
Gastrointestinal lymphomas: Morphology, immunophenotype and molecular features
Introduction
Primary lymphoma of the gastrointestinal (GI) tract accounts for 30-40% of lymphomas arising extranodally and comprises 10-15% of all non-Hodgkin lymphomas (NHL) (1). It occurs in the stomach in about 60-75% of the cases, followed by the small intestines, ileum, cecum, colon and rectum (2). Mature B cell, T cell and NK/T-cell lymphomas are encountered, of which mucosa-associated lymphoid tissue (MALT) and diffuse large B cell lymphomas (DLBCL) are the two histologic subtypes most commonly observed (1). MALT lymphoma usually arises in a background of chronic inflammation in particular, infection with Helicobacter pylori (H. pylori) (3); as such eradication of H. pylori has been documented to result in disease remission (4-6). Immunoproliferative small intestinal disease (IPSID) is a variant of MALT lymphoma that arises in the small bowel and is usually associated with Campylobacter jejuni (C. jejuni) infection (7). Other mature B cell lymphomas that can arise in the GI tract are Burkitt lymphoma (BL), follicular lymphoma (FL), mantle cell lymphoma (MCL), anaplastic lymphoma kinase (ALK)-positive large B cell lymphoma, and lymphomatoid granulomatosis (LG). Characteristic molecular abnormalities are expressed by a number of these mature B cell lymphomas.
T cell lymphoma may arise in the setting of celiac sprue, notably type I enteropathy-associated T cell lymphoma (EATL) (8). Molecular studies for T cell gene rearrangement confirm clonal evolution of malignant neoplastic T cells. Extranodal NK/T cell lymphoma (ENKTL) of the GI tract is commonly associated with Epstein-Barr virus (EBV) infection and demonstrates aggressive clinical behavior and thus, like most T cell lymphomas, confers a poor prognosis (9). On the other hand, a few cases of benign, indolent and EBV negative, NK-cell lymphoproliferative disorder/enteropathy or lymphomatoid gastropathy, a recently described entity, have been documented (10,11).
Other hematopoietic neoplasms may also involve the GI tract although incidence is extremely rare compared to mature B cell lymphomas. These include but are not limited to extramedullary plasmacytoma (EMP) (12,13), or primary amyloidosis associated with myeloma (14), plasmablastic lymphoma (PBL) (15,16), Hodgkin lymphoma (HL) (17,18), histiocytic sarcoma (HS) (19-21) and mast cell sarcoma (MCS) (22,23). This review concentrates on the selected primary GI tract lymphomas as mentioned, focusing on the characteristic morphologic, immunophenotypic and molecular or cytogenetic features, with a brief description of a few selected hematopoietic malignancies, other than lymphoma, that may also be encountered in the GI tract.
Clinical findings
Symptoms may vary from each patient who may present with any or combination of any of the following: dyspepsia, epigastric pain, abdominal pain, nausea, vomiting, diarrhea, weight loss, malabsorption (8), obstruction, anemia, and to a lesser extent ulceration, perforation (2,21) and intussusception (24,25). Hematochezia has been reported in some patients who were later diagnosed with rectal lymphoma. Conversely, a few patients were reportedly asymptomatic (10).
Imaging
On endoscopy, lymphoma may be nodular similar to a reactive lymph node or manifest as mucosal ulceration, hyperplasia, polyp, or as an infiltrative lesion (26). In rare occasion, lymphoma can present as incidental thickening of GI luminal wall on computed tomography (CT) (27). Biopsy is frequently performed on mucosal abnormalities as it is difficult to differentiate neoplastic lymphoid nodules from benign reactive follicles or mucosal polyps.
Endoscopic ultrasonography (EUS) demonstrates four types of patterns indicative of gastric lymphoma: superficially spreading, diffusely infiltrating, mass forming and mixed. Low-grade MALT lymphoma characteristically appears as a superficially spreading or diffusely infiltrative lesion (28). EUS is a valuable adjunct for initial GI lymphoma staging as it allows visualization of all layers of the gastric wall and thus, permits evaluation of tumor depth. Gastric MALT lymphoma staging by EUS is as follows: T1a and T1b correspond to invasion of the superficial mucosa, and infiltration through the muscularis mucosa, respectively. Invasion to submucosa conforms to T2 stage, whereas involvement beyond submucosa conveys T3 (28). Similarly, it has been proven as an indispensable tool in disease follow-up after treatment (28,29). However, a study by Ribeiro and colleagues revealed that lymphoma subclassification by EUS-fine needle aspiration (FNA) and Tru-cut biopsy (TCB) showed lower accuracy (60% of cases) in distinguishing low-grade lymphomas in comparison to subclassifying high-grade DLBCLs (78% of cases) (30).
Mature B cell lymphomas
Extranodal marginal zone mucosa associated lymphoid tissue (MALT) lymphoma
MALT lymphomas comprise over 50% of primary gastric non-Hodgkin lymphomas, occurring predominately in patients older than 50 years, with a noted peak in the seventh decade and a male: female ratio of about 1.5:1 (1). Patients commonly present with nonspecific gastritis and/or a peptic ulcer. Endoscopy commonly demonstrates erythematous and slightly thickened rugae with superficial spreading of lesions without formation of a distinct mass. The gastric lesions commonly are multifocal, and most patients have stage I or II disease (1,3,6). Cases of MALT transformation to DLBCL have also been recognized (1).
Pathogenesis
A strong association between chronic H. pylori infection and MALT gastric lymphoma has been demonstrated in 80% to 90% of cases, and it is widely accepted that the bacterial infection plays a crucial role in the pathogenesis of this tumor (1,3,4). Chronic H. pylori infection provides the antigenic stimulus, resulting in the clonal expansion of lymphoid cells leading to the evolution of MALT lymphoma. According to the study by Arnold and colleagues, H. pylori strains expressing the cytotoxin-associated gene A (CagA) protein carry the major histocompatibility complex (MHC) class II T cell epitope. Therefore, infection with this specific strain induces activation of CD4+ T cells which has been postulated to instigate neoplasia (31).
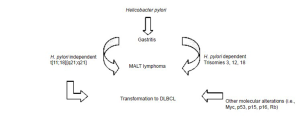
On one hand, lymphomagenesis has also been hypothesized to evolve independent of H. pylori infection (32,33), particularly in the setting of translocation [11;18] [q21;q21]. This aberration is further described under molecular abnormalities. Figure 1 outlines possible pathways of MALT lymphomagenesis. Transformation to DLBCL has been documented in cases independent of H. pylori infection, as well as in cases harboring genomic alterations of the Myc, p53, p15, p16, and retinoblastoma (Rb) genes (32).
Morphology and immunophenotype
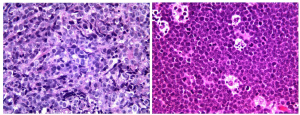
Gastric MALT lymphomas are characterized by lymphoepithelial lesions (LEL) (Figure 2, left) with glandular invasion by neoplastic centrocyte-like cells or small lymphoid cells with irregular nuclear contour, nuclear clefting, hyperchromasia, and with scant to fair amount of cytoplasm. Occasional atypical plasmacytoid tumor cells may also be observed. Nevertheless, care must be taken to avoid overinterpretation of LELs as these lesions may also appear in benign settings including reactive lymphoid infiltrates. Reactive germinal centers, common in the deeper mucosa associated with H. pylori gastritis, may be colonized by lymphoma cells, with obliteration of mantle zone and the appearance of so-called “naked” follicles. The atypical lymphoid infiltrate usually expands the lamina propria or submucosa (Figure 2, right). Muscularis mucosae infiltration and disruption can be a useful clue to the diagnosis in small biopsy specimens. In more extensive cases, the lymphoma can create mucosal ulcers and can infiltrate through the muscularis propria.

While MALT lymphoma does not show a specific immunohistochemical profile, there is usually an overabundance of neoplastic B cells as highlighted by CD20 immunostain. Large series have demonstrated that up to 50% of the cases may also aberrantly co-express CD43 and/or BCL2 by these neoplastic B cells (1,4,6). The tumor cells show variable surface and cytoplasmic immunoglobulin reactivity, with most cases expressing IgM, and a few cases showing IgA or IgG reactivity, whereas IgD expression is rare. The neoplastic B cells are negative for CD10, CD23, and cyclin D1, and typically do not co-express CD5, although rare cases of CD5-positive MALT lymphomas have been documented (34). In cases with extensive or nearly complete plasmacytic differentiation, IHC for kappa and lambda light chains can be extremely useful in highlighting possible restricted plasma cell population.
Molecular abnormalities
For MALT lymphomas in general, the genetic abnormalities encompass trisomies 3, 12 and 18, as well as balanced translocations, specifically t[11;18][q21;q21], t[14;18][q32;q21], t[1;14] [p22;q32] and t[3;14] [p14;q32]. The most common translocation in gastric MALT lymphoma, arising in approximately 20-30% of cases (although lower in North America) is t[11;18] [q21;q21], in which the t[11;18] fuses with the amino terminal of the apoptosis inhibitor API2 at 11q21 to the carboxyl terminal of MALT1 at 18q21 leading to a chimeric fusion protein. MALT1 is involved in antigen receptor-mediated nuclear factor kB (NF-κB) activation (32,33). However, t[11;18] [q21;q21] is usually not associated with H. pylori gastritis; hence, such cases are believed to show resistance to antibiotic therapy (1). Table 1 provides a detailed description, frequency and clinical implications for the chromosomal abnormalities frequently detected in MALT lymphoma.
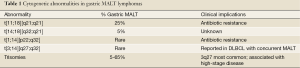
Full table
All aforementioned translocations induce activation of the nuclear factor κB (NF-κB) oncogenic pathway (1). It has been postulated that chronic inflammation leads to activation of NF-κB pathway via the antigen receptor signaling in MALT lymphoma cells. Antigen stimulation and CD40 triggering synergize NF-κB activation through formation of CARMA1-BCL10-MALT1 ternary complex. In addition, the continuous and sustained antiapoptotic stimuli driven by API2-MALT1 are most likely to play key roles in the pathogenesis of MALT lymphomas (32,33).
Prognosis
The response of low grade MALT lymphoma to H. pylori eradication is predicted by stage. Complete regression of low-grade, early stage MALT lymphoma following successful H. pylori eradication has been confirmed in about 75-80% of cases (4-6,35). Studies have documented that complete response has been achieved in nearly all patients where disease is limited to the gastric mucosa or submucosa. Complete response rates have decreased in cases where disease extended to the muscularis propria or serosa (35). Furthermore, it has been shown that no patients with nodal disease achieved complete response with H. pylori eradication alone (4-6,36-39).
It is important to note, however, that approximately 10% of gastric MALT lymphomas with t[11;18] [q21;q21] translocation are resistant to H. pylori antibiotic therapy, suggesting importance of strict follow up, and if clinically indicated, a trial of chemotherapy, immunotherapy (i.e., Rituximab), and/or radiotherapy for localized disease, may be pursued (6,36-40). Studies suggest that medical therapy alone is superior to surgery, although surgical intervention may be appropriate in specific circumstances such as in cases with gastric outlet obstruction and/or other complications (35).
Immunoproliferative small intestinal disease (IPSID)
IPSID has also been acknowledged as alpha heavy chain disease (αHCD), and is a variant form of MALT lymphoma arising in the small intestine. IPSID or αHCD is the most common form of the heavy chain diseases (HCD). It accounts for about one-third of all GI lymphomas in the middle-east. IPSID occurs in a younger age population, with most patients presenting at the age of 20 to 30 years (7).
Pathogenesis
As in cases of H. pylori associated MALT lymphoma, an infectious etiology has been suspected in cases of IPSID. Studies have mirrored the efficacy of antimicrobial therapy in disease regression. Lecuit et al. demonstrated C. jejuni as a possible stimulus for this proliferation. C. jejuni has been shown to persist in Peyer’s patches and mesenteric lymph nodes, and is capable of eliciting strong IgA mucosal response. Persistent infection may lead to sustained stimulation of B cells eventually resulting in the production of monotypic IgA such as that seen in IPSID (7).
Morphology and immunophenotype
IPSID is morphologically characterized by small bowel infiltration by a monotypic lymphoplasmacytic population, and is associated with a variety of histopathological changes which range from small to medium atypical lymphoid propagation to DLBCL. The centrocyte-like lymphocytes are CD20 positive, and both atypical lymphocytic and plasmacytic populations will stain strongly with IgA heavy chain, with absence of light chain staining (7).
Molecular abnormality
Much like H. pylori associated MALT lymphoma, IPSID appears to arise from monoclonal overgrowth secondary to chronic immune stimulation by an infectious organism in this case by C. jejuni (7). Deletions of alpha heavy chain gene are observed which lead to expression of a faulty heavy chain that precludes binding of light chain to form an intact immunoglobulin molecule (7,41).
Prognosis
In the early phases, the disease may completely resolve following antibiotic therapy; however, transformation to DLBCL is not uncommon (7).
Diffuse large B cell lymphoma (DLBCL)
DLBCL of the gastrointestinal tract is an aggressive lymphoma which may arise de novo or from transformation of another lymphoma, commonly MALT lymphoma. It constitutes 40% to70% of all gastric lymphomas, more commonly affecting males with a median age range of 50 to 60 years (1,2).
Pathogenesis
No definite risk factors have been identified, although some evidences suggest that this neoplasm may arise in a background of atrophic gastritis, particularly in the setting of immunodeficiency. Foci of DLBCL may be found in MALT lymphomas, ranging from small number of transformed cells to predominant large cell population with minimal residual MALT lymphoma (2). Distinction of the latter from DLBCL can be difficult, and may require correlation of identical rearranged immunoglobulin (Ig) genes with co-existent low-grade MALT lymphoma (1).
Morphology and immunophenotype
DLBCL is characterized by large lymphoid cells, with nuclei greater than twice the size of a small lymphocyte, and frequently larger than nuclei of tissue macrophage. The tumor cells are medium to large sized cells and contain round, oval, or slightly irregular nuclei with vesicular nuclear chromatin, prominent nucleoli, and ample amount of basophilic cytoplasm (Figure 3, left), and show a moderate to high proliferation index as evident by tumor cell nuclear positivity for Ki-67 immunostain. In most cases, the predominant cells resemble either large centroblasts or immunoblasts; nonetheless, a mixture of these two cell types is also commonly encountered. Histologically, there is an intense cellular infiltration of the lamina propria.
Transformed MALT lymphomas may be distinguished from de novo germinal center DLBCL by immunophenotype. Both transformed MALT lymphomas and DLBCLs show BCL6 positivity; however, DLBCLs with a germinal center-like phenotype are frequently CD10 and BCL2 positive, whereas transformed MALT lymphomas are CD10 and BCL2 negative (2).
Molecular abnormalities
A number of genetic variability in DLBCLs has been documented. Studies continue to subdivide these processes into separate disease entities with associated overall clinical circumstances. However, approximately 30% of DLBCL has been demonstrated to show BCL6 abnormalities. BCL2 translocation has been documented in about 25%, and presence of c-MYC rearrangements have been postulated to occur at an average of about 10% of patients (42,43).
Prognosis
Several factors affect the prognosis of gastrointestinal DLBCL. Age, stage of disease, lactate dehydrogenase (LDH) level, and use of chemotherapy are independently and significantly associated with survival. A more aggressive clinical course has been reported in patients with more extensive disease, such as presence of systemic symptoms, bulky lymphadenopathy, and elevated serum LDH levels. Interestingly, patients with CD10-positive disease showed a significantly higher survival rate compared to patients with CD10-negative lymphomas. The prognostic and diagnostic roles of some molecular variables, like microsatellite instability, allelic imbalance and chromosomal trisomies, are matters of continued investigation (1,2).
Burkitt lymphoma (BL)
Burkitt lymphoma is a substantially aggressive mature B cell neoplasm mainly in children and young adults. This entity has three recognized clinical variants: endemic form which is usually associated with EBV infection, sporadic variant where only about 30% of the cases are related to EBV infection, and immunodeficiency-associated BL (44). Extranodal disease is frequently observed but GI tract involvement varies among the three clinical subtypes, with the sporadic variant usually presenting as an abdominal mass, commonly in the terminal ileum (43). Rare cases of gastric (45,46) and cecal (47) BL have also been described.
Pathogenesis
All three variants harbor chromosomal rearrangement of c-MYC oncogene which modifies cell cycle regulation, cellular metabolism, adhesion, differentiation and apoptosis ultimately leading to tumor formation (44). Baumgaertner and colleagues reported a case of H. pylori-associated Burkitt lymphoma with complete disease remission after H. pylori eradication therapy. This occurrence may imply probable role of H. pylori in BL (45).
Morphology and immunophenotype
BL displays a diffuse, monotonous infiltrate of medium-sized neoplastic lymphoid cells with round nuclei showing finely clumped and dispersed, with multiple basophilic nucleoli. The profoundly basophilic cytoplasm generally encloses multiple lipid vacuoles on Wright-Giemsa or Diff-Quick stained smears. Frequent mitotic figures and apoptotic bodies are encountered; the apoptotic body-containing tangible body macrophages impart the characteristic “starry sky” morphology (Figure 3, right).
The tumor cells co-express pan B-cell markers such as CD19, CD20, as well as CD10, BCL6, and demonstrate light chain restriction, but are typically negative for BCL2 and TdT. The neoplastic cells show an extremely high proliferation index with nearly 100% of tumor cells showing nuclear accentuation by Ki-67 (45).
Molecular abnormalities
As previously mentioned, all three subtypes of BL typically demonstrate any of three c-MYC translocations at band 8q24; the most common of which is with immunoglobulin heavy (IgH) chain gene at 14q32, and infrequently with Ig kappa (IgK) at 2p12 or Ig lambda (IgL) at 22q11. However, c-MYC rearrangement is not specific for BL. Approximately 28-50% of GI tract, de novo DLBCLs, and DLBCL, unclassifiable, with features intermediate between DLBCL and BL (DLBCL/BL) show c-MYC translocation with a non-Ig gene partner, complex karyotype, and simultaneous BCL2, BCL6 and/or PAX5 translocations, referred to as “double or triple hit” lymphoma (43). Morphological overlap exists between BL and high-grade DLBCL and/or DLBCL/BL; therefore, it is imperative to differentiate BL from DLBCL and DLBCL/BL, particularly since the latter two entities are more resistant to chemotherapy and carry a poorer prognosis overall (43).
Prognosis
BL is chemosensitive and the advent of high intensity, multi-agent chemotherapeutic regimen has led to an astoundingly high remission rate. As observed in one case, patients with concomitant H. pylori infection may also benefit from H. pylori eradication treatment (45).
Epstein-Barr virus positive diffuse large B-cell lymphoma (EBV-positive DLBCL) of the elderly
EBV-positive DLBCL is a clonal B-cell neoplasm in patients older than 50 years without known immunodeficiency or prior lymphoma (48,49). About 70% of these patients present with extranodal EBV-positive B-cell lymphomas in a number of locations, including the stomach in approximately 9% of cases (48).
Pathogenesis
EBV-positive DLBCL is believed to arise in the context of declining immunity related to senescence (48-50). As with other variants of DLBCL, a clear etiology is not yet known.
Morphology and immunophenotype
Age-related EBV-positive lymphomas generally show large lymphoid cells in a background of smaller, reactive components (small lymphocytes, plasma cells, histiocytes, and epithelioid cells). There may also be patchy necrosis and a relatively broad range of B cell maturation, including morphologic centroblasts, immunoblasts, and Hodgkin Reed Sternberg-like (HRS-like) giant cells with distinct nucleoli (49). This variability distinguishes the disease into two subtypes: large-cell and polymorphic. Large cell lymphoma is characterized by relatively monomorphic large lymphoid cells, while polymorphic lymphoma shows scattered large cells in a polymorphous background consisting of smaller lymphocytes, plasma cells, and histiocytes. The histologic findings may be widely variable, indicating tumor heterogeneity as a spectrum rather than true divergence; a finding supported by similar clinical and immunophenotypic characteristics between groups (48).
The tumor cells express B cell markers (CD20 and/or CD79a) and harbor EBV gene [latent membrane protein-1 (LMP1) and/or EBV nuclear antigen-2 (EBNA2)]. Additionally, variations in CD30 and CD10 expression have been observed in comparison to EBV-negative DLBCL. CD30 is seen in about 75% of age-related EBV-positive DLBCL compared to 13% in EBV-negative DLBCL, while CD10 expression is decreased (18% and 38%, respectively) (48).
Molecular abnormalities
Molecular studies will typically detect the clonality of immunoglobulin genes and EBV genomes (50).
Prognosis
Typically the prognosis for EBV-positive B-cell lymphomas in the elderly is significantly poorer than that of EBV-negative tumors, with a mean survival of about 2 years. Advanced age (>70 years) and presence of B symptoms such as fever, weight loss, lymphadenopathy confers worse prognosis (48,49). The general performance status of elderly patients also plays a role in the clinical course of the disease; inasmuch as many of these patients may not be able undergo intensive therapies. As such, EBV-positive DLBCL of the elderly warrants separate consideration due to the diagnostic and therapeutic challenges they pose (48-50).
Follicular lymphoma (FL)
FL is the second most common type of lymphoma among adults in western countries, typically occurring in lymph nodes with splenic, hepatic and bone marrow involvement. Primary extranodal FL is uncommon, constituting less than 7% of GI tract lymphomas (51). FL of the GI tract most frequently occurs in middle-aged adults with a slight female predominance (2:1). The tumor typically arises in the duodenum followed by the ileum and colon (52).
Pathogenesis
The translocation (14;18) places the anti-apoptotic or proto-oncogene BCL2 locus located on chromosome 18, under the control of the IgH locus which is situated on chromosome 14, resulting in over expression of anti-apoptotic proteins and immortalization of tumor cells (52,53).
Morphology and immunophenotype
Histologically, FL of the GI tract consists of relatively uniform, medium sized neoplastic follicles which involve the mucosa. These nodules are composed of small, monotonous lymphoid cells with characteristic cleaved nuclei (centrocytes) admixed with variable numbers of centroblasts which are larger lymphoid cells with vesicular chromatin, presence of nucleoli, with fair amount of cytoplasm. Deeper infiltration into the muscularis mucosae or submucosa can also be seen, as can superficial involvement of surface epithelium with or without ulceration (51). Immunohistochemically, BCL2 and CD20 are nearly uniformly positive, with most cases negative for CD3, CD5, CD23, CD43, and cyclin D1. CD10 positivity generally highlights both neoplastic follicles and interfollicular tumor cells in cases with a follicular, as well as mixed follicular and diffuse growth patterns (53).
Molecular abnormality
The cytogenetic hallmark of FL is t[14;18] [q32;21], with rearrangement of the BCL2 gene seen in up to 90% of cases (53).
Prognosis
In a case report of 26 primary GI follicular lymphomas, Shia and associates demonstrated no deaths related to FL, suggesting an indolent clinical course, relatively similar to nodal FL (53).
Mantle cell lymphoma (MCL)
MCL commonly presents with advanced-stage disease, with about 80% of patients showing involvement of extranodal sites, including bone marrow, spleen, Waldeyer’s ring and GI tract. GI tract involvement has been documented in only about 20% of MCL cases, and presents as numerous small mucosal excrescences referred to as multiple lymphomatous polyposis (MLP) (54). Studies by Salar’s (55) and Romaguera’s (56) groups have since demonstrated microscopic involvement of the lower GI tract in 77% to 88%, and 43% to 77% of cases with involvement of upper GI tract, respectively.
Pathogenesis
MCL is characterized by the translocation t[11;14] [q13;q32], which joins the IgH gene sequences with the BCL1 locus, leading to CCND1 gene up-regulation and cyclin D1 over expression (55).
Morphology and immunophenotype
In the gastrointestinal tract, MCL demonstrates microscopic infiltration by nodular lymphoid aggregates in the lamina propria or submucosa of the stomach or colon. These infiltrates may be present, though difficult to find in endoscopically normal mucosa. Cyclin D1 over-expression is a highly characteristic and specific feature of MCL. It is usually detected in CD20+ and CD5+ lymphoid infiltrates, but not in CD20+ and CD5- lymphoid infiltrates, and is therefore most helpful in distinguishing malignant mantle lymphoid cells from normal mucosa-associated lymphoid tissue or reactive B cell lymphocytes of chronic gastritis (55).
Molecular abnormality
The translocation t[11;14] [q13;q32] is detected in 50% to 70% of MCL patients by conventional cytogenetic analysis, in 90% to 100% by fluorescence in situ hybridization (FISH). Polymerase chain reaction (PCR) is less sensitive, demonstrating the translocation in only 30% to 50% of cases (55).
Prognosis
Compared to primary GI tract FL and MALT lymphoma, primary GI tract MCL or MLP confers a worse prognosis, with a median overall survival of eight to 20 months (54).
Anaplastic lymphoma kinase (ALK) - positive large B cell lymphoma
Identification of plasmablastic or anaplastic cells can prompt extensive immunohistochemical examination to exclude poorly differentiated carcinoma, melanoma, plasmablastic myeloma, and lymphoma. The presence of characteristic large cells with prominent centrally placed nucleoli and abundant amphophilic cytoplasm should also prompt ALK immunostaining in consideration for this rare presentation of ALK+ DLBCL in the GI tract.
Pathogenesis
The clathrin (CLTC) gene encodes a protein involved in receptor-mediated endocytosis in coated vesicles. Translocation of the CLTC and ALK gene loci [t(2;17) (p23;q23)] results in movement of ALK from the cell membrane to the cytoplasm resulting in constitutional activation (57).
Morphology and immunophenotype
Characteristically this malignancy shows large cells with plasmablastic morphology involving the subcapsular sinus of lymph nodes. As indicated by the name, these lymphomas are positive for ALK in a finely granular cytoplasmic pattern (58). This cytoplasmic pattern supports the function of CLTC in moving ALK expression from the cell membrane. Furthermore, this neoplasm shows variable CD30 expression, with frequent CD38 and CD138 co-expression in the absence of earlier B cell antigens such as CD19, CD20, CD22, CD79a and CD79b (57). Distinction of this entity from plasmablastic lymphoma is largely based on ALK positivity and lack of Epstein-Barr early ribonucleoprotein 1 (EBER1) expression, which are typical features of ALK-positive large B cell lymphoma (58).
Molecular abnormality
The presence of t[2;17] [p23;q23] has been suggested as a genetic mechanism for ALK-positive B cell lymphoma, including cases that occur in the GI tract. The translocation joins clathrin (CLTC) and ALK, resulting in a fusion gene (57,58).
Prognosis
In the more commonly encountered CD30-positive anaplastic large cell lymphoma of T-cell or null type, ALK expression is generally regarded as a good prognostic factor. In ALK-positive large B-cell lymphoma, however, only four cases have been fully characterized, with a documented median survival of 11 months in patients with stages III-IV disease (59).
Lymphomatoid granulomatosis (LG)
Lymphomatoid granulomatosis is an extranodal angiodestructive disease composed of EBV-positive B cells within a dominant background reactive T-cell population. It most commonly occurs in the lung; however, the gastrointestinal tract may rarely be involved (60).
Pathogenesis
EBV has been hypothesized to play a role in disease pathogenesis. As such, immunocompromised patients are at increased risk in developing this lesion.
Morphology and immunophenotype
LG is an angiocentric/angiodestructive infiltrate of polymorphous lymphocytes. Admixed plasma cells and histiocytes are usually observed, however, neutrophils and eosinophils are not typically conspicuous. Invasion of vascular walls by lymphocytes may lead to adjacent necrosis due to compromised vascular integrity.
Distinction between the similarly angiodestructive sinonasal NK/T-cell lymphoma is paramount and can be accomplished by immunophenotypic analysis. LG typically consists of mature, CD20+ B cell population, frequently co-expressing EBV-encoded RNA, in a background of reactive, CD4+ and CD8+ T cells (60).
Molecular abnormalities
Molecular techniques detect both clonality of immunoglobulin genes and presence of EBV-encoded RNA. Key to the differential of NK/T-cell lymphomas, T cell receptor (TCR) gene rearrangement analysis will show germline configuration in true NK-cell lesion (i.e., no evidence of monoclonality) (60).
Prognosis
Typically the disease is aggressive, with median survival falling below two years. High-grade lesions have a risk of progression to EBV-positive DLBCL.
Mature T cell, NK/T cell lymphomas
Enteropathy-associated T cell lymphoma (EATL)
EATL is an intestinal intraepithelial T cell malignancy most commonly occurring in the jejunum or ileum. Rarely, it may present in the duodenum, stomach or colon. This entity is particularly common in Northern Europe where celiac disease is highly prevalent. Multiple raised, ulcerated mucosal nodules are often seen on endoscopy but in some instances may appear as an exophytic mass (61). EATL consists of two types of disorders: type I EATL which is a complication of celiac disease and the less frequent, type II EATL which is unrelated to celiac sprue (8).
Pathogenesis
In cases of refractory celiac disease, type I EATL is thought to arise from activation of intraepithelial T lymphocytes where malignant transformation with down-regulation of T cell receptor (TCR)-CD3, loss of CD8 expression, and TCR gene rearrangement may occur (8). Interleukin (IL)-15, an inflammatory cytokine is typically over-expressed in the intestinal mucosa of patients with celiac disease. Studies have demonstrated the role of IL-15 in the development of T cell lymphoma (62,63). In addition, propagation of small clonal T cells (microlymphomas) in the setting of ulcerative jejunitis, a complication of celiac sprue, has been hypothesized in the neoplastic transformation of T cells (8).
Morphology and immunophenotype
Type I EATL consists of a polymorphous population of neoplastic lymphoid cells of varying sizes with predominant large lymphoid cells demonstrating irregular, angulated, vesicular nuclei with distinct nucleoli and moderate to ample faintly staining cytoplasm admixed with inflammatory cells such as histiocytes, plasma cells and eosinophils. Pleomorphism with large, multinucleated lymphoid cells resembling anaplastic large cell lymphoma is observed in rare cases. However, necrosis is commonly present which may lead to transmural perforation. Villous atrophy, crypt hyperplasia and increase in intraepithelial inflammatory cells are frequently observed. The neoplastic T cells are positive for CD3, CD7 and CD103, but are typically negative for CD4 and CD5, and show variable reactivity with CD8, CD30 and TCRβ. They may also co-express cytotoxic markers such as granzyme B, perforin and/or TIA1. The adjacent intraepithelial lymphocytes may also express abnormal immunophenotype with loss of CD5, CD4 and CD8 expression (8,61).
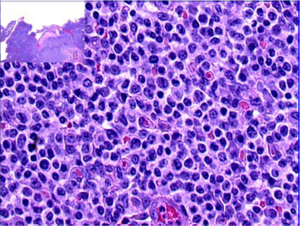
Type II EATL on the other hand consists of a monomorphous infiltrate of medium-sized lymphoid cells with hyperchromatic nuclei enclosed by scant, pale cytoplasm (Figure 4). There is usually marked infiltration of the surface epithelium (Figure 4, inset) and crypts. Nonetheless, background inflammation and necrosis are less frequently observed (8,61). The monomorphic neoplastic T cells characteristically show CD8 positivity with co-expression of CD56 (Figure 5, top right, bottom left, respectively). Reactivity with CD3 (Figure 5, top left) and TCRβ are noted. The tumor cell nuclei usually show a quite high Ki-67 nuclear proliferative index (Figure 5, bottom right). However, the tumor cells are usually negative for CD4. Similarly, the intraepithelial lymphocytes in close proximity may express the same aberrant phenotypes (8,10,61).
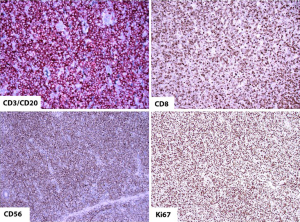
Molecular abnormalities
Rearrangements of TCRβ and TCRγ are present in both type I and II EATLs. Approximately 58-70% of the cases show complex chromosomal aberrations including gains at 9q31.3-qter or deletions in 16q12.1. Moreover, type I EATL demonstrates gains at 5q, or gains or partial trisomy of 1q22-q44, while amplification of the MYC locus at 8q24 is more commonly expressed in type II EATL (8,61).
The HLA-DQA1*0501, DQB1*0201 genotype is identified in greater than 90% of patients with celiac disease. This genotype is also noted in patients with EATL (61) further supporting the association between the two diseases.
Prognosis
Both type I and type II EATL have poor clinical outcomes (61) with a reported 5-year survival of 8-20% (64). Death often results from malabsorption and other abdominal or clinical complications.
Extranodal NK/T cell lymphoma, nasal type (ENKTL)
This aggressive entity primarily occurs in the nasal cavity, nasopharynx and paranasal sinuses but also involves a number of extranasal locations including the GI tract where it may present as ulceration (65) or perforation. The disease is more commonly seen in Asians, Mexicans and natives of Central and South America, and more frequently affects males than females. Virtually all cases of ENKTL are associated with EBV infection (8,9).
Pathogenesis
Although evolution of this entity remains uncertain, studies attribute to the probable role of EBV which presents in a clonal episomal form resulting in the production of cytokines such as IL-9 and IL-10 inducing oncogenicity. This clonal EBV harbored by the tumor cells typically shows type II latency pattern with Epstein-Barr nuclear antigen 1 (EBNA-1) and LMP1 positivity and non-reactivity for EBNA-2 (8,9,66).
Morphology and immunophenotype
The infiltrate often effaces the mucosal architecture and consists of varying sizes of pleomorphic neoplastic lymphoid cells with irregular, convoluted nuclear contour with indistinct nucleoli. The larger lymphoid cells show irregular nuclei with vesicular chromatin. The moderate to abundant cytoplasm is usually clear or faint. In particular, the neoplastic lymphoid cells characteristically show an angiocentric and angiodestructive pattern where they aggregate around and infiltrate blood vessel wall. Admixed inflammatory cells consisting of histiocytes, plasma cells and small lymphocytes, ulceration of the overlying mucosa and geographic necrosis are frequently observed.
The tumor cells are distinctively CD2, CD56, cytoplasmic CD3 positive and express cytotoxic molecules (Granzyme B, TIA-1 and perforin) but are negative for surface CD3 and other T or NK cell markers such as CD4, CD5, CD8, TCRδ, βF1, CD16 and CD57. Some cases demonstrate reactivity for CD7 or CD30 (8,9).
Molecular abnormalities
The majority of cases demonstrate TCR and immunoglobulin genes in the usual germline pattern, with only a minor percentage of cases expressing clonal TCR rearrangement. The cases with TCR rearrangement possibly represent a true cytotoxic T cell origin (8,9). Various cytogenetic alterations have been documented but the two most frequent aberrations noted are del (6)[q21q25] and i(6)(p10), and other cytogenetic abnormalities identified via array comparative genomic hybridization analyses include gain of 2q, and loss of 1p36.23-p36.33, 6q16.1-q27, 4q12, 5q34-q35.3, 7q21.3-q22.1, 11q22.3-q23.3 and 15q11.2-q14 (55,60). Some cases of ENKTL have also been documented to harbor abnormal methylation of promoter CpG domains particularly of the p73 gene, mutation of TP53, KRAS, KIT or β-catenin, and partial deletion of FAS gene (9).
Prognosis
ENKTL is an aggressive disease and confers poor prognosis. EBV-DNA level in plasma and peripheral blood mononuclear cells have been recently proposed as a probable prognostic factor. Detectable or a higher titer of plasma EBV-DNA level has been shown to be associated with widespread disease, poor therapeutic response and an overall higher mortality rate (9,67,68).
NK-cell enteropathy or lymphomatoid gastropathy
Rare cases of benign, indolent NK-cell enteropathy or lymphomatoid gastropathy have been recently described and therefore should be differentiated from the aggressive ENKTL. Mansoor and associates documented eight cases of atypical NK-cell proliferation limited to the GI tract (stomach, duodenum and colon) (10). Tanaka and colleagues reported a similar gastric lesion from a 50-year-old man; hence, the designation “lymphomatoid gastropathy” (11). Clinical presentations vary from asymptomatic states to vague abdominal discomfort, constipation, diarrhea, hematochezia and melena (10,11).
Pathogenesis and molecular abnormalities
The exact etiology of this entity is still yet to be elucidated. Polymerase chain reaction (PCR) analysis performed in the nine documented cases of NK-cell enteropathy and/or lymphomatoid gastropathy showed absence of TCR-gamma (γ) gene rearrangement (10,11).
Morphology and immunophenotype
The lamina propria is usually distended by a fairly well-circumscribed atypical cellular infiltrate consisting of medium to large round to ovoid cells with irregular nuclear contour, with hyperchromasia, small nucleoli, and an ample amount of cytoplasm. There is infrequent involvement of the submucosa. The atypical cellular infiltrate distorts the glandular architecture in the early stages of the disease, whereas glandular destruction is noted in advanced disease. Nevertheless, the atypical cells rarely infiltrate glandular epithelium (absence of epitheliotropism). In addition, angiocentric or angiodestructive growth pattern, a characteristic feature noted in ENKTL, is generally not observed in NK-cell enteropathy or lymphomatoid gastropathy (10,11).
The atypical cells express NK cell markers such as CD56, cytoplasmic CD3, CD7, TIA-1 and/or Granzyme B, but are non-reactive for CD4, CD8, CD5, CD10, CD20, CD30, CD68, or CD138. The proliferative index as evident by Ki-67 nuclear staining is usually low. Furthermore, in contrast to ENKTL, NK-cell enteropathy or lymphomatoid gastropathy is not typically associated with EBV infection (10,11).
Prognosis
This lesion clinically behaves in a benign and an indolent manner. Disease persistence was observed in 67% to 75% of the patients, with recurrence in one patient two years after spontaneous regression of the disease (11). Moreover, none of the patients showed evidence of disease progression, and there was no reported mortality (10,11).
It is therefore essential to distinguishing this entity from the more aggressive NK/T-cell lymphomas in order to avoid unnecessary therapy and its associated risks.
Other GI hematopoietic neoplasms
Extramedullary plasmacytoma (EMP)
Extramedullary plasmacytoma is a neoplastic proliferation of monoclonal plasma cells outside of the peripheral blood circulation and bone marrow. The tumor cells show eccentrically located nuclei with speckled chromatin. The abundant deeply basophilic cytoplasm forms a distinct, partial perinuclear hoff or clearing. The tumor cells are arranged in large sheets or clusters. Propagation of larger, immature plasma cells (plasmablasts) as well as pleomorphism with bi-nucleated and frequently tri-nucleated forms is typically noted in advanced or more extensive disease process. The neoplastic plasma cells express plasma cell markers such as CD138 and CD38 with monotypic cytoplasmic immunoglobulin light chain (either kappa or lambda) but lacking surface immunoglobulin. Approximately 67-79% of the cases show aberrant co-expression of CD56 (69).
EMP is a recognized occurrence; however, involvement of the GI tract, particularly colon is rare with less than 25 documented cases in the literature (13). Amyloidosis, a systemic disease associated with plasma cell dyscrasia particularly the light chain or amyloid light chain (AL) subtype, may also be encountered in the GI tract and most frequently occurs in the small bowel (14). Amyloid deposit shows characteristic dull brick red staining with Congo red and demonstrates apple-green birefringence on polarized light, features that differentiate amyloid from collagen.
Morphologically, EMP may appear similar to plasmablastic lymphoma (PBL) which is a more aggressive entity and is typically associated with immunodeficiency. Clinical and immunophenotypic features are necessary in differentiating these two entities.
Plasmablastic lymphoma (PBL)
Plasmablastic lymphoma, originally discovered in the oral cavity, has since shown a predilection for extranodal, mucosal sites including the GI tract (15,16). PBL has been documented arising in the stomach, small bowel, colon, rectum and anus (15). This entity is most frequently associated with immunodeficiency particularly in the context of human immunodeficiency virus (HIV) infection (15).
PBL demonstrates diffuse, cohesive cell aggregates with variable morphologic differentiation ranging from immunoblastic to more mature plasmacytic features. Mitotic activity is usually brisk and apoptotic bodies are frequently encountered (16). The tumor cells are positive for plasma cell antigens CD138, CD38, Vs38c, and MUM1, but are negative for pan-B cell markers such as CD20 and PAX5. The neoplastic cells are also non-reactive for the germinal center marker BCL6 (15). CD45 reactivity may be weak or variable, and CD79a is seen in 50-85% of cases (15,16). Co-expression of cytoplasmic Ig, particularly IgG is detected in a subset of cases (15). The tumor cells characteristically demonstrate EBV encoded RNA (EBER) positivity but are non-reactive for EBNA-2 or LMP1; a feature noted in all cases of documented HIV-associated PBL (15,16). Morphology may appear similar to plasmablastic or anaplastic plasmacytoma/myeloma; however, EBER reactivity distinguishes PBL from the aforementioned entity. In addition, the lack of pan-B cell expression differentiates PBL from DLBCL with immunoblastic or plasmacytic features (15).
PBL is an aggressive disease, with a number of patients dying within a year of diagnosis despite advances in HIV treatment (15,16).
Hodgkin lymphoma (HL)
Primary extranodal Hodgkin lymphoma of the GI tract is a rare occurrence. Of the GI organs, the stomach is the most frequently involved, followed by the small bowel, colon and esophagus (17). Although infrequent, primary GI tract HL may mimic the clinical presentation as well as radiographic and endoscopic impressions of inflammatory bowel disease (IBD) which may pose a diagnostic challenge (17). Criteria for the diagnosis of primary extranodal HL of the GI tract include predominant GI tract lesion, lack of concomitant superficial and mediastinal lymphadenopathy, unremarkable blood cell counts, and absence of liver and spleen involvement (18).
Histologically, the mucosa and submucosa are infiltrated by nodular and diffuse polymorphous cellular population, consisting of small to medium-sized lymphocytes, plasma cells, histiocytes and eosinophils. Often, scattered characteristic binucleated HRS cells with large prominent eosinophilic nucleoli and their mononuclear variants are encountered. The polymorphous infiltrate may spread to the muscularis propria and penetrate the serosa resulting in perforation (18). In classical HL, the HRS cells and variants usually lack expression of CD45 but are positive for PAX5 and MUM1. CD30 and CD15 highlight the HRS cells and variants with characteristic membranous and Golgi staining patterns. The characteristic HRS cells and variants typically show reactivity for EBER indicating association with EBV infection as a consequence of immunosuppression or immunodeficiency (18).
Histiocytic sarcoma (HS)
This a rare neoplasm consisting of diffuse, medium to large and round to oval epithelioid cells with convoluted nuclei and abundant pale to eosinophilic, vacuolated cytoplasm. Although some cases may demonstrate monomorphous proliferation, pleomorphism is commonly encountered. Histiocytic sarcoma (HS) may morphologically mimic DLBCL or anaplastic large cell lymphoma (ALCL), and while histiocytic sarcoma usually presents as a non-cohesive infiltrate, the tumor cells may occasionally show cohesion and thus, imitate carcinoma or melanoma (19). Hence, immunohistochemistry is frequently utilized for characterization and distinction from several differential diagnoses. The histiocytic tumor cells usually express CD163, CD68 and lysozyme and lack specific lymphoid (i.e., CD3, CD20), myeloid (i.e., myeloperoxidase, CD33, CD13) or Langerhans cell (i.e., CD1a, langerin) markers (70). CD30 and epithelial membrane antigen (EMA) are also useful in distinguishing HS from ALCL; these two markers are usually positive in ALCL (19) and negative in HS. Moreover, HS is negative for pancytokeratin, whereas carcinomas typically express this marker.
Although occurrence in the GI tract is rare, HS has been documented in the stomach, colon, ileum, rectum and anus, and are often behaves in a clinically aggressive fashion (15,16,19,20). One case had widespread disease infiltration involving the liver, spleen, bone marrow and lymph nodes and showed moderate tumor pleomorphism with multinucleated giant cells. Consequently, multiple ulcerations with critical perforations were identified in the esophagus and duodenum but tumor cells were not found in these regions. It was postulated that ischemic embolism associated with the malignant process instigated mucosal damage (21).
Mast cell sarcoma (MCS)
Mast cell sarcoma (MCS), an exceedingly rare entity is one of the variants of systemic mastocytosis (SM). It consists of a unifocal, destructive growth of atypical mast cells in aggregates and sheets demonstrating convoluted hyperchromatic nuclei which are often bi- or multilobated, with ample amount of finely granular cytoplasm. MCS may morphologically mimic other malignancies such as histiocytic or myeloid neoplasms, as well as sarcomas with epithelioid features. Immunohistochemistry is essential in differentiating MCS from these other lesions. MCS is reactive for tryptase, CD117 and show co-expression of CD2 and CD25; the latter two highlight neoplastic mast cells (71).
At least six cases of MCS have been reported in the literature, thus far, and only two of these primarily involved the GI tract, mainly the small bowel (22) and ascending colon (23). This disease is usually aggressive with high frequency of transformation to mast cell leukemia (71).
Treatment
Treatment for GI lymphomas primarily depends on the grade and stage at presentation and association with H. pylori infection. For instance, low-grade, stage I MALT lymphoma associated with H. pylori follows a conservative approach with triple antibiotics to eradicate the microorganism. This process has been repeatedly documented in producing excellent clinical outcome with approximately 75-80% remission rate; thus, it has been considered the primary mode of treatment in stage I, H. pylori-associated cases (4-6,35-38,40). Chemotherapy with or without radiation therapy is reserved for more advanced diseases or in H. pylori-associated primary GI lymphomas which are resistant to antibiotics, and also in cases without H. pylori association (37-39). Surgery is rarely performed and is only pursued in cases with severe complications (40) such as obstruction and perforation, or in localized disease with prior resistance to neoadjuvant chemotherapy.
Conclusions
Primary lymphomas of the GI tract may consist of mature B, T or NK/T cell neoplasms, of which, the two most commonly encountered morphologic subtypes are extranodal MALT lymphoma and DLBCL (1). The stomach is the most frequent site involved (2). Moreover, association of primary GI lymphomas with H. pylori infection, particularly observed in extranodal MALT lymphoma and in a few cases of DLBCL has revolutionized treatment approach, with conservative antibiotic regimen as the primary therapeutic method in low-grade, stage I diseases (4-6,35-38). On the other hand, T cell and NK/T cell GI tract lymphomas often entail a more aggressive clinical behavior (8,9,57,58,61-63). However, cases of benign, indolent NK-cell enteropathy or lymphomatoid gastropathy have been described recently (10,11), and thus, it is imperative to distinguish this entity from true NK/T cell neoplasm in order to initiate proper clinical management.
Acknowledgments
The authors would like to thank Dr. Jeffrey D. Cao for sharing GI lymphoma cases from the Loma Linda Veterans Hospital, and Dr. Craig Zuppan for his help and instructions in photomicrograph editing.
Disclosure: The authors declare no confict of interest.
References
- Psyrri A, Papageorgiou S, Economopoulos T. Primary extranodal lymphomas of stomach: clinical presentation, diagnostic pitfalls and management. Ann Oncol 2008;19:1992-9. [PubMed]
- Ferreri AJ, Montalbán C. Primary diffuse large B-cell lymphoma of the stomach. Crit Rev Oncol Hematol 2007;63:65-71. [PubMed]
- Swerdlow SH, Campo E, Harris NL, et al. Extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon:IARC Press, 2008:214-7.
- Stolte M, Bayerdörffer E, Morgner A, et al. Helicobacter and gastric MALT lymphoma. Gut 2002;50:III19-24. [PubMed]
- Jezersek Novaković B, Vovk M, Juznic Setina T. A single-center study of treatment outcomes and survival in patients with primary gastric lymphomas between 1990 and 2003. Ann Hematol 2006;85:849-56. [PubMed]
- Nakamura T, Seto M, Tajika M, et al. Clinical features and prognosis of gastric MALT lymphoma with special reference to responsiveness to H. pylori eradication and API2-MALT1 status. Am J Gastroenterol 2008;103:62-70. [PubMed]
- Lecuit M, Abachin E, Martin A, et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N Engl J Med 2004;350:239-48. [PubMed]
- de Leval L, Gaulard P. Pathology and biology of peripheral T-cell lymphomas. Histopathology 2011;58:49-68. [PubMed]
- Chan JKC, Quintanilla-Martinez L, Ferry JA, et al. Extranodal NK/T-cell lymphoma, nasal type. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:285-8.
- Mansoor A, Pittaluga S, Beck PL, et al. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood 2011;117:1447-52. [PubMed]
- Tanaka T, Megahed N, Takata K, et al. A case of lymphomatoid gastropathy: An indolent CD56-positive atypical gastric lymphoid proliferation, mimicking aggressive NK/T cell lymphomas. Pathol Res Pract 2011;207:786-9. [PubMed]
- Islam SR, Attaya MN, Parupudi S, et al. Sigmoid plasmacytoma mimicking colon cancer in a patient with multiple myeloma: case report and review of literature. Gastrointest Endosc 2010;71:655-7. [PubMed]
- Kakati BR, Krishna K, Krishna SG, et al. Extensive Extramedullary Disease Involving the Colon in Multiple Myeloma: A Case Report and Review of Literature. J Gastrointest Cancer 2012;43:379-81. [PubMed]
- Ebert EC, Nagar M. Gastrointestinal manifestations of amyloidosis. Am J Gastroenterol 2008;103:776-87. [PubMed]
- Dong HY, Scadden DT, de Leval L, et al. Plasmablastic lymphoma in HIV-positive patients: an aggressive Epstein-Barr virus-associated extramedullary plasmacytic neoplasm. Am J Surg Pathol 2005;29:1633-41. [PubMed]
- Stein H, Harris NL, Campo E. Plasmablastic lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon:IARC Press, 2008:256-7.
- Kashi MR, Belayev L, Parker A. Primary extranodal Hodgkin lymphoma of the colon masquerading as new diagnosis of Crohn's disease. Clin Gastroenterol Hepatol 2010;8:A20. [PubMed]
- Gandhi JS, Mehta A, Sharma A, et al. Primary Hodgkin lymphoma of the ileum. J Cancer Res Ther 2010;6:342-3. [PubMed]
- Hornick JL, Jaffe ES, Fletcher CD. Extranodal histiocytic sarcoma: clinicopathologic analysis of 14 cases of a rare epithelioid malignancy. Am J Surg Pathol 2004;28:1133-44. [PubMed]
- Vos JA, Abbondanzo SL, Barekman CL, et al. Histiocytic sarcoma: a study of five cases including the histiocyte marker CD163. Mod Pathol 2005;18:693-704. [PubMed]
- Akishima Y, Akasaka Y, Yih-Chang G, et al. Histiocytic sarcoma with fatal duodenal ulcers. Pathol Res Pract 2004;200:473-8. [PubMed]
- Bugalia A, Abraham A, Balasubramanian P, et al. Mast cell sarcoma of the small intestine: a case report. J Clin Pathol 2011;64:1035-7. [PubMed]
- Kojima M, Nakamura S, Itoh H, et al. Mast cell sarcoma with tissue eosinophilia arising in the ascending colon. Mod Pathol 1999;12:739-43. [PubMed]
- Ioannidis O, Cheva A, Kakoutis E, et al. Acute adult intussusception caused by primary cecal non Hodgkin lymphoma. Acta Gastroenterol Belg 2011;74:451-3. [PubMed]
- England RJ, Pillay K, Davidson A, et al. Intussusception as a presenting feature of Burkitt lymphoma: implications for management and outcome. Pediatr Surg Int 2012;28:267-70. [PubMed]
- Kelessis NG, Vassilopoulos PP, Tsamakidis KG, et al. Is gastroscopy still a valid diagnostic tool in detecting gastric MALT lymphomas? A dilemma beyond the eye. Mucosa-associated lymphoid tissue. Surg Endosc 2003;17:469-74. [PubMed]
- Tellez-Avila FI, García-Osogobio S, Chavez-Tapia NC, et al. Utility of endoscopy in patients with incidental gastrointestinal luminal wall thickening detected with CT. Surg Endosc 2009;23:2191-6. [PubMed]
- Mehra M, Agarwal B. Endoscopic diagnosis and staging of mucosa-associated lymphoid tissue lymphoma. Curr Opin Gastroenterol 2008;24:623-6. [PubMed]
- Janssen J. The impact of EUS in primary gastric lymphoma. Best Pract Res Clin Gastroenterol 2009;23:671-8. [PubMed]
- Ribeiro A, Pereira D, Escalón MP, et al. EUS-guided biopsy for the diagnosis and classification of lymphoma. Gastrointest Endosc 2010;71:851-5. [PubMed]
- Arnold IC, Hitzler I, Engler D, et al. The C-terminally encoded, MHC class II-restricted T cell antigenicity of the Helicobacter pylori virulence factor CagA promotes gastric preneoplasia. J Immunol 2011;186:6165-72. [PubMed]
- Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol 2005;23:6370-8. [PubMed]
- Sagaert X, De Wolf-Peeters C, Noels H, et al. The pathogenesis of MALT lymphomas: where do we stand? Leukemia 2007;21:389-96. [PubMed]
- Terada T. CD5-positive marginal zone B-cell lymphoma of the mucosa-associated lymphoid tissue (MALT) of the lung. Diagn Pathol 2012;7:16. [PubMed]
- Yoon SS, Coit DG, Portlock CS, et al. The diminishing role of surgery in the treatment of gastric lymphoma. Ann Surg 2004;240:28-37. [PubMed]
- Nakamura T, Inagaki H, Seto M, et al. Gastric low-grade B-cell MALT lymphoma: treatment, response, and genetic alteration. J Gastroenterol 2003;38:921-9. [PubMed]
- Chen LT, Lin JT, Tai JJ, et al. Long-term results of anti-Helicobacter pylori therapy in early-stage gastric high-grade transformed MALT lymphoma. J Natl Cancer Inst 2005;97:1345-53. [PubMed]
- Levy M, Copie-Bergman C, Traulle C, et al. Conservative treatment of primary gastric low-grade B-cell lymphoma of mucosa-associated lymphoid tissue: predictive factors of response and outcome. Am J Gastroenterol 2002;97:292-7. [PubMed]
- Fischbach W. Long-term follow-up of gastric lymphoma after stomach conserving treatment. Best Pract Res Clin Gastroenterol 2010;24:71-7. [PubMed]
- Aleman BM, Haas RL, van der Maazen RW. Role of radiotherapy in the treatment of lymphomas of the gastrointestinal tract. Best Pract Res Clin Gastroenterol 2010;24:27-34. [PubMed]
- Harris NL, Isaacson PG, Grogan TM, et al. Heavy chain diseases. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:198.
- Stein H, Chan J, Warnke R, et al. Diffuse large B-cell lymphoma, not otherwise specified. In: Swerdlow S, Campo E, Harris N, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:233-7.
- Choi SY, Kim SJ, Kim WS, et al. Aggressive B cell lymphomas of the gastrointestinal tract: clinicopathologic and genetic analysis. Virchows Arch 2011;459:495-502. [PubMed]
- Pagano L, Caira M, Valentini CG, et al. Clinical aspects and therapy of sporadic burkitt lymphoma. Mediterr J Hematol Infect Dis 2009;1:e2009030. [PubMed]
- Baumgaertner I, Copie-Bergman C, Levy M, et al. Complete remission of gastric Burkitt’s lymphoma after eradication of Helicobacter pylori. World J Gastroenterol 2009;15:5746-50. [PubMed]
- Colović N, Radovanović N, Vidović A, et al. Primary Burkitt’s lymphoma of the stomach. Srp Arh Celok Lek 2011;139:523-6. [PubMed]
- Siani LM, Siani A, Ricci V, et al. Burkitt’s lymphoma of the caecum in a patient with AIDS: clinical case and review of the literature. Minerva Chir 2009;64:229-33. [PubMed]
- Oyama T, Yamamoto K, Asano N, et al. Age-related EBV-associated B-cell lymphoproliferative disorders constitute a distinct clinicopathologic group: a study of 96 patients. Clin Cancer Res 2007;13:5124-32. [PubMed]
- Wong HH, Wang J. Epstein-Barr virus positive diffuse large B-cell lymphoma of the elderly. Leuk Lymphoma 2009;50:335-40. [PubMed]
- Nakamura S, Jaffe ES, Swerdlow SH. EBV positive diffuse large B-cell lymphoma of the elderly. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:243-4.
- Damaj G, Verkarre V, Delmer A, et al. Primary follicular lymphoma of the gastrointestinal tract: a study of 25 cases and a literature review. Ann Oncol 2003;14:623-9. [PubMed]
- Misdraji J, Harris NL, Hasserjian RP, et al. Primary follicular lymphoma of the gastrointestinal tract. Am J Surg Pathol 2011;35:1255-63. [PubMed]
- Shia J, Teruya-Feldstein J, Pan D, et al. Primary follicular lymphoma of the gastrointestinal tract: a clinical and pathologic study of 26 cases. Am J Surg Pathol 2002;26:216-24. [PubMed]
- Nguyen V, Nguyen B, Petris GD, et al. Education and imaging. Gastrointestinal: gastrointestinal involvement of mantle cell lymphoma. J Gastroenterol Hepatol 2012;27:617. [PubMed]
- Salar A, Juanpere N, Bellosillo B, et al. Gastrointestinal involvement in mantle cell lymphoma: a prospective clinic, endoscopic, and pathologic study. Am J Surg Pathol 2006;30:1274-80. [PubMed]
- Romaguera JE, Medeiros LJ, Hagemeister FB, et al. Frequency of gastrointestinal involvement and its clinical significance in mantle cell lymphoma. Cancer 2003;97:586-91. [PubMed]
- McManus DT, Catherwood MA, Carey PD, et al. ALK-positive diffuse large B-cell lymphoma of the stomach associated with a clathrin-ALK rearrangement. Hum Pathol 2004;35:1285-8. [PubMed]
- Zhang D, Denley RC, Filippa DA, et al. ALK-positive diffuse large B-cell lymphoma with the t(2;17)(p23;q23). Appl Immunohistochem Mol Morphol 2009;17:172-7. [PubMed]
- Reichard KK, McKenna RW, Kroft SH. ALK-positive diffuse large B-cell lymphoma: report of four cases and review of the literature. Mod Pathol 2007;20:310-9. [PubMed]
- Pittaluga S, Wilson WH, Jaffe ES. Lymphomatoid granulomatosis. In: Swerdlow SH, Campo E, Harris NL, et al, eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:247-9.
- Isaacson PG, Chott A, Ott G, et al. Enteropathy associated T-cell lymphoma. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:289-91.
- Mention JJ, Ben Ahmed M, Bègue B, et al. Interleukin 15: a key to disrupted intraepithelial lymphocyte homeostasis and lymphomagenesis in celiac disease. Gastroenterology 2003;125:730-45. [PubMed]
- Malamut G, El Machhour R, Montcuquet N, et al. IL-15 triggers an antiapoptotic pathway in human intraepithelial lymphocytes that is a potential new target in celiac disease-associated inflammation and lymphomagenesis. J Clin Invest 2010;120:2131-43. [PubMed]
- van de Water JM, Cillessen SA, Visser OJ, et al. Enteropathy associated T-cell lymphoma and its precursor lesions. Best Pract Res Clin Gastroenterol 2010;24:43-56. [PubMed]
- Yu JH, Choi KD, Koh YW, et al. A case of CD56+ extranodal NK/T-cell lymphoma, nasal type, presenting as a duodenal ulcer bleeding. Korean J Gastroenterol 2009;54:174-9. [PubMed]
- Gualco G, Domeny-Duarte P, Chioato L, et al. Clinicopathologic and molecular features of 122 Brazilian cases of nodal and extranodal NK/T-cell lymphoma, nasal type, with EBV subtyping analysis. Am J Surg Pathol 2011;35:1195-203. [PubMed]
- Nakashima Y, Tagawa H, Suzuki R, et al. Genome-wide array-based comparative genomic hybridization of natural killer cell lymphoma/leukemia: different genomic alteration patterns of aggressive NK-cell leukemia and extranodal Nk/T-cell lymphoma, nasal type. Genes Chromosomes Cancer 2005;44:247-55. [PubMed]
- Suzuki R, Yamaguchi M, Izutsu K, et al. Prospective measurement of Epstein-Barr virus-DNA in plasma and peripheral blood mononuclear cells of extranodal NK/T-cell lymphoma, nasal type. Blood 2011;118:6018-22. [PubMed]
- McKenna RW, Kyle RA, Kuehl WM, et al. Plasma cell neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:202-8.
- Grogan TM, Pileri SA, Chan JKC, et al. Histiocytic sarcoma. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:356-7.
- Horny HP, Metcalfe DD, Bennett JM, et al. Mastocytosis. In: Swerdlow SH, Campo E, Harris NL, et al. eds. World Health Organization Classification of Tumours of Haematopoietic and Lymphoid Tissues (4th edition). Lyon: IARC Press, 2008:54-63.


