Difference in toxicity between HIV-positive and HIV-negative patients with squamous-cell cancer of the anal canal treated with concomitant radio-chemotherapy
Introduction
The incidence of anal canal cancer (ACC) has been increasing over the last three decades. According to the 2018 GLOBOCAN study (1), around 48,541 new cases are diagnosed worldwide, with 19,129 deaths and a 5-year prevalence of 127,599 cases. The etiopathological behavior of ACC is more similar to that of malignant tumors of the genital tract than to tumors of the gastrointestinal tract (2). About 85% to 90% of ACCs are squamous cell carcinomas. Patients positive for HIV have been described to be at higher risk of developing this disease (3,4).
The number of HIV-infected patients with squamous-cell (SC) ACC has been increasing, mostly among younger patients (mean age at presentation: 40 years), and with a predominance among the male population (4).
The current standard of treatment for patients with ACC is radio-chemotherapy (RTCT), while surgery plays a role as salvage treatment when this first line of curative management fails (5-12). Although HIV positivity is an exclusion criterion for randomized clinical trials in patients with ACC, some series including HIV-positive patients treated with radiotherapy (RT) alone or in combination with chemotherapy (CT) report similar oncological results for HIV-negative and HIV-positive patients, with locoregional control rates of 65% or more. However, other series report lower rates of local control and sphincter preservation for HIV-positive patients despite early-stage diseases and good initial tumor responses (13-20). Regarding treatment toxicity in HIV-positive patients, some series report more severe side effects (13,15,20).
The current recommendation is that HIV-positive patients should be treated similarly to HIV-negative patients, taking into account the complications that patients with HIV/AIDS can present and the consequent possible need for treatment modification (21,22).
This systematic review aims at evaluating the available literature describing observed toxicities, their impact in treatment compliance and published data on quality of life for HIV-positive and HIV-negative patients, treated with RTCT for non-relapsing SC-ACC stages T1-T4N0/+ M0.
Methods
Information search
We carried out a systematic literature review following the guidelines of the Cochrane Handbook for Systematic Reviews of Interventions (23). In order to identify relevant studies, a systematic search was conducted in electronic databases (MEDLINE, EMBASE, COCHRANE, DARE, LILACS). Syntaxes were adapted for each database through the use of controlled terms (MeSH, EMTREE and DeCS), free language terms and Boolean and proximity operators. No restrictions by language, publication date or type of design were applied. References from selected studies were evaluated and experts in the field were consulted, as additional search sources. Special collections of the International Journal of Radiation Oncology, Biology & Physics, Radiotherapy & Oncology, and Lancet Oncology were also reviewed.
Study selection
Titles and summaries were independently reviewed by two authors who also independently verified inclusion and exclusion criteria; inconsistencies were resolved by a third author. Selection criteria were: (I) experimental or analytical observational studies on patients diagnosed with SC ACC treated with RTCT; (II) two comparison arms of HIV-positive vs. HIV-negative patients; (III) any RT techniques, regardless of volumes, doses and fractionations; (IV) any CT drugs. Studies including metastatic disease were excluded from our review as were single-arm studies not comparing HIV-positive vs. HIV-negative patients.
Qualification, extraction and synthesis of information
Bias risk assessment was performed according to the type of design. Randomized studies were evaluated by Cochrane Collaboration criteria and non-randomized studies were evaluated using the Scottish Intercollegiate Guidelines Network (SIGN) tool (23,24). Information was extracted in a format previously designed for independent review by an author and subsequent review by a second author. Information was extracted related to the defined variables.
A summary estimator of primary outcomes was generated to estimate heterogeneity by I2 statistic, using the Review Manager program, version 5.3 (Cochrane Collaboration). Publication bias was assessed by performing a funnel plot using the Egger test.
To obtain data missing in the published articles, the authors were contacted by email. When this was not possible, the data were analyzed omitting the missing information. The data included in the analysis were those reported by intention-to-treat analysis.
Results
Study description
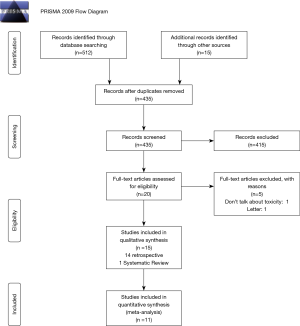
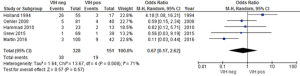
As a result of the search process, 527 references were found. Of them, 14 retrospective studies and one systematic review complied with our inclusion and exclusion criteria (15,19,25-37). Of the 1,395 patients included in the retrospective studies reviewed, 372 were HIV-positive and 1,023 HIV-negative. Figure 1 shows the study selection process.
Table 1 describes the characteristics of the selected studies. The studies compared HIV-positive vs. HIV-negative patients with SC ACC managed with RTCT. The main outcome was toxicity and the secondary one was quality of life. Since no quality of life study comparing the two arms was found at the time of the search, only differences in terms of toxicities between HIV-positive versus HIV-negative patients were analyzed.
Full table
Bias risk of selected studies
Since all the studies were retrospective, they were evaluated according to SIGN criteria. Overall, a high risk of confusion and detection biases was found. Furthermore, since they were all retrospective studies, it was not possible to evaluate performance and attrition biases. Table 2 shows the results of the qualification.
Full table
In order to avoid publication bias, gray literature and reports cited in clinical trials were reviewed; no new publications or records were found (38,39). Egger test and funnel plot graph were also performed, finding no publication bias.
RTCT-related toxicity
Regarding RT, a study showed no significant differences in the duration of irradiation between HIV-positive and negative patients (P=0.67) (36). Three studies showed that there were no differences in terms of the dose received in both HIV-positive and HIV-negative patients. Of these, one showed that there were no significant differences in terms of total dose (P=0.91), another showed that there were no significant differences in the total RT dose to the pelvis (P=0.53) or in the boost dose (P=0.53) and another showed that there were no significant differences in radiation dose to electively treated lymph nodes or tumors, without calculating statistical significance (26,35,36).
Regarding RT technique, three studies used the box technique, three studies administered conformational RT and one study applied intensity modulated radiation therapy (IMRT); of the remaining studies, one switched from the box technique to IMRT after 2007, and two other studies used both conformational RT and IMRT, while four studies did not specify the RT technique used (15,19,25-37). Five studies used brachytherapy as boost (15,28,30,31,36).
Regarding the drugs used, eight studies administered 5-fluorouracil (5-FU) and mitomycin-C (MMC), two studies used 5-FU plus cisplatin (CDDP), two studies used 5-FU in combination with either MMC or CDDP, one study used 5-FU either alone or combined with MMC, and one study used 5-FU alone or combined with either MMC or CDDP (15,19,25-37).
One study showed that there was no significant difference in CT dose reduction between HIV-positive and HIV-negative patients (P=0.74) (36). However, another study showed that the full dose of 5-FU/MMC was administered to 72% of HIV-positive patients compared to 91% of HIV-negative patients (P=0.04). CT had to be stopped after the second cycle due to hematological toxicity and/or infections in 4 HIV-positive patients (16%), and the CT dose reduced after the second cycle in 3 HIV-positive patients (12%), while in HIV-negative patients a CT-dose reduction after the second cycle was necessary in only 4 cases (9%) (26). One study showed significantly lower tolerance to MMC (P≤0.001) in HIV-positive patients, while another showed no differences in the administration of MMC in both groups (32,35).
One study reported lower, non-significant compliance in HIV-positive patients, due to a higher toxicity rate in this population, particularly in patients with low CD4 counts or severe thrombocytopenia before or during treatment (30). Another study reported no statistically significant differences in compliance between the two groups but did not report the statistical magnitude (35).
Hospitalization, treatment interruption and duration
Four studies reported the need for hospitalization, three of them by study arm, showing a significantly higher rate of hospitalization in HIV-positive patients compared to HIV-negative patients (25,28,34,35). Grew reported a hospitalization rate of 33% vs. 15% (P=0.024) in HIV-positive vs. HIV-negative patients respectively (35); a second article reported a hospitalization rate of 48% and 41% in HIV-positive vs. HIV-negative patients respectively, which was not significant (P=0.62) (34); a third study reported a 43% vs. 7% hospitalization rate in HIV-positive vs. HIV-negative patients, without reporting statistical significance (25). Finally, one study reported overall hospitalization in 18% of patients, without differentiating between the two groups (28).
Treatment interruption was also reported in five studies (25), of which, one reported an overall 30% rate in RT pause (mean interruption 7 days) (28), three studies showed that pauses were longer in HIV-positive patients (25,31,34), and one study showed that they were longer in HIV-negative patients (26). Of the three studies that showed longer interruptions in HIV-positive patients, one reported that 34 patients required treatment pauses for more than five days, 43% HIV-positive and 38% HIV-negative patients (P=0.80, not significant) (34); in another study, all HIV-positive patients required pausing treatment vs. 55% of HIV-negative patients, no statistical significance was reported (25); the third study reported a complete stop of the RTCT treatment at 45 Gy in 2 HIV-infected patients due to local infections and in 1 patient not infected with HIV due to perianal necrosis (31). This same study showed that 26 patients (11 infected and 15 not infected with HIV) received prolonged RTCT treatment (31). The study that reported a higher interruption rate in HIV-negative patients showed that temporary (>3 days) interruption of RT was necessary in 8% of HIV-positive patients and in 11% of HIV-negative patients, without reporting any statistical significance (26).
Regarding treatment duration, one study showed that due to the acute toxicity of RTCT there was an increase in the duration of treatment in HIV-infected patients compared to uninfected patients (P=0.027) while another paper showed that RTCT duration did not significantly differ between the cohorts (median 96.5 days, IQR 73.0 to 118.8 for the HIV-positive cohort and 88.5 days, IQR 73.0 to 118.8, for the HIV-negative cohort, P =0.57 (31,32).
Acute toxicity
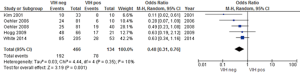
Overall acute toxicity was evaluated in five studies (15,28,30,31,34) where statistically significant differences were reported between HIV-positive and HIV-negative patients (P=0.001) with a low heterogeneity (Figure 2). However, when reviewing the publications, two showed that acute toxicity was higher in HIV-positive patients; one of them registered a non-significant difference of 81% vs. 73%, and the other showed that although the frequency of grade 3–4 acute 3toxicities doubled in seropositive individuals (60% vs. 30%) this difference was not significant (30,34). Two studies divided acute toxicity into hematological and non-hematological toxicity (dermatitis, diarrhea) and did not report any statistically significant grade 3-4 events among HIV-positive or HIV-negative patients (P=0.43) (25,28).
Hematological toxicity was reported in 5 studies, four of which showed no statistically significant differences between the two arms (15,25,28,33).
A paper did show statistically significant results, finding that HIV-positive patients experienced a significantly lower mean white blood cell nadir than HIV-negative patients (2.15 versus 3.05 thousand cells/µL; P=0.004); however, there were no significant differences in neutrophils, platelets or hemoglobin (35).
CT-induced grade 3–4 hematological toxicity was higher in HIV-positive (33%) compared to HIV-negative patients who received MMC (12%; P=0.08); two of the four HIV-positive patients with severe hematological adverse effects had CD4 >200 cells/µL; no severe hematological toxicity was observed in HIV-positive patients who received cisplatin (15). Another study that also assessed CT-induced hematologic toxicity showed that 50% of HIV-positive patients receiving antiretrovirals who were treated with MMC CT developed acute grade-3 thrombocytopenia, compared with 0% of HIV-negative patients (P=0.05) (30). It should be noted that two of the patients had a CD4 count >200 cells/µL, suggesting adequate compliance with antiretroviral treatment (30).
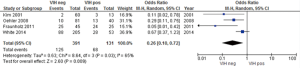
Four studies reported similar risks of hematological toxicity; we assessed whether HIV-positivity posed a higher risk, finding a statistically significant (P=0.009) higher risk for hematological toxicity in HIV-positive patients, despite highly heterogeneous data (Figure 3) (15,26,28,33).
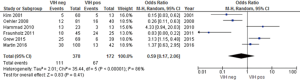
Four studies reported no statistically significant differences in grade 3–4 skin toxicities between HIV-positive and –negative patients (15,19,27,35), while two studies reported that acute grade 3–4 dermatitis was more frequent among HIV-positive patients without reporting statistical data (26,33). The meta-analysis results showed that the risk of grade 3–4 skin toxicity among HIV-positive patients was not significantly different compared to HIV-negative patients (Figure 4). Severe skin toxicity induced by RT was observed in 35% of HIV-positive and 17% of HIV-negative patients (P=0.04) (15,19,26,27,33,35).
Five studies did not report any significant differences in gastrointestinal toxicity between the two arms (15,19,25,27,35), while one showed that HIV-positive patients had a higher risk of diarrhea (4% versus 16%) without reporting the significance of the finding and another study showed that acute gastrointestinal toxicity was higher in HIV-positive patients than in HIV-negative patients (48% and 24%, respectively; P=0.04) (26,34).
The need for colostomy was reported in two studies that showed no difference between the two arms (26,34). The risk of developing diarrhea was evaluated in five studies (15,19,25,27,35), with no statistically significant differences between HIV-positive and HIV-negative patients (Figure 5).
None of the studies documenting higher toxicity for HIV-positive patients specified what the CD4 count was for the patients who tolerated the treatment poorly, nor did they report whether medical follow-up with CD4 count was performed. The studies that reported use of antiretrovirals did not specify whether a change in drugs was necessary for patients presenting opportunistic infections due to the RTCT treatment (15,19,25-36).
Late and chronic toxicity
Overall, four studies reported the risk of chronic toxicity, finding no statistically significant differences in the risk of developing chronic toxicity between HIV-positive and HIV-negative patients (15,28,34,38) (Figure 6).
Two studies reported a higher rate of toxicity to the abdomino-pelvic area in HIV positive patients. Vatra et al. reported 40% abdomino-pelvic toxicities in HIV-positive vs. 12% in HIV-negative (P=0.04) while Fraunholz et al. reported a higher risk of serious late gastrointestinal toxicities (diarrhea, perforation, enteritis, infection) in HIV-positive patients (11% vs. 4.4%) (26,29).
Furthermore, one study reported that anal stenosis was the main cause of late toxicity in the HIV-positive group (34). Another study found that a late grade-4 large perineal ulcer was more frequent in patients with HIV, while another study reported higher gastrointestinal toxicity in HIV-negative patients, and the authors suggested that this was possibly because they received higher RT doses (33,34).
Antiretrovirals use
Of all the studies, only three did not report the use of antiretrovirals in HIV-positive patients (25,29,33). Although an equitable comparison cannot be made, since most studies used antiretrovirals, the three studies that did not report antiretroviral use registered higher toxicity rates among HIV-positive patients, while in the studies that used antiretrovirals, most of the toxicities evaluated did not yield any statistically significant differences.
Additionally, it was evaluated whether RTCT-induced toxicity in patients with ACC was related to overall survival. This data was reported in 6 studies. Kim et al. (33) reported non-significant overall survival of 3.1 years in HIV+ patients versus 5.3 years in uninfected patients (P=0.06); Hammad et al. (19) reported median overall survival of 33.5 months for HIV+ patients, with a 58% rate in 24 months (90% CI, 34–82%) versus 71.8 months, with a 77% rates in 24 months for HIV-negative patients (90% CI, 65–90%), Holland et al. (25) showed a 71% 4-year overall survival rate in uninfected patients versus a 29% 2-year survival rate in HIV-positive patients, but did not report statistical data; Vatra et al. (29) documented a 40% 3-year survival rate for HIV-positive vs. 21% for HIV-negative patients (data available for 10/20 infected patients and for 22/24 of HIV-negative patients), median survival for HIV+ patients was 18 months (range, 10–43 months), which was significantly shorter (P<0.01) than the 28-month (range, 18–172 month) median survival observed in the HIV-group. Abramowitz et al. (36) did not find any statistical differences between the two groups (P=0.92) while White et al. (28) reported a trend toward worse 3-year overall survival in the HIV-positive group at univariate analysis (72% vs. 84%; P=0.06).
None of these studies reported whether survival was negatively influenced by treatment-related toxicity, however, four studies assessed the possible impact of CD4 counts on overall survival. Vatra et al. (29) found that HIV+ patients with CD4 counts <250/mm3 at diagnosis survived an average of 9 months; two studies found no differences in overall survival between patients with CD4 counts < or >200 (P=0.57) (34) and HR: 1.34 (0.32–5.96; P=0.67) (28), while a forth study showed no impact of CD4 counts on overall survival, without reporting CD4 range or statistical values (31).
Discussion
This review compiles the evidence published until June 2019 on the different toxicities from RTCT treatment in HIV-positive compared to HIV-negative patients with SC ACC. It is important to take into account that all RT techniques and CT agents used for the disease were included. All studies were retrospective; a systematic review published in 2018 was also found (15,19,25-37). We planned to assess whether there were any differences in quality of life between these two groups of patients, however, no studies were found that evaluated this item, which was therefore excluded from the results.
Regarding treatment toxicity in HIV-positive patients, some series report more severe side effects, particularly to the perineal skin, anorectal mucosa and hematological system, especially with RT doses over 30 Gy (13,15,40). Two factors that can predict the increase in acute toxicity to normal tissues have been found: a Cluster of Differentiation 4 (CD4) count <200/µL at the start of treatment, and the presence of acquired immune deficiency syndrome (AIDS), although these are not always associated with poor tolerance (25,41). Small observational studies have assessed the relationship between low CD4 count and treatment tolerance. One series found that patients with <200 cells/µL were more often hospitalized for myelosuppression, diarrhea or moist desquamation, and colostomy for treatment-related complications or persistent/recurrent disease, while three other series where patients received modern antiretroviral therapy found no significant relationship between CD4 count and treatment-related toxicity, even when CD4 was <200 to 300 cells/µL (42).
RTCT studies in patients with ACC including patients with CD4 counts >300 cells/mm3 show similar results between HIV-positive and -negative patients (43,44). Another therapeutic option that has been assessed is the use of a immunomodulators for HIV+ patients. Some studies including subjects with CD4 counts >200 cells/mm3 and others with counts >300 cells/mm3, have shown promising results: some studies are still ongoing (45-47). To date, there are no randomized clinical studies including HIV+ patients with ACC treated with RTCT.
The role of modern antiretrovirals in the improvement of treatment tolerance and cancer outcomes for HIV-positive patients with ACC is contradictory (15,19,20,23-29,48-50). It is important to remember that when antiretrovirals are administered concomitant to antineoplastic drugs, the risk for cross-toxicity must be taken into account, as well as the possible pharmacokinetics and pharmacodynamics of these drugs, since a drug accumulation can occur, as well as possible toxicity or decreased efficacy of one or both drug groups. This, however, should not be considered an obstacle to standard-dose treatment; it should only be taken as a warning for a strict surveillance of this subset of patients so as to avoid adverse reactions (21).
This review found that hematological and acute toxicities are significantly higher in HIV positive patients; however, several studies reported other events occurring more frequently, although without any statistical significance, in HIV positive patients, such as reduced treatment compliance, higher hospitalization and interruption rates. One of the possible reasons that can explain this, and that some authors mentioned, is the low number of HIV-positive patients compared to HIV-negative ones in the reviewed studies (372 vs. 1,023). However, with the use of more effective antiretrovirals, the performance status of HIV-positive patients has improved, potentially contributing to a significant decrease in toxicity rates.
When comparing studies that used antiretrovirals and those that did not, it was found that the three studies that did not use antiretrovirals showed higher toxicity rates for HIV-positive patients, while the studies where antiretroviral drugs were used reported mostly non-significant toxicity differences between the two groups, suggesting that antiretrovirals may play an important role in the reduction of toxicity (15,19,25-36). It was not possible to establish whether the RT technique used or the drugs used for CT played a role in toxicity, given the high heterogeneity of data.
Regarding overall survival, none of the studies that reported this outcome document that it was affected by toxicities during the course of treatment (19,25,28,29,33,36). However, some studies analyzed if overall survival was impacted by CD4 count in HIV-positive patients, finding conflicting results (28,29,31,34).
One of the limitations of our analysis is that all the evaluated studies were retrospective. There was also a large difference between the number of HIV-positive and HIV-negative patients.
When comparing the results of this systematic review with a recently published one, which analyzes whether there are any differences in terms of cancer outcomes between HIV-positive and HIV-negative patients, we found that in terms of dermatological and gastrointestinal toxicity, our results are consistent with those found by Camandaroba et al. Hematologic toxicity, on the other hand, cannot be compared because in our review we report it as a whole, while the other systematic review evaluates it depending on the cellularity affected (37).
Current guidelines recommend that HIV-positive patients should be treated the same way as HIV-negative ones, encouraging the use of antiretrovirals to diminish the side-effects caused by lower CD4 counts. However, a strict surveillance is necessary during treatment in order to detect any toxicity, as well as to carefully assess any possible drug interaction between antiretrovirals and antineoplastic agents (21,22).
In conclusion, the available literature comparing treatment-related toxicity between HIV-positive and HIV-negative patients treated by concurrent chemo-radiation for an ACC is scarce and disparate. Available data suggest that HIV-positive patients may be at higher risk of developing hematological toxicity than HIV-negative patients; they also show a higher trend of non-compliance with the treatment, treatment interruptions and need of hospitalization. Regarding the impact of antiretrovirals, a precise conclusion cannot be drawn given the high heterogeneity of the data analyzed. However, a beneficial effect might exist reflecting the importance of a better control of the HIV infection.
We hope randomized clinical trials for ACC in the future will include HIV-positive patients treated with RTCT, so as to clarify all doubts on oncological outcomes and treatments tolerance in this population.
Acknowledgments
We thank Ms. Nordiana Baruzzi for the translation to English. We thank the International Atomic Energy Agency for sponsoring the Master in Advanced Radiation Oncology. This study was supported by the Fundación Arturo Lopez Perez.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Myerson RJ, Karnell LH, Menck HR. The National Cancer Data Base report on carcinoma of the anus. Cancer 1997;80:805-15. [Crossref] [PubMed]
- Palefsky JM. Anal human papillomavirus infection and anal cancer in HIV-positive individuals: an emerging problem. AIDS 1994;8:283-95. [Crossref] [PubMed]
- Simard EP, Pfeiffer RM, Engels EA. Spectrum of cancer risk late after AIDS onset in the United States. Arch Intern Med 2010;170:1337-45. [Crossref] [PubMed]
- Epidermoid anal cancer: results from the UKCCCR randomised trial of radiotherapy alone versus radiotherapy, 5-fluorouracil, and mitomycin. UKCCCR Anal Cancer Trial Working Party. UK Co-ordinating Committee on Cancer Research. Lancet 1996;348:1049-54. [Crossref] [PubMed]
- Northover J, Glynne-Jones R, Sebag-Montefiore D, et al. Chemoradiation for the treatment of epidermoid anal cancer: 13-year follow-up of the first randomised UKCCCR Anal Cancer Trial (ACT I). Br J Cancer 2010;102:1123-8. [Crossref] [PubMed]
- Bartelink H, Roelofsen F, Eschwege F, et al. Concomitant radiotherapy and chemotherapy is superior to radiotherapy alone in the treatment of locally advanced anal cancer: results of a phase III randomized trial of the European Organization for Research and Treatment of Cancer Radiotherapy and Gastrointestinal Cooperative Groups. J Clin Oncol 1997;15:2040-9. [Crossref] [PubMed]
- Flam M, John M, Pajak TF, et al. Role of mitomycin in combination with fluorouracil and radiotherapy, and of salvage chemoradiation in the definitive nonsurgical treatment of epidermoid carcinoma of the anal canal: results of a phase III randomized intergroup study. J Clin Oncol 1996;14:2527-39. [Crossref] [PubMed]
- Ajani JA, Winter KA, Gunderson LL, et al. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA 2008;299:1914-21. [Crossref] [PubMed]
- Gunderson LL, Winter KA, Ajani JA, et al. Long-term update of US GI intergroup RTOG 98-11 phase III trial for anal carcinoma: survival, relapse, and colostomy failure with concurrent chemoradiation involving fluorouracil/mitomycin versus fluorouracil/cisplatin. J Clin Oncol 2012;30:4344-51. [Crossref] [PubMed]
- James R, Wan S, Glynne-Jones R, et al. A randomized trial of chemoradiation using mitomycin or cisplatin, with or without maintenance cisplatin/5-FU in squamous cell carcinoma of the anus (ACT II). J Clin Oncol 2009. [Crossref]
- Conroy T, Ducreux M, Lemanski C, et al. Treatment intensification by induction chemotherapy (ICT) and radiation dose escalation in locally advanced squamous cell anal canal carcinoma (LAAC): Definitive analysis of the intergroup ACCORD 03 trial. J Clin Oncol 2009;27:4033.
- Hoffman R, Welton ML, Klencke B, et al. The significance of pretreatment CD4 count on the outcome and treatment tolerance of HIV-positive patients with anal cancer. Int J Radiat Oncol Biol Phys 1999;44:127-31. [Crossref] [PubMed]
- Place RJ, Gregorcyk SG, Huber PJ, et al. Outcome analysis of HIV-positive patients with anal squamous cell carcinoma. Dis Colon Rectum 2001;44:506-12. [Crossref] [PubMed]
- Oehler-Jänne C, Huguet F, Provencher S, et al. HIV-specific differences in outcome of squamous cell carcinoma of the anal canal: a multicentric cohort study of HIV-positive patients receiving highly active antiretroviral therapy. J Clin Oncol 2008;26:2550-7. [Crossref] [PubMed]
- Cleator S, Fife K, Nelson M, et al. Treatment of HIV-associated invasive anal cancer with combined chemoradiation. Eur J Cancer 2000;36:754-8. [Crossref] [PubMed]
- Edelman S, Johnstone PA. Combined modality therapy for HIV-infected patients with squamous cell carcinoma of the anus: outcomes and toxicities. Int J Radiat Oncol Biol Phys 2006;66:206-11. [Crossref] [PubMed]
- Peddada AV, Smith DE, Rao AR, et al. Chemotherapy and low-dose radiotherapy in the treatment of HIV-infected patients with carcinoma of the anal canal. Int J Radiat Oncol Biol Phys 1997;37:1101-5. [Crossref] [PubMed]
- Hammad N, Heilbrun LK, Gupta S, et al. Squamous Cell Cancer of the Anal Canal in HIV-Infected Patients Receiving Highly Active Antiretroviral Therapy: A Single Institution Experience. Am J Clin Oncol 2011;34:135-9. [PubMed]
- Chiao EY, Giordano TP, Richardson P, et al. Human immunodeficiency virus-associated squamous cell cancer of the anus: epidemiology and outcomes in the highly active antiretroviral therapy era. J Clin Oncol 2008;26:474-9. [Crossref] [PubMed]
- NCCN Clinical Practice Guidelines in Oncology 2019. Available online: https://www.nccn.org/professionals/physician_gls/default.aspx
- ESTRO. ESTRO - European Society for Radiotherapy & Oncology 2019. Available online: https://www.estro.org/Science/Guidelines
- Higgins JPT, Thomas J, Chandler J, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2019.
- .The Scottish Intercollegiate Guidelines Network (SIGN). 2019.
- Holland JM, Swift PS. Tolerance of patients with human immunodeficiency virus and anal carcinoma to treatment with combined chemotherapy and radiation therapy. Radiology 1994;193:251-4. [Crossref] [PubMed]
- Fraunholz I, Rabeneck D, Gerstein J, et al. Concurrent chemoradiotherapy with 5-fluorouracil and mitomycin C for anal carcinoma: are there differences between HIV-positive and HIV-negative patients in the era of highly active antiretroviral therapy? Radiother Oncol 2011;98:99-104. [Crossref] [PubMed]
- Martin D, Balermpas P, Fokas E, et al. Are there HIV-specific Differences for Anal Cancer Patients Treated with Standard Chemoradiotherapy in the Era of Combined Antiretroviral Therapy? Clin Oncol (R Coll Radiol) 2017;29:248-55. [Crossref] [PubMed]
- White EC, Khodayari B, Erickson KT, et al. Comparison of Toxicity and Treatment Outcomes in HIV-positive Versus HIV-negative Patients With Squamous Cell Carcinoma of the Anal Canal. Am J Clin Oncol 2017;40:386-92. [Crossref] [PubMed]
- Vatra B, Sobhani I, Aparicio T, et al. Gastroenterol Clin Biol 2002;26:150-6. [Anal canal squamous-cell carcinomas in HIV positive patients: clinical features, treatments and prognosis]. [PubMed]
- Oehler-Jänne C, Seifert B, Lütolf UM, et al. Local tumor control and toxicity in HIV-associated anal carcinoma treated with radiotherapy in the era of antiretroviral therapy. Radiat Oncol 2006;1:29. [Crossref] [PubMed]
- Munoz-Bongrand N, Poghosyan T, Zohar S, et al. Anal carcinoma in HIV-infected patients in the era of antiretroviral therapy: a comparative study. Dis Colon Rectum 2011;54:729-35. [Crossref] [PubMed]
- Wieghard N, Hart KD, Kelley K, et al. HIV positivity and anal cancer outcomes: A single-center experience. Am J Surg 2016;211:886-93. [Crossref] [PubMed]
- Kim JH, Sarani B, Orkin BA, et al. HIV-positive patients with anal carcinoma have poorer treatment tolerance and outcome than HIV-negative patients. Dis Colon Rectum 2001;44:1496-502. [Crossref] [PubMed]
- Hogg ME, Popowich DA, Wang EC, et al. HIV and anal cancer outcomes: a single institution's experience. Dis Colon Rectum 2009;52:891-7. [Crossref] [PubMed]
- Grew D, Bitterman D, Leichman CG, et al. HIV Infection Is Associated With Poor Outcomes for Patients With Anal Cancer in the Highly Active Antiretroviral Therapy Era. Dis Colon Rectum 2015;58:1130-6. [Crossref] [PubMed]
- Abramowitz L, Mathieu N, Roudot-Thoraval F, et al. Epidermoid anal cancer prognosis comparison among HIV+ and HIV- patients. Aliment Pharmacol Ther 2009;30:414-21. [Crossref] [PubMed]
- Camandaroba MPG, de Araujo RLC, Silva VSE, et al. Treatment outcomes of patients with localized anal squamous cell carcinoma according to HIV infection: systematic review and meta-analysis. J Gastrointest Oncol 2019;10:48-60. [Crossref] [PubMed]
- ClinicalTrials.gov. Nimotuzumab With Radiotherapy in the Treatment of Anal Canal Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT01382745
- ClinicalTrials.gov. Topical MTS-01 for Dermatitis During Radiation and Chemotherapy for Anal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01324141
- Formenti SC, Chak L, Gill P, et al. Increased radiosensitivity of normal tissue fibroblasts in patients with acquired immunodeficiency syndrome (AIDS) and with Kaposi's sarcoma. Int J Radiat Biol 1995;68:411-2. [Crossref] [PubMed]
- Place RJ, Gregorcyk SG, Huber PJ, et al. Outcome analysis of HIV-positive patients with anal squamous cell carcinoma. Dis Colon Rectum 2001;44:506-12. [Crossref] [PubMed]
- Seo Y, Kinsella MT, Reynolds HL, et al. Outcomes of chemoradiotherapy with 5-Fluorouracil and mitomycin C for anal cancer in immunocompetent versus immunodeficient patients. Int J Radiat Oncol Biol Phys 2009;75:143-9. [Crossref] [PubMed]
- ClinicalTrials.gov. Cisplatin and Fluorouracil Compared With Carboplatin and Paclitaxel in Treating Patients With Inoperable Locally Recurrent or Metastatic Anal Cancer (InterAACT). Accessed February 1, 2019.https://clinicaltrials.gov/ct2/show/NCT02560298
- Rao SR, Sclafani F, Guren MG, et al. InterAACT: a multicentre open label randomised phase II advanced anal cancer trial of cisplatin (CDDP) plus 5-fluorouracil (5-FU) vs carboplatin (C) plus weekly paclitaxel (P) in patients (pts) with inoperable locally recurrent (ILR) or metastatic treatment na¨ıve disease - an International Rare Cancers Initiative (IRCI) trial. Ann Oncol 2018. [Crossref]
- ClinicalTrials.gov. Nivolumab After Combined Modality Therapy in Treating Patients With High Risk Stage II-IIIB Anal Cancer. Accessed February 1, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT03233711
- Morris VK, Eng C. Role of immunotherapy in the treatment of squamous cell carcinoma of the anal canal. J Natl Compr Canc Netw 2018;16:903-8. [Crossref] [PubMed]
- ClinicalTrials.gov. DNA Plasmid-encoding Interleukin-12/HPV DNA Plasmids Therapeutic Vaccine INO-3112 and Durvalumab in Treating Patients With Recurrent or Metastatic Human Papillomavirus Associated Cancers. Available online: https://clinicaltrials.gov/ct2/show/NCT03439085
- Stadler RF, Gregorcyk SG, Euhus DM, et al. Outcome of HIV-infected patients with invasive squamous-cell carcinoma of the anal canal in the era of highly active antiretroviral therapy. Dis Colon Rectum 2004;47:1305-9. [Crossref] [PubMed]
- Wexler A, Berson AM, Goldstone SE, et al. Invasive anal squamous-cell carcinoma in the HIV-positive patient: outcome in the era of highly active antiretroviral therapy. Dis Colon Rectum 2008;51:73-81. [Crossref] [PubMed]
- Klencke BJ, Palefsky JM. Anal cancer: an HIV-associated cancer. Hematol Oncol Clin North Am 2003;17:859-72. [Crossref] [PubMed]