
Inherited syndromes involving pancreatic neuroendocrine tumors
Multiple endocrine neoplasia type 1 (MEN1) is one of the most well-known hereditary endocrine syndromes, first described in 1903 with an estimated prevalence of 2 per 100,000 in the general population (1,2). However, in patients with gastrinomas the incidence of MEN1 is 16−38%, which highlights the opportunity for syndrome recognition in this disease population (3-5). The clinical diagnostic criteria include: a patient with two or more classic MEN1-associated tumors (parathyroid, gastroenteropancreatic neuroendocrine, pituitary) or a patient with a single MEN1-associated tumor and a first-degree relative with MEN1 (6). A molecular diagnosis is given when a pathogenic variant in the MEN1 gene is identified, genetic testing criteria are provided in Figure 1 (6,7).
As MEN1 syndrome is inherited in an autosomal dominant pattern, there is a 50% chance for affected individuals to pass the disease on to each offspring. Approximately 10% of cases are considered sporadic, where neither parent has any manifestations of MEN1. Since the majority of cases are familial, it is appropriate to consider non-paternity and occult or unreported disease in a parent if no family history is reported. MEN1 syndrome is also a highly penetrant disease, over 90% individuals with MEN1 syndrome develop hyperparathyroidism by the age of 60, nearly 70% develop gastroenteropancreatic neuroendocrine tumors (GEP-NET), and 30% to 40% develop pituitary adenoma (Table 1) (8,9). The first presenting manifestation in individuals with MEN1 is typically hyperparathyroidism which is often multiglandular disease. Gastrinoma is the most commonly identified GEP-NET, seen in approximately 40% of patients with MEN1 syndrome, followed by insulinoma at a 10% frequency (6). Cutaneous manifestations are common in MEN1 syndrome and include facial angiofibromas, collagenomas and lipomas. Individuals with MEN1 syndrome also have an increased risk for developing thymic and bronchial carcinoid tumors, adrenocortical tumors (mostly nonfunctioning) and meningiomas. Comprehensive guidelines for screening and management of MEN1 syndrome are available from expert sources including The Endocrine Society and The National Comprehensive Cancer Network (6,10).
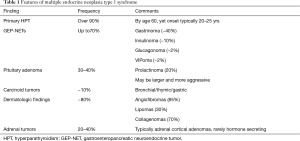
Full table
MEN1 is a tumor suppressor gene, identified in 1997 at the 11q13.1 locus, encoding the menin protein consisting of 10 exons and 610 amino acids. Menin localizes to the nucleus and is involved in several important cell functions including transcriptional regulation, genome stability, cell division, and proliferation. Over 250 germline pathogenic/likely-pathogenic variants in the MEN1 gene have been reported in the ClinVar database (11). There are no known specific mutational hot spots and pathogenic variants are found in all the coding exons. Frameshift and nonsense variants, which generate a truncated menin protein, are the most frequent type of variant identified in MEN1 syndrome (12-14). Most recently a genotype-phenotype association was reported for large rearrangements in the MEN1 gene and an earlier onset of disease when compared with patients who harbored truncating or missense variants (15).
The current genetic testing technologies, including MEN1 gene sequencing and deletion/duplication analysis, identify a pathogenic variant in approximately 90% of familial cases and 65% in apparently sporadic instances of MEN1 syndrome. Unidentified causative variants may exist in non-coding and regulatory regions in and around the MEN1 gene, these areas of the genome are not analyzed in routine clinical genetic testing. It is also possible, in the case of sporadic MEN1 syndrome, that the condition is not due to a genetic cause, but rather an incidental co-occurrence of 2 endocrine tumors. Subsequent research in familial cases of phenotypic MEN1 syndrome with no previously identified causative gene variant led to the discovery of MEN4 syndrome. Patients with MEN4 are identified as having a pathogenic variant in the cyclin-dependent kinase (CDK) inhibitor 1b gene (CDKN1B) and not in the MEN1 gene (16-18). As there are a limited number of published MEN4 syndrome cases to date, the natural history, penetrance and expressivity of the disease is uncertain (19). Primary hyperparathyroidism is the most commonly reported manifestation in MEN4 syndrome patients, yet GEP-NETs, pituitary tumors, carcinoid tumor have also been observed (19-24).
von Hippel Lindau (VHL) disease is a multifaceted tumor predisposition syndrome, with the symptoms first described in the early 1800s (25). The VHL tumor spectrum includes malignant and benign tumors comprised of: renal cell carcinoma, pheochromocytoma, serous cystadenoma and neuroendocrine tumors of the pancreas, endolymphatic sac tumors, epididymal and broad ligament cysts and hemangioblastomas of the retina and central nervous system (Figure 2). The VHL gene is a tumor suppressor with many functions, cloned in 1993 on the short of chromosome 3, consisting of 3 exons and 213 amino acids (26). Pathogenic variants in the VHL gene are the only known cause of VHL syndrome and can be identified through gene sequencing and deletion/duplication analysis in up to 100% of individuals meeting the clinical diagnostic criteria (26,27). Over 250 pathogenic/likely pathogenic variants have been described in the ClinVar database and detailed in the medical literature (11,28,29). Genetic testing is highly reliable and should be offered to individuals meeting diagnostic criteria (Figure 3) (26). Additionally, unexpected germline VHL pathogenic variants have been identified at a high rate in seemingly sporadic tumors, leading to the recommendation for offering genetic testing in simplex cases of retinal or brain/spinal cord hemangioblastomas, pheochromocytoma or endolymphatic sac tumors, as well as clear-cell renal carcinoma with any of the following features: diagnosed at an age ≤46 years, bilateral or multifocal tumors, or ≥1 close relatives with clear-cell renal carcinoma (30).
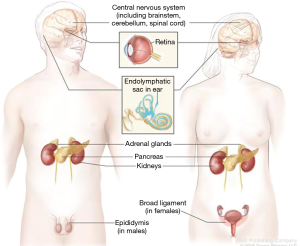
The incidence of VHL syndrome is approximately 1 in 36,000, the inheritance pattern is autosomal dominant, yet a de novo occurrence seen in 3−20% of cases (28,31). The condition is highly penetrant, with 90% of affected individuals displaying symptoms by 65 years of age (32,33). Although the disease has variable expressivity, some genotype-phenotype associations are appreciated (34-36). Large deletions and truncating pathogenic variants in the VHL gene are associated with a lower risk for pheochromocytoma, whereas missense pathogenic variants, at codon 167 especially, are associated with a high risk (36,37). However, at this time it is recommended that all individuals with a diagnosis of VHL syndrome follow the same surveillance protocol, as the understanding of genotype and phenotype implications is still evolving. Medical management recommendations have been developed through expert consensus and updated based on published outcomes of comprehensive surveillance programs (38,39).
The prevalence of PNET in individuals with VHL syndrome ranges from 9% to 17% and the tumors are most often nonfunctional and may be numerous (40-42). Risk factors for PNET metastasis in VHL syndrome include: greatest tumor diameter >3 cm, blood type O, tumor doubling time <500 days, and pathogenic missense variants or any pathogenic variant in exon 3 of the VHL gene (43-46). PNETs in the setting of VHL syndrome typically have a better prognosis compared to sporadic PNETs (47). Cystic lesions in the pancreas are common and nearly always benign (40). The pancreatic lesions that can be seen in VHL syndrome are almost never the presenting feature in an individual, rather they are discovered incidentally during surveillance for individuals known to have disease (48).
Less commonly, pancreatic involvement in neurofibromatosis (NF1) and tuberous sclerosis complex (TSC) may be observed. NF1 is an autosomal dominant tumor predisposition syndrome that has a population frequency of 1 in 3,000 births with half of cases due to a de novo mutation (49). NF1 syndrome is caused by germline pathogenic variants in the NF1 gene, which is a tumor suppressor located on chromosome 17q encoding for the neurofibromin protein (50,51). Manifestations of NF1 involve multiple organs, there is highly variable expressivity, but the condition is predominantly characterized by nervous system involvement including neurofibromas and cutaneous findings. GEP-NET tumors may be reported in up to 10% of patients with NF1 syndrome, most frequently nonfunctioning somatostatinomas arising from the duodenum where there is risk for obstruction (52-54). The somatostatinoma histologic features are characteristic with glandular formation and psammomatous calcifications (55). These tumors are not a significant cause of mortality in patients with NF1 but do increase morbidity (56).
TSC is an autosomal-dominant syndrome characterized by the development of hamartomas in almost every organ, disabling neurologic disorders, and dermatologic features (57). Both functional and nonfunctional PNETs are a rare manifestation, involved in only 1% of cases (56,58). TSC is caused by pathogenic variants in either the TSC1 gene, which encodes hamartin or the TSC2 gene, which encode tuberin (59).
Given the complexities of these multi-organ hereditary syndromes, a multidisciplinary approach to care coordination is essential for optimal patient outcomes. Although no gene-therapy nor preventative treatment is yet available for these syndromes, comprehensive surveillance programs can lead to reduced morbidity and mortality through early detection and appropriately timed intervention (6,60-62). Since there are tumor risks for young children, genetic testing should be pursued early in childhood, before the age of 5. Genetic counseling and testing are important for at risk relatives to identify carriers who require lifelong screening and noncarriers who can discontinue screening. Importantly, genetic counseling has been shown to improve adherence to genetic testing recommendations as genetic counselors aid in risk communication for family members, provide education, and work through barriers to genetic testing (63-65). All patients with a suspicion for a hereditary endocrine neoplasia syndrome should be referred for pre-test genetic counseling. Additionally, close family members should receive genetic counseling and genetic testing for risk stratification and appropriate management. When possible, it is ideal to begin the genetic testing process in an affected family member, as identification of a causative pathogenic variant in the index case can improve interpretation for family members and is more cost effective.
The discoveries and increased knowledge from our understanding of hereditary endocrine neoplasia syndromes provide a model to help scientists and clinicians have insight into the pathogenesis of sporadic PNETs as well. The observance of intrafamilial phenotypic variable expressivity in both MEN1 and VHL syndromes suggest the disease is influenced by environmental factors, personal risk factors and/or modifying genetic and epigenetic factors that have yet to be discovered. The era of precision medicine and somatic molecular analysis will bring identification of novel biomarkers for prognosis and new treatment targets for PNETs.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Callisia N. Clarke, Douglas B. Evans) for the series “Pancreatic Neuroendocrine Tumors” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: The author has completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2020.03.09). The series “Pancreatic Neuroendocrine Tumors” was commissioned by the editorial office without any funding or sponsorship. The author has no other conflicts of interest to declare.
Ethical Statement: The author is accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Erdheim J. Zur normalen und pathologischen histologie der glandula thyroidea, parathyreoidea und Hypophysis. Beitr z path Anat u z allg Path 1903;33:158-236.
- Brandi ML, Gagel RF, Angeli A, et al. Guidelines for diagnosis and therapy of MEN type 1 and type 2. J Clin Endocrinol Metab 2001;86:5658-71. [Crossref] [PubMed]
- Bardram L, Stage JG. Frequency of endocrine disorders in patients with the Zollinger-Ellison syndrome. Scand J Gastroenterol 1985;20:233-8. [Crossref] [PubMed]
- Farley DR, van Heerden JA, Grant CS, et al. The Zollinger-Ellison syndrome. A collective surgical experience. Ann Surg 1992;215:561-9; discussion 569-70. [Crossref] [PubMed]
- Ito T, Sasano H, Tanaka M, et al. Epidemiological study of gastroenteropancreatic neuroendocrine tumors in Japan. J Gastroenterol 2010;45:234-43. [Crossref] [PubMed]
- Thakker RV, Newey PJ, Walls GV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1). J Clin Endocrinol Metab 2012;97:2990-3011. [Crossref] [PubMed]
- de Laat JM, van Leeuwaarde RS, Valk GD. The Importance of an Early and Accurate MEN1 Diagnosis. Front Endocrinol (Lausanne) 2018;9:533. [Crossref] [PubMed]
- Machens A, Schaaf L, Karges W, et al. Age-related penetrance of endocrine tumours in multiple endocrine neoplasia type 1 (MEN1): a multicentre study of 258 gene carriers. Clin Endocrinol (Oxf) 2007;67:613-22. [PubMed]
- Kouvaraki MA, Lee JE, Shapiro SE, et al. Genotype-phenotype analysis in multiple endocrine neoplasia type 1. Arch Surg 2002;137:641-7. [Crossref] [PubMed]
- NCCN. Neuroendocrine and Adrenal Tumors (Version 1.2019). 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf
- ClinVar. Archive for interpretations of clinical significance of variants in genes. National Center for Biotechnology Information. Accessed 01/03/2020. Available online: https://www.ncbi.nlm.nih.gov/clinvar/
- Marini F, Giusti F, Fossi C, et al. Multiple endocrine neoplasia type 1: analysis of germline MEN1 mutations in the Italian multicenter MEN1 patient database. Endocrine 2018;62:215-33. [Crossref] [PubMed]
- Concolino P, Costella A, Capoluongo E. Multiple endocrine neoplasia type 1 (MEN1): An update of 208 new germline variants reported in the last nine years. Cancer Genet 2016;209:36-41. [Crossref] [PubMed]
- Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat 2008;29:22-32. [Crossref] [PubMed]
- Romanet P, Mohamed A, Giraud S, et al. UMD-MEN1 Database: An Overview of the 370 MEN1 Variants Present in 1676 Patients From the French Population. J Clin Endocrinol Metab 2019;104:753-64. [Crossref] [PubMed]
- Occhi G, Regazzo D, Trivellin G, et al. A novel mutation in the upstream open reading frame of the CDKN1B gene causes a MEN4 phenotype. PLoS Genet 2013;9:e1003350. [Crossref] [PubMed]
- Pellegata NS, Quintanilla-Martinez L, Siggelkow H, et al. Germ-line mutations in p27Kip1 cause a multiple endocrine neoplasia syndrome in rats and humans. Proc Natl Acad Sci U S A 2006;103:15558-63. [Crossref] [PubMed]
- Agarwal SK, Mateo CM, Marx SJ. Rare germline mutations in cyclin-dependent kinase inhibitor genes in multiple endocrine neoplasia type 1 and related states. J Clin Endocrinol Metab 2009;94:1826-34. [Crossref] [PubMed]
- Frederiksen A, Rossing M, Hermann P, et al. Clinical Features of Multiple Endocrine Neoplasia Type 4: Novel Pathogenic Variant and Review of Published Cases. J Clin Endocrinol Metab 2019;104:3637-46. [Crossref] [PubMed]
- Costa-Guda J, Marinoni I, Molatore S, et al. Somatic mutation and germline sequence abnormalities in CDKN1B, encoding p27Kip1, in sporadic parathyroid adenomas. J Clin Endocrinol Metab 2011;96:E701-6. [Crossref] [PubMed]
- Belar O, De La Hoz C, Perez-Nanclares G, et al. Novel mutations in MEN1, CDKN1B and AIP genes in patients with multiple endocrine neoplasia type 1 syndrome in Spain. Clin Endocrinol (Oxf) 2012;76:719-24. [Crossref] [PubMed]
- Tichomirowa MA, Lee M, Barlier A, et al. Cyclin-dependent kinase inhibitor 1B (CDKN1B) gene variants in AIP mutation-negative familial isolated pituitary adenoma kindreds. Endocr Relat Cancer 2012;19:233-41. [Crossref] [PubMed]
- Elston MS, Meyer-Rochow GY, Dray M, et al. Early Onset Primary Hyperparathyroidism Associated with a Novel Germline Mutation in CDKN1B. Case Rep Endocrinol 2015;2015:510985.
- Pardi E, Mariotti S, Pellegata NS, et al. Functional characterization of a CDKN1B mutation in a Sardinian kindred with multiple endocrine neoplasia type 4 (MEN4). Endocr Connect 2015;4:1-8. [Crossref] [PubMed]
- Richard S, Graff J, Lindau J, et al. Von Hippel-Lindau disease. Lancet 2004;363:1231-4. [Crossref] [PubMed]
- van Leeuwaarde RS AS, Links TP, et al. Von Hippel-Lindau Syndrome. GeneReviews® [Internet]. Seattle (WA): University of Washington, 2000 May 17 [Updated 2018 Sep 6].
- Hes FJ, van der Luijt RB, Janssen AL, et al. Frequency of Von Hippel-Lindau germline mutations in classic and non-classic Von Hippel-Lindau disease identified by DNA sequencing, Southern blot analysis and multiplex ligation-dependent probe amplification. Clin Genet 2007;72:122-9. [Crossref] [PubMed]
- Nordstrom-O'Brien M, van der Luijt RB, van Rooijen E, et al. Genetic analysis of von Hippel-Lindau disease. Hum Mutat 2010;31:521-37. [PubMed]
- Beroud C, Joly D, Gallou C, et al. Software and database for the analysis of mutations in the VHL gene. Nucleic Acids Res 1998;26:256-8. [Crossref] [PubMed]
- Hampel H, Bennett RL, Buchanan A, et al. A practice guideline from the American College of Medical Genetics and Genomics and the National Society of Genetic Counselors: referral indications for cancer predisposition assessment. Genet Med 2015;17:70-87. [Crossref] [PubMed]
- Richards FM, Payne SJ, Zbar B, et al. Molecular analysis of de novo germline mutations in the von Hippel-Lindau disease gene. Hum Mol Genet 1995;4:2139-43. [Crossref] [PubMed]
- Maher ER, Iselius L, Yates JR, et al. Von Hippel-Lindau disease: a genetic study. J Med Genet 1991;28:443-7. [Crossref] [PubMed]
- Maher ER, Yates JR, Harries R, et al. Clinical features and natural history of von Hippel-Lindau disease. Q J Med 1990;77:1151-63. [Crossref] [PubMed]
- Hes F, Zewald R, Peeters T, et al. Genotype-phenotype correlations in families with deletions in the von Hippel-Lindau (VHL) gene. Hum Genet 2000;106:425-31. [Crossref] [PubMed]
- Chen F, Kishida T, Yao M, et al. Germline mutations in the von Hippel-Lindau disease tumor suppressor gene: correlations with phenotype. Hum Mutat 1995;5:66-75. [Crossref] [PubMed]
- McNeill A, Rattenberry E, Barber R, et al. Genotype-phenotype correlations in VHL exon deletions. Am J Med Genet A 2009;149a:2147-51. [Crossref] [PubMed]
- Maher ER, Webster AR, Richards FM, et al. Phenotypic expression in von Hippel-Lindau disease: correlations with germline VHL gene mutations. J Med Genet 1996;33:328-32. [Crossref] [PubMed]
- Rednam SP, Erez A, Druker H, et al. Von Hippel-Lindau and Hereditary Pheochromocytoma/Paraganglioma Syndromes: Clinical Features, Genetics, and Surveillance Recommendations in Childhood. Clin Cancer Res 2017;23:e68-75. [Crossref] [PubMed]
- Alliance V. The VHL Handbook. 5th ed. Boston, MA: VHL Alliance, 2015.
- Keutgen XM, Hammel P, Choyke PL, et al. Evaluation and management of pancreatic lesions in patients with von Hippel–Lindau disease. Nat Rev Clin Oncol 2016;13:537-49. [Crossref] [PubMed]
- Libutti SK, Choyke PL, Alexander HR, et al. Clinical and genetic analysis of patients with pancreatic neuroendocrine tumors associated with von Hippel-Lindau disease. Surgery 2000;128:1022-7; discussion 1027-8. [Crossref] [PubMed]
- Weisbrod AB, Kitano M, Thomas F, et al. Assessment of tumor growth in pancreatic neuroendocrine tumors in von Hippel Lindau syndrome. J Am Coll Surg 2014;218:163-9. [Crossref] [PubMed]
- Tirosh A, Sadowski SM, Linehan WM, et al. Association of VHL Genotype With Pancreatic Neuroendocrine Tumor Phenotype in Patients With von Hippel-Lindau Disease. JAMA Oncol 2018;4:124-6. [Crossref] [PubMed]
- Blansfield JA, Choyke L, Morita SY, et al. Clinical, genetic and radiographic analysis of 108 patients with von Hippel-Lindau disease (VHL) manifested by pancreatic neuroendocrine neoplasms (PNETs). Surgery 2007;142:814-8; discussion 818.e1-2.
- Weisbrod AB, Liewehr DJ, Steinberg SM, et al. Association of type O blood with pancreatic neuroendocrine tumors in Von Hippel-Lindau syndrome. Ann Surg Oncol 2012;19:2054-9. [Crossref] [PubMed]
- Krauss T, Ferrara AM, Links TP, et al. Preventive medicine of von Hippel-Lindau disease-associated pancreatic neuroendocrine tumors. Endocr Relat Cancer 2018;25:783-93. [Crossref] [PubMed]
- de Mestier L, Gaujoux S, Cros J, et al. Long-term Prognosis of Resected Pancreatic Neuroendocrine Tumors in von Hippel-Lindau Disease Is Favorable and Not Influenced by Small Tumors Left in Place. Ann Surg 2015;262:384-8. [Crossref] [PubMed]
- Safo AO, Pambuccian SE. Pancreatic manifestations of von Hippel-Lindau disease. Arch Pathol Lab Med 2010;134:1080-3. [PubMed]
- Evans DG, Howard E, Giblin C, et al. Birth incidence and prevalence of tumor-prone syndromes: estimates from a UK family genetic register service. Am J Med Genet A 2010;152A:327-32. [Crossref] [PubMed]
- Goldgar DE, Green P, Parry DM, et al. Multipoint linkage analysis in neurofibromatosis type I: an international collaboration. Am J Hum Genet 1989;44:6-12. [PubMed]
- Viskochil D, Buchberg AM, Xu G, et al. Deletions and a translocation interrupt a cloned gene at the neurofibromatosis type 1 locus. Cell 1990;62:187-92. [Crossref] [PubMed]
- Caiazzo R, Mariette C, Piessen G, et al. Type I neurofibromatosis, pheochromocytoma and somatostatinoma of the ampulla. Literature review. Ann Chir 2006;131:393-7. [Crossref] [PubMed]
- Cantor AM, Rigby CC, Beck PR, et al. Neurofibromatosis, phaeochromocytoma, and somatostatinoma. Br Med J (Clin Res Ed) 1982;285:1618-9. [Crossref] [PubMed]
- Relles D, Baek J, Witkiewicz A, et al. Periampullary and duodenal neoplasms in neurofibromatosis type 1: two cases and an updated 20-year review of the literature yielding 76 cases. J Gastrointest Surg 2010;14:1052-61. [Crossref] [PubMed]
- Burke AP, Sobin LH, Shekitka KM, et al. Somatostatin-producing duodenal carcinoids in patients with von Recklinghausen's neurofibromatosis. A predilection for black patients. Cancer 1990;65:1591-5. [Crossref] [PubMed]
- Jensen RT, Berna MJ, Bingham DB, et al. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer 2008;113:1807-43. [Crossref] [PubMed]
- Crino PB, Nathanson KL, Henske EP. The tuberous sclerosis complex. N Engl J Med 2006;355:1345-56. [Crossref] [PubMed]
- Eledrisi MS, Stuart CA, Alshanti M. Insulinoma in a patient with tuberous sclerosis: is there an association? Endocr Pract 2002;8:109-12. [Crossref] [PubMed]
- Schwartz RA, Fernandez G, Kotulska K, et al. Tuberous sclerosis complex: advances in diagnosis, genetics, and management. J Am Acad Dermatol 2007;57:189-202. [Crossref] [PubMed]
- Maher ER, Neumann HP, Richard S. von Hippel-Lindau disease: a clinical and scientific review. Eur J Hum Genet 2011;19:617-23. [Crossref] [PubMed]
- Priesemann M, Davies KM, Perry LA, et al. Benefits of screening in von Hippel-Lindau disease--comparison of morbidity associated with initial tumours in affected parents and children. Horm Res 2006;66:1-5. [PubMed]
- Kamilaris CDC, Stratakis CA. Multiple Endocrine Neoplasia Type 1 (MEN1): An Update and the Significance of Early Genetic and Clinical Diagnosis. Front Endocrinol (Lausanne) 2019;10:339. [Crossref] [PubMed]
- Kasparian NA, Rutstein A, Sansom-Daly UM, et al. Through the looking glass: an exploratory study of the lived experiences and unmet needs of families affected by Von Hippel-Lindau disease. Eur J Hum Genet 2015;23:34-40. [Crossref] [PubMed]
- Gallagher TM, Bucciarelli M, Kavalukas SL, et al. Attitudes toward genetic counseling and testing in patients with inherited endocrinopathies. Endocr Pract 2017;23:1039-44. [Crossref] [PubMed]
- Matloff ET, Barnett RE. The growing role for genetic counseling in endocrinology. Curr Opin Oncol 2011;23:28-33. [Crossref] [PubMed]




