (86–100%) and specificity (79–100%) compared to Octreoscan (34). However, one notable limitation of Ga-68 labeled somatostatin analogues is their inability to detect insulinomas, having a sensitivity of only 25% (35). At our institution, the Ga-68 dotatate scan is used to help stage patients with PanNETs (36).
Resection of nonfunctional PanNETs
Surgical management of PanNETs is indicated for nonfunctional tumors ≥2 centimeters, tumors that are functional, symptomatic, or have evidence of aggressive features (local invasion, lymphatic metastases). With the increasing incidence of PanNETs 37). Pancreatic surgery, despite having low mortality, is associated with high morbidity rates of almost 50% (38). Postoperative complications range from short term pancreatic fistula, intraabdominal fluid collections, or surgical site infections to long term pancreatic endocrine or exocrine insufficiencies.
Surgical options for the approach to PanNET resection depends on tumor size, anatomical location, and proximity to the pancreatic duct. These factors guide the surgeon in approaching the tumor with planned enucleation, pancreaticoduodenectomy (PD), distal pancreatectomy (DP), central pancreatectomy, or total pancreatectomy (TP). Additionally, patients that undergo splenectomy with DP should receive perioperative vaccines. The previously mentioned morbidities and mortality vary across these surgical techniques.
Enucleation is a technique limited to nonfunctional tumors less than 2 cm or peripheral insulinomas. One of the caveats with enucleation is that lymph nodes are not often sampled and the decision to proceed is examining the risk of having locoregional lymph node involvement. Enucleation mainly involves blunt dissection along the pancreas for superficial lesions. The pancreatic capsule is routinely closed but is left to surgeon discretion. Falconi et al. reported that atypical resection/enucleation had a lower incidence of endocrine or exocrine insufficiency, but did have a higher incidence of pancreatic fistula that were clinically relevant (grade B-C based on the International Study Group on Pancreatic Fistula) (39). A meta-analysis by Finkelstein in 2016 supported these findings across eleven studies with a total of 1,491 patients and also found that enucleation had shorter operative time and lower blood loss (40).
Surgical resections of DP, PD, and TP with nodal dissection are indicated for large, malignant lesions, or PanNETs close to the pancreatic duct where injury to the duct is likely. For non-functional PanNETs between 1–2 cm, treatment remains controversial. Studies have shown that 7–26% of small, nonfunctional PanNETs 1–2 cm in size can have lymph node involvement (41). Hashim et al. found that 12% of tumors 42). Furthermore, patients with tumors >1.5 cm were almost 5× as likely to have regional lymph node metastases than smaller tumors (odds ratio 4.7, 95% CI: 1.81 to 12.35, P=0.002). Based on these data, not every tumor et al. developed a novel lymph node risk score for NF-PanNETs 3%, based on this, they developed a 3-tiered risk system. Patients in the low risk group had an incidence of 3.2%, intermediate with 13.8%, and high with 20.5% of positive lymph nodes (43). At our institution, we consider resection of NF-PanNETs between 1–2 cm if they have high Ki-67 (>3%), increase in size, or evidence of local invasion.
There have been multiple studies looking at the utility of minimally invasive techniques in pancreatic surgery. Zhang et al. found in a retrospective multi-institutional matched study that the incidence of minimally invasive DP for PanNETs is becoming more popular in comparison to open pancreatectomy (44). Furthermore, minimally invasive surgery resulted in similar oncologic outcomes compared to open, while having equivalent to lower blood loss, lower Clavien-Dindo III or greater complications, and shorter hospital stay. These data were also seen several other studies (45,46).
Resection of functional PanNETs
Functional PanNETs are sub-classified based on the endocrine hormone they secrete and resulting clinical syndrome they produce. F-PanNETs are less prevalent (1) with an annual incidence of 1–5 per 100,000 people (16). As seen in Table 1, the incidence of the all F-PanNETs varies, and so do the preoperative workup, management, and outcomes. All F-PanNETs should be respected. The following sections will focus on specific F-PanNETs.
Insulinomas
Insulinomas can be visualized by cross-sectional imaging, but are often 47,48). Surgical resection is the treatment of choice for insulinomas. Preoperative management as shown in Table 1 includes Diazoxide to inhibit the release of insulin, promote glycogenolysis, palliate Whipple’s Triad, and is used for palliation in patients with advanced disease (16). Although, octreotide is used to control the symptoms of many functional PanNETs, for insulinomas, it can be dangerous if used as initial treatment as it can exacerbate the hypoglycemic episodes. Enucleation can be used for lesions that are exophytic and away from the main pancreatic duct due to the relative low incidence of lymph node involvement. For any lesion close to the pancreas duct, having a larger size, or if there is evidence of local invasion or nodal involvement, a formal pancreatectomy is warranted. In the OR, if a discrete tumor is not identified, a pancreatic biopsy should be taken to rule out other disease etiologies such as beta cell hyperplasia or adult nesidioblastosis, and the procedure should be aborted. If a lesion is still suspected, intraoperative ultrasound can help identify lesions in up to 90% of cases (47,48). With routine use of cross-sectional imaging, these scenarios are rare.
Gastrinomas
Approximately 75% of gastrinomas are sporadic, while the remaining are associated with Multiple Endocrine Neoplasia Type-1 (MEN1). Gastrinomas are most commonly malignant (60–90%), and distant metastases, particularly to the liver, is the strongest predictor of long-term survival (17). With surgical resection though, 15-year disease free survival can reach up to 98% (49). During workup of gastrinomas, proton pump inhibitors, histamine-2 receptor blockers, and somatostatin may be necessary, with the occasional use of Carafate to decrease gastrin hypersecretion to prevent gastric or duodenal perforation.
Surgical resection for gastrinomas always includes a formal pancreatectomy with regional lymph node dissection due to a high rate of nodal metastasis at operation. Intraoperatively, a thorough exploration should be performed and include ultrasound of the liver and pancreas with kocherization of the duodenum. If the lesion still cannot be found despite a clinical diagnosis, at the time of exploration, a lateral duodenotomy is performed with digital palpation for duodenal tumors, and peritoneal exploration for extra-pancreatic locations. Prior to performing a duodenotomy, endoscopy with transillumination should be performed first as creating a duodenotomy prevents subsequent insufflation.
VIPomas
Vasoactive Intestinal Polypeptide (VIP)-secreting tumors (VIPomas) are rare tumors as seen in Table 1. They are located 80–95% of the time in the pancreas, while 10% are localized to the periganglionic, adrenal, or neural regions (50,51). Typical preoperative imaging modalities are used, but portal venous sampling can be used to confirm pancreatic location. Preoperatively, patients need to be resuscitated to correct electrolyte derangements commonly associated with the diarrhea including hyponatremia, hypokalemia, and a metabolic acidosis. Additionally, octreotide can be used to control symptoms in preparation for the operating room. Surgical resection with a formal pancreatectomy is the treatment of choice with regional lymph node dissection due to the high incidence of malignancy.
Glucagonomas
Glucagonomas have a low incidence however, 70% of patients at the time of diagnosis have necrolytic migratory erythema, and 70% of glucagonomas are malignant and frequently have metastasis at diagnosis (18). Labs can assist in diagnosis but is typically confirmed by biopsy of the necrolytic migratory erythema (52). Surgical resection with regional lymph node dissection is the treatment of choice and consideration can be given to perform a metastasectomy when possible. For patients with necrolytic migratory erythema, somatostatin analogues can be used for treatment.
Somatostatinomas
Somatostatinomas are typically malignant and can be found in the duodenum, pancreas, or jejunum. Soga et al. showed that 5-year OS following resection is 100% for localized disease (19). They are typically large, solitary lesions located in the head of the pancreas and identifiable on cross-sectional imaging. Surgical resection of the lesion remains the primary treatment. Due to the increased incidence of cholelithiasis in patients with somatostatinoma should undergo a cholecystectomy at the time of resection.
Surgical management of PanNETs with genetic associations
PanNETs can be associated with an inherited genetic abnormality, and the most common associations include MEN1, von Hippel-Lindau disease (VHL), tuberous sclerosis (TS), and von Recklinghausen Disease—Neurofibromatosis 1 (NF1) (20). When a patient is diagnosed with PanNET, a thorough family history should be performed, and genetic counseling considered. Additionally, their surgical management is complicated by the potential requirement for multiple resections predisposing patients to endocrine insufficiencies such as diabetes (39). MEN1 will be discussed separately.
VHL is an autosomal dominant mutation in the 3p25 gene with an incidence of PanNETs in 10–17% of patients. Patients present younger and greater than 98% are non-functional (53). They are also prone to the development of pancreatic cysts which makes imaging the pancreas difficult. Concurrent diseases in VHL include hemangioblastomas, retinal angiomas, pheochromocytomas, and clear cell renal carcinomas. Surgical resection is recommended like for sporadic PanNET patients. Blansfield et al. reviewed outcomes of 108 patients with VHL and PanNETs, and recommended observation if lesions were less than three centimeters, had a low mutational burden of exon 3, and slow doubling time (54). They recommend screening with cross-sectional imaging of PanNETs meeting these criteria every 6–12 months.
TS and NF1 are two less common genetic associations with PanNETs. TS is caused by an autosomal dominant mutation in 9q34 and is associated with the rare (1 per 10,000) development of PanNETs. These are most commonly non-functional malignant PanNETs. Management is the same as sporadic PanNETs.
NF1 is a mutation in a tumor suppressor protein on 17q11.2, and these patients commonly develop pheochromocytoma. PanNETs in NF1 are rare (7 reported to date) (55), and management is the same as sporadic. Preoperative workup should include ruling out pheochromocytomas as missing this lesion could result in disastrous intraoperative complications.
Surgical management of PanNETs associated with MEN1
MEN1 is an autosomal dominant genetic abnormality and caries a prevalence of PanNET of 1–10 per 100,000 patients. Additionally, 20–25% of patients diagnosed with gastrinomas and 4% of patients with insulinomas have MEN1 (20). MEN1 is also associated with pituitary adenomas, parathyroid lesions, and PanNETs, with the most common functional lesion being gastrinomas. These patients often have multifocal microscopic functional and/or non-functional PanNETs at time of diagnosis.
MEN1 most commonly presents in patients during their second to third decades of life and primary hyperparathyroidism is typically the first endocrine disorder diagnosed. Following workup and treatment of hyperparathyroidism, they should be assessed for PanNETs, which occur in 50–75% of patients with MEN1 (56). Currently, guidelines recommend screening with MRI or CT for PanNETs in MEN1 patients beginning at ten years old and subsequently every ten years. Biochemical screening starts at 20 years old (56). Malignant degeneration of PanNETs with subsequent metastasis is the most common cause of death (56). Patients typically present at an earlier age with PanNETs, and the majority are gastrinomas. Surgical management is controversial as they will often recur, and resection is rarely curative.
For MEN1 patients with a gastrinoma, only rarely is a solitary lesion identified as they are often multiple and scattered throughout the pancreas. For these patients, surgery does remain an option. Originally described by Dr. Norman Thompson, the Thompson Procedure involves performing a DP for body tail lesions, enucleation of head lesions, duodenotomy for excision of duodenal gastrinomas, and lymph node dissection. This procedure aims to avoid pancreatic endocrine and exocrine insufficiency associated with the surgical alternative i.e., TP (52). From Thompson’s initial description in 1996, he described 68% of patients were eugastrinaemic at 19-year follow-up. Additionally, only one patient had recurrence requiring hepatic resection and there was no mortality associated with the surgery.
Patients who have MEN1 and known hyperparathyroidism, parathyroidectomy has been proven to reduce end organ effects of hypergastrinemia and is often performed before a pancreatectomy (57). Additionally, symptoms from gastrinomas can be well controlled with anti-acids (58). The second most common functional PanNET associated with MENI are insulinomas. Like for sporadic insulinomas, treatment consists of surgical resection, but more aggressive surgical management should be undertaken with resection due to their higher incidence of malignancy in MEN1.
MEN1 is also associated with NF-PanNETs. Almost all patients with MEN1 have multi-focal, asymptomatic NF-PanNETs (20,59). They confer a worse prognosis than F-PanNETs, and malignant PanNETs are the most common cause of death in patients with MEN1 (14). Currently ENETs recommends surgical resection for radiographically visible PanNETs, but due to the high rate of recurrence and risk for insufficiencies strategies should be individualized (34).
Resection versus observation?
Surveillance for PanNETs that do not meet obvious criteria for surgery remains a controversial area with mixed evidence for optimal management. This group are typically nonfunctional lesions et al. found that NF-PanNET size is correlated with malignancy, with only 6% of PanNETs 60). In contrast, Gratian et al. showed, 5-year OS for surveillance vs. partial pancreatectomy to be 27.6% vs. 83.0% for PanNETs less than two centimeters using the National Cancer Database (NCDB) (8).
All in all, there is concern regarding the morbidity associated with pancreatic resections; therefore, the risks and benefits of surgical management versus surveillance must be weighed. Kazanjian et al. found that among 70 patients that underwent resection for PanNETs, complications occurred in 48% of patients undergoing PD, 12.5% after DP, and 0% in those undergoing enucleation (61).
In contrast, Sadot et al. in a matched case-control study of 104 patients with PanNETs 62). They did find that 26 of the 104 observation patients eventually underwent surgery with a median of 30-month observation for patient preference, increase in tumor size, physician’s preference, and/or pancreatic ductal dilatation. This demonstrated that observation can be feasible in the carefully selected patient with small PanNETs and is supported by multiple other studies (62).
The rate of screening for changes in tumor size or new concerning features is still debatable. EUS or cross-sectional imaging is the preferred surveying studies. The ENETs Guidelines recommend surgical intervention if lesions increase in size by 0.5 cm over a period of 6 to 12 months (34). This is an area that needs more studies however, the data does show it is safe to observe patients with small NF-PanNETs and Ki-67
The Asymptomatic Small Pancreatic Endocrine Neoplasms (ASPEN) Trial in Europe is currently examining the best treatment for sporadic asymptomatic non-functioning pancreatic neuroendocrine neoplasms (NF-PNEN) ≤2 cm (63).
At the University of Cincinnati, resection is recommended for NF-PanNETs ≥2 cm and a multidisciplinary discussion is performed for lesions between 1–2 cm.
Surgical management of locally advanced (non-metastatic) PanNETs
PanNETs that are larger than 2 cm, invasive, or have radiographically evident positive lymph nodes are classified as locally advanced. Surgical management for these patients by the NCCN guidelines includes PD or DP with splenectomy and regional nodes based on tumor location if complete resection is possible (41). Patients with resectable localized disease have a median survival of approximately 7.1 years compared to those with locally advanced without undergoing resection have a median survival of 5.2 years (64).
In 2014, aggressive surgical resection for locally advanced PanNETs is possible even when tumors involve the portal or superior mesenteric vein as long as revascularization is achievable. Birnbaum et al. showed in a multi-institutional study, the role of extended resections for PNETs in 134 patients. They found among patients that underwent extended resection for local and/or metastatic disease severe morbidity was 21% and mortality was 5% (65). At the time of resection, ion, due to the likely use of somatostatin analogues postoperatively, cholecystectomy is recommended. In patients with locally advanced resectable disease, neoadjuvant systemic therapy is often utilized to test disease biology and potentially downstage tumors.
For patients that have a local recurrence, aggressive surgical management is still possible and result in improved long-term survival (66).
The data for locally advanced, non-metastatic disease is not sufficient to support debulking surgery. However, bypass operations for patients with severe symptoms including gastric outlet obstruction and biliary obstruction should be considered when endoscopic procedures are not possible.
Surgical management of metastatic PanNETs
Up to 77% of patients with PanNETs develop liver metastasis (67). Initial management of these patients begins with determining the differentiation of the tumor. Well-differentiated lesions that are G1 or G2 are potential candidates for metastatectomy (Table 2). Additionally patients with functional, resectable liver metastasis, absence of extra-hepatic intraabdominal disease, or Carcinoid syndrome are candidates for resection (34).

Full table
Sarmiento et al. first examined the feasibility of hepatic resection in 2003 showing that among 170 patients that underwent hepatic resection for metastatic PanNETs, 96% of patients had partial or complete response to their hormonal symptoms (68). Recurrences did occur in 84% of patients at 5 years and OS was 61% at 5 years. Following this in 2009, a Cochrane systemic review examined the benefit of cytoreductive surgery in patients with unresectable liver metastases. They found that there was insufficient data to recommend cytoreductive surgery (69,70). In 2012, Cusati et al. examined the outcomes of 72 patients with metastatic PanNETs that underwent R0 vs. R1 (>90%) resection. They found no significant differences in 5-year overall or disease free survival (21-23). Despite these data, current guidelines recommend that resection for metastatic PanNETs should be considered only when R0 resection it possible with “debulking surgery” remaining controversial.
In addition to hepatic resections, radiofrequency ablation (RFA), arterial embolization, and transarterial chemoembolization (TACE) are alternatives that are used to treat patients with metastatic disease (24). For patients with metastatic or locally advanced unresectable disease, systemic chemotherapy with streptozocin and doxorubicin or 5-flurouracil, everolimus, and long acting octreotide are treatment options (41).
Orthotopic liver transplantation (OLT) is a controversial subject in management of patients with metastatic PanNETs. The NCCN practice guidelines state OLT as investigational for metastatic PanNETs. Although a meta-analysis showed that OLT may provide survival benefit, patient selection is critical due to the high rates of recurrence and scarcity of grafts (25,26).
Surveillance and adjuvant therapy
Recurrence following resection of a PanNET occurs in approx. 12–15% of patients and is linked with reduced OS (27,28). Focus has been shifted to detecting recurrence early and developing potential salvage therapies. Consensus guidelines currently vary in recommendations on surveillance post resection. NANETs recommend cross-sectional imaging 3 to 6 months initially, followed by every 6 to 12. ENETs and others tailor imaging based on tumor grade varying from 3 to 6 months on severity for life.
Recently, Zaidi et al. established a recurrence risk scoring system (RRS) from 1,006 international patients. They found that tumors >2 cm, Ki67 >3%, positive lymph nodes, and symptomatic tumors were predictive recurrence (Table 3) (71). Their RRS stratified patients into three categories and found a 33% predictability of recurrence in their high-risk group versus 2% and 14% in their low and intermediate-risk groups respectively.

Full table
Additionally, Dong et al. have studied predictive factors for patterns and time of recurrence following curative resection (28). In the retrospective review, of the 1,020 patients that underwent curative resection, 53.3% had recurrence within two years. They found that 49.4% recurred in the liver, 22.7% pancreatic, and the remaining in multiple other organs. Tumor factors that were predictive of liver recurrence included Ki-67, perineural invasion, and major vascular resection, while pancreatic recurrences were only associated with positive surgical margins.
Because of the high risk of recurrences, patients should undergo follow up every 3–12 months or sooner should the patients develop any symptoms. This evaluation includes a history and physical, biochemical testing, and cross-sectional imaging for every 6–12 months with increased vigilance the first two postoperative years.
Currently there are no recommendations for adjuvant therapy for resectable PanNETs.
The Eastern Cooperative Oncology Group (ECOG) Trial E2211, tested the combination of adjuvant capecitabine and temozolomide in patients with advanced PanNETs (72). They found that patients had improvement in OS and progression-free survival. Based on these data, there are currently studies under way to examine the benefits of adjuvant capecitabine and temozolomide in patients with high risk resectable PNETs (high Ki-67, lymph node positive, high grade, and lymphovascular invasion).
In the advanced setting and for recurrences, treatment with long acting octreotide analogues, mTOR inhibitors (Everolimus) and cytotoxic systemic chemotherapies is standard of care (73). Recently, the use of peptide receptor radionucleotide therapy with 177Lu-Dotatate for advanced midgut NETs was performed and found an improvement progression free survival compared to octreotide alone (74,75).
Conclusions
PanNETs are a heterogenous group of tumors that vary in type, workup, surgical management and outcomes. With the increasing incidence of these lesions, knowledge of the disease biology, genetic associations, perioperative management/workup, and novel treatment strategies under way are crucial for the surgeon.
Acknowledgments
Thank you to Dr. Choe and the Department of Radiology at the University of Cincinnati Medical Center for the radiologic images.
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Callisia N. Clarke, Douglas B. Evans) for the series “Pancreatic Neuroendocrine Tumors” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2019.12.09). The series “Pancreatic Neuroendocrine Tumors” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Halfdanarson TR, Rabe KG, Rubin J, et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 2008;19:1727-33. [Crossref] [PubMed]
- Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol 2007;14:3492-500. [Crossref] [PubMed]
- Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol 2017;3:1335-42. [Crossref] [PubMed]
- Kuo EJ, Salem RR. Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 2013;20:2815-21. [Crossref] [PubMed]
- Modlin IM, Oberg K, Chung DC, et al. Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol 2008;9:61-72. [Crossref] [PubMed]
- Scarpa A, Mantovani W, Capelli P, et al. Pancreatic endocrine tumors: improved TNM staging and histopathological grading permit a clinically efficient prognostic stratification of patients. Mod Pathol 2010;23:824-33. [Crossref] [PubMed]
- Pancreatic Neuroendocrine Tumor (NET) 20.19. Available online: https://www.cancer.org/cancer/pancreatic-neuroendocrine-tumor/about.html
- Gratian L, Pura J, Dinan M, et al. Impact of extent of surgery on survival in patients with small nonfunctional pancreatic neuroendocrine tumors in the United States. Ann Surg Oncol 2014;21:3515-21. [Crossref] [PubMed]
- Lv Y, Han X, Zhang C, et al. Combined test of serum CgA and NSE improved the power of prognosis prediction of NF-pNETs. Endocrine Connections 2018;7:169-78. [Crossref] [PubMed]
- Giusti M, Sidoti M, Augeri C, et al. Effect of short-term treatment with low dosages of the proton-pump inhibitor omeprazole on serum chromogranin A levels in man. Eur J Endocrinol 2004;150:299-303. [Crossref] [PubMed]
- Cloyd JM, Poultsides GA. Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol 2015;21:9512-25. [Crossref] [PubMed]
- Kulke MH, Anthony LB, Bushnell DL, et al. NANETS treatment guidelines: Well-differentiated neuroendocrine tumors of the stomach and pancreas. Pancreas 2010;39:735-52. [Crossref] [PubMed]
- Metz DC, Jensen RT. Gastrointestinal Neuroendocrine Tumors: Pancreatic Endocrine Tumors. Gastroenterology 2008;135:1469-92. [Crossref] [PubMed]
- Chiruvella A, Kooby DA. Surgical Management of Pancreatic Neuroendocrine Tumors. Surg Oncol Clin N Am 2016;25:401-21. [Crossref] [PubMed]
- Lewis RB, Lattin GE Jr, Paal E. Pancreatic endocrine tumors: radiologic-clinicopathologic correlation. Radiographics 2010;30:1445-64. [Crossref] [PubMed]
- Mathur A, Gorden P, Libutti SK. Insulinoma. The Surgical clinics of North America 2009;89:1105-21. [Crossref] [PubMed]
- Yu F, Venzon DJ, Serrano J, et al. Prospective study of the clinical course, prognostic factors, causes of death, and survival in patients with long-standing Zollinger-Ellison syndrome. J Clin Oncol 1999;17:615-30. [Crossref] [PubMed]
- O'Grady HL, Conlon KC. Pancreatic neuroendocrine tumours. Eur J Surg Oncol 2008;34:324-32. [Crossref] [PubMed]
- Soga J, Yakuwa Y. Somatostatinoma/inhibitory syndrome: a statistical evaluation of 173 reported cases as compared to other pancreatic endocrinomas. J Exp Clin Cancer Res 1999;18:13-22. [PubMed]
- Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 2008;113:1807-43. [Crossref] [PubMed]
- Cusati D, Zhang L, Harmsen WS, et al. Metastatic nonfunctioning pancreatic neuroendocrine carcinoma to liver: surgical treatment and outcomes. J Am Coll Surg 2012;215:117-24; discussion 124-5. [Crossref] [PubMed]
- Mayo SC, de Jong MC, Pulitano C, et al. Surgical management of hepatic neuroendocrine tumor metastasis: results from an international multi-institutional analysis. Ann Surg Oncol 2010;17:3129-36. [Crossref] [PubMed]
- Norton JA, Cromack DT, Shawker TH, et al. Intraoperative ultrasonographic localization of islet cell tumors. A prospective comparison to palpation. Ann Surg 1988;207:160-8. [Crossref] [PubMed]
- Chan MY, Ma KW, Chan A. Surgical management of neuroendocrine tumor-associated liver metastases: a review. Gland surgery 2018;7:28-35. [Crossref] [PubMed]
- Moris D, Tsilimigras DI, Ntanasis-Stathopoulos I, et al. Liver transplantation in patients with liver metastases from neuroendocrine tumors: A systematic review. Surgery 2017;162:525-36. [Crossref] [PubMed]
- Chan G, Kocha W, Reid R, et al. Liver transplantation for symptomatic liver metastases of neuroendocrine tumours. Curr Oncol 2012;19:217-21. [Crossref] [PubMed]
- Marchegiani G, Landoni L, Andrianello S, et al. Patterns of Recurrence after Resection for Pancreatic Neuroendocrine Tumors: Who, When, and Where? Neuroendocrinology 2019;108:161-71. [PubMed]
- Dong DH, Zhang XF, Lopez-Aguiar AG, et al. Resection of pancreatic neuroendocrine tumors: defining patterns and time course of recurrence. HPB (Oxford) 2020;22:215-23. [Crossref] [PubMed]
- Jensen RT, Cadiot G, Brandi ML, et al. ENETS consensus guidelines for the management of patients with digestive neuroendocrine neoplasms: Functional pancreatic endocrine tumor syndromes. Neuroendocrinology 2012;95:98-119. [Crossref] [PubMed]
- Ito T, Igarashi H, Jensen RT. Therapy of metastatic pancreatic neuroendocrine tumors (pNETs): Recent insights and advances. Journal of Gastroenterology 2012;47:941-60. [Crossref] [PubMed]
- Poultsides GA, Huang LC, Chen Y, et al. Pancreatic neuroendocrine tumors: Radiographic calcifications correlate with grade and metastasis. Ann Surg Oncol 2012;19:2295-303. [Crossref] [PubMed]
- Sundin A, Vullierme MP, Kaltsas G, et al. ENETS consensus guidelines for the standards of care in neuroendocrine tumors: Radiological examinations. Neuroendocrinology 2009;90:167-83. [Crossref] [PubMed]
- Qian ZR, Li T, Ter-Minassian M, et al. Association Between Somatostatin Receptor Expression and Clinical Outcomes in Neuroendocrine Tumors. Pancreas 2016;45:1386-93. [Crossref] [PubMed]
- Falconi M, Eriksson B, Kaltsas G, et al. ENETS consensus guidelines update for the management of patients with functional pancreatic neuroendocrine tumors and non-functional pancreatic neuroendocrine tumors. Neuroendocrinology 2016;103:153-71. [Crossref] [PubMed]
- Scherübl H, Streller B, Stabenow R, et al. Clinically detected gastroenteropancreatic neuroendocrine tumors are on the rise: Epidemiological changes in Germany. World J Gastroenterol 2013;19:9012-9. [Crossref] [PubMed]
- Krenning EP, Kwekkeboom DJ, Bakker WH, et al. Somatostatin receptor scintigraphy with [111In-DTPA-D-Phe1]- and [123I-Tyr3]-octreotide: the Rotterdam experience with more than 1000 patients. Eur J Nucl Med 1993;20:716-31. [Crossref] [PubMed]
- Hayward R. VOMIT (victims of modern imaging technology)—an acronym for our times. BMJ 2003;326:1273. [Crossref]
- Jilesen APJ, van Eijck CHJ. Postoperative Complications, In-Hospital Mortality and 5-Year Survival After Surgical Resection for Patients with a Pancreatic Neuroendocrine Tumor: A Systematic Review. World J Surg 2016;40:729-48. [Crossref] [PubMed]
- Falconi M, Mantovani W, Crippa S, et al. Pancreatic insufficiency after different resections for benign tumours. Br J Surg 2008;95:85-91. [Crossref] [PubMed]
- Finkelstein P, Sharma R, Picado O, et al. Pancreatic Neuroendocrine Tumors (panNETs): Analysis of Overall Survival of Nonsurgical Management Versus Surgical Resection. J Gastrointest Surg 2017;21:855-66. [Crossref] [PubMed]
- Network NCC. Neuroendocrine and Adrenal Tumors 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- Hashim YM, Trinkaus KM, Linehan DC, et al. Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg 2014;259:197-203. [Crossref] [PubMed]
- Lopez-Aguiar AG, Ethun CG, Zaidi MY, et al. The conundrum of Crossref] [PubMed]
- Zhang XF, Lopez-Aguiar AG, Poultsides G, et al. Minimally invasive versus open distal pancreatectomy for pancreatic neuroendocrine tumors: An analysis from the U.S. neuroendocrine tumor study group. J Surg Oncol 2019;120:231-40. [PubMed]
- Tamburrino D, Partelli S, Renzi C, et al. Systematic review and meta-analysis on laparoscopic pancreatic resections for neuroendocrine neoplasms (PNENs). Expert Rev Gastroenterol Hepatol 2017;11:65-73. [Crossref] [PubMed]
- Drymousis P, Raptis DA, Spalding D, et al. Laparoscopic versus open pancreas resection for pancreatic neuroendocrine tumours: a systematic review and meta-analysis. HPB (Oxford) 2014;16:397-406. [Crossref] [PubMed]
- Hashimoto LA, Walsh RM. Preoperative localization of insulinomas is not necessary. J Am Coll Surg 1999;189:368-73. [Crossref] [PubMed]
- Lo CY, Lam KY, Kung AW, et al. Pancreatic insulinomas. A 15-year experience. Arch Surg 1997;132:926-30. [Crossref] [PubMed]
- Norton JA, Fraker DL, Alexander HR, et al. Surgery increases survival in patients with gastrinoma. Annals of surgery 2006;244:410-9. [PubMed]
- Park SK, O'Dorisio MS, O'Dorisio TM. Vasoactive intestinal polypeptide-secreting tumours: biology and therapy. Baillieres Clin Gastroenterol 1996;10:673-96. [Crossref] [PubMed]
- Ro C, Chai W, Yu VE, et al. Pancreatic neuroendocrine tumors: biology, diagnosis,and treatment. Chin J Cancer 2013;32:312-24. [Crossref] [PubMed]
- Stacpoole PW. The glucagonoma syndrome: clinical features, diagnosis, and treatment. Endocr Rev 1981;2:347-61. [Crossref] [PubMed]
- Tamura K, Nishimori I, Ito T, et al. Diagnosis and management of pancreatic neuroendocrine tumor in von Hippel-Lindau disease. World J Gastroenterol 2010;16:4515-8. [Crossref] [PubMed]
- Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery 2007;142:814-8; discussion 818.e1-2.
- Nishi T, Kawabata Y, Hari Y, et al. A case of pancreatic neuroendocrine tumor in a patient with neurofibromatosis-1. World J Surg Oncol 2012;10:153. [Crossref] [PubMed]
- Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 2012;97:2990-3011. [Crossref] [PubMed]
- Norton JA, Cornelius MJ, Doppman JL, et al. Effect of parathyroidectomy in patients with hyperparathyroidism, Zollinger-Ellison syndrome, and multiple endocrine neoplasia type I: A prospective study. Surgery 1987;102:958-66. [PubMed]
- Bartsch DK, Langer P, Rothmund M. Surgical aspects of gastrinoma in multiple endocrine neoplasia type 1. Wiener Klinische Wochenschrift 2007;119:602-8. [Crossref] [PubMed]
- Pipeleers-Marichal M, Somers G, Willems G, et al. Gastrinomas in the duodenums of patients with multiple endocrine neoplasia type 1 and the Zollinger-Ellison syndrome. N Engl J Med 1990;322:723-7. [Crossref] [PubMed]
- Bettini R, Partelli S, Boninsegna L, et al. Tumor size correlates with malignancy in nonfunctioning pancreatic endocrine tumor. Surgery 2011;150:75-82. [Crossref] [PubMed]
- Kazanjian KK, Reber HA, Hines OJ. Resection of Pancreatic Neuroendocrine Tumors: Results of 70 Cases. Arch Surg 2006;141:765-9; discussion 769-70. [Crossref] [PubMed]
- Sadot E, Reidy-Lagunes DL, Tang LH, et al. Observation versus Resection for Small Asymptomatic Pancreatic Neuroendocrine Tumors: A Matched Case–Control Study. Ann Surg Oncol 2016;23:1361-70. [Crossref] [PubMed]
- Falconi M. Asymptomatic Small Pancreatic Endocrine Neoplasms. (ASPEN). ClinicalTrialsgov Identifier: NCT03084770. [Clinical Trial]. Available online: https://clinicaltrials.gov/ct2/show/NCT03084770
- Solorzano CC, Lee JE, Pisters PWT, et al. Nonfunctioning islet cell carcinoma of the pancreas: Survival results in a contemporary series of 163 patients. Surgery 2001;130:1078-85. [Crossref] [PubMed]
- Birnbaum DJ, Turrini O, Vigano L, et al. Surgical management of advanced pancreatic neuroendocrine tumors: short-term and long-term results from an international multi-institutional study. Ann Surg Oncol 2015;22:1000-7. [Crossref] [PubMed]
- Fendrich V, Langer P, Celik I, et al. An aggressive surgical approach leads to long-term survival in patients with pancreatic endocrine tumors. Ann Surg 2006;244:845-51; discussion 852-3. [Crossref] [PubMed]
- Pavel M, Baudin E, Couvelard A, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology 2012;95:157-76. [Crossref] [PubMed]
- Sarmiento JM, Heywood G, Rubin J, et al. Surgical treatment of neuroendocrine metastases to the liver: a plea for resection to increase survival. J Am Coll Surg 2003;197:29-37. [Crossref] [PubMed]
- Gurusamy KS, Ramamoorthy R, Sharma D, et al. Liver resection versus other treatments for neuroendocrine tumours in patients with resectable liver metastases. Cochrane Database Syst Rev 2009.CD007060. [PubMed]
- Gurusamy KS, Pamecha V, Sharma D, et al. Palliative cytoreductive surgery versus other palliative treatments in patients with unresectable liver metastases from gastro-entero-pancreatic neuroendocrine tumours. Cochrane Database Syst Rev 2009.CD007118. [PubMed]
- Zaidi MY, Lopez-Aguiar AG, Switchenko JM, et al. A Novel Validated Recurrence Risk Score to Guide a Pragmatic Surveillance Strategy After Resection of Pancreatic Neuroendocrine Tumors: An International Study of 1006 Patients. Ann Surg 2019;270:422-33. [Crossref] [PubMed]
- Kunz PL, Catalano PJ, Nimeiri H, et al. A randomized study of temozolomide or temozolomide and capecitabine in patients with advanced pancreatic neuroendocrine tumors: A trial of the ECOG-ACRIN Cancer Research Group (E2211). J Clin Oncol 2018;36:abstr 4004.
- Pusceddu S, Verzoni E, Prinzi N, et al. Everolimus treatment for neuroendocrine tumors: latest results and clinical potential. Ther Adv Med Oncol 2017;9:183-8. [Crossref] [PubMed]
- Strosberg JR, Halfdanarson TR, Bellizzi AM, et al. The North American Neuroendocrine Tumor Society Consensus Guidelines for Surveillance and Medical Management of Midgut Neuroendocrine Tumors. Pancreas 2017;46:707-14. [Crossref] [PubMed]
- Rinke A, Muller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol 2009;27:4656-63. [Crossref] [PubMed]


