Minimally invasive complete mesocolic excision and central vascular ligation (CME/CVL) for right colon cancer
Introduction
Total mesorectal excision (TME), which was pioneered by Heald et al., revolutionized rectal cancer surgery by significantly reducing local recurrence rates and improving survival (1-4).
Complete mesocolic excision with central vascular ligation (CME/CVL) for right sided colonic cancers is an operation that is increasingly performed by colorectal units worldwide. Especially for units from East Asia and Europe, CME/CVL is often regarded as a standard operation that is performed via a minimally invasive method (laparoscopic or robotically). It is reasonable to consider CME/CVL as a procedure that is analogous to TME.
Laparoscopic colorectal cancer resection has emerged as a feasible and safe alternative to open surgery due to advantages such as less postoperative pain, faster return of bowel function, lower incidence of wound infection and reduction in length of hospital stay. In comparison of oncologic outcomes, laparoscopic surgery has also shown to be equivalent to open surgery (5-14).
CME/CVL is a challenging procedure due to the technical difficulties that it poses. In this article, the authors explore the background and evolution of CME/CVL. Current literature pertaining to the benefits and risks of performing CME/CVL for right sided colonic cancers will be examined. Technical details on how to perform the operation safely via laparoscopy will also be discussed. (Clarification to reader: CME/CVL is a surgical technique that is applicable to both left and right sided colonic resections. For ease of description and clarity, the term “CME/CVL” in this article henceforth refers to performing CME/CVL in right hemicolectomies for adenocarcinoma located from the caecum to the middle third of the transverse colon.)
Background
The curative treatment of colon cancer mandates the en bloc resection of the primary tumour with appropriate proximal and distal bowel margins. Lymph nodes, blood vessels and neural tissue that are located within the bowel mesentery are resected with the tumour as well.
In a conventional right colectomy, the extent of lymphadenectomy is usually determined by the surgeon, who would divide the mesentery and colon at an anatomically convenient location to remove lymph nodes which are detectable. Vessel ligation is usually performed at the mid-mesenteric level, which is approximately the midpoint between the colon and the superior mesenteric vessels.
Prior to the advocacy of CME/CVL, apart from the Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines, there was no other international criteria that guides the extent of surgery during colon cancer resections.
Concept of CME/CVL
The entire colon and its mesentery are enveloped by the mesocolic fascia, which is a physical direct continuation of the mesorectal fascia. Between the mesocolic fascia and the parietal retroperitoneal fascia is a potential space known as Toldt’s fascia.
Hohenberger et al. (15) advocated CME/CVL for resection of right sided colon cancers which comprises two parts. First, it which involves sharp dissection along Toldt’s fascia with the eventual goal of removing the primary tumour, its mesentery and a complete undisrupted envelope of mesocolic fascia. Within the resected specimen would also contain adjacent blood vessels, draining lymphatics and neural tissue, which are potential pathways through which the tumour may spread (8). The duodenum is also fully kocherized.
The second component is CVL whereby the tumour supplying vessels are dissected free and ligated at their origin. This ensures that all regional lymph nodes in the central (vertical) direction are maximally harvested.
Technical considerations
For the senior/corresponding author’s (WTC) institution, laparoscopic CME/CVL is the standard of care. Details of the procedure are as follows:
Pre-operative
The patient undergoes a complete colonoscopic examination to biopsy the tumour in order to obtain histological confirmation of cancer. Location of the tumour is also ascertained. Tattooing of the tumour is performed routinely to facilitate tumour identification during the operation; this is especially useful for small lesions. Computed tomography (CT) of the thorax, abdomen and pelvis is done to assess for distant metastasis. An anaesthetic assessment is performed for the patient prior to the operation. Bowel preparation is not required. Invasive monitoring (intraarterial and central venous pressure monitoring lines) is carried out at the discretion of the anaesthetist.
Patient positioning
After general anaesthesia, the patient is placed in a modified Lloyd Davis position with both upper limbs tucked beside the body. A nasogastric tube is inserted to decompress the stomach. The primary surgeon stands between the patient’s legs while the camera assistant is positioned on the surgeon’s right. Should another assistant be required, he/she will be positioned on the surgeon’s left.
Port placement
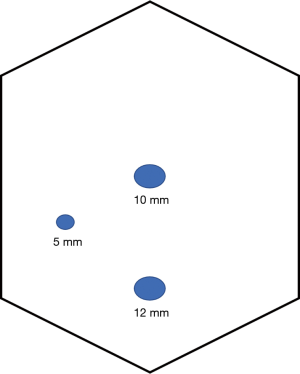
The camera is placed through a 10 mm port at the umbilicus. A 12 mm right hand working port is placed in suprapubic region about two finger breadths superior to the symphysis pubis at the midline while a 5 mm left hand working port is placed in right iliac fossa over McBurneys’ point. An additional 5 mm port may be placed at the right hypochondrium for assistance if needed. Refer to Figure 1.
Right colon mobilisation (inferior to superior)
After establishing pneumoperitoneum, a diagnostic laparoscopy is performed to look for metastases. The patient is then placed in a Trendelenburg position with left side down. Next, the greater omentum, transverse colon and small bowel are retracted in the cephalad direction. The ileocecal junction is retracted anteriorly towards the abdominal wall.
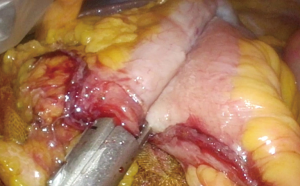
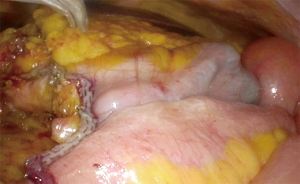
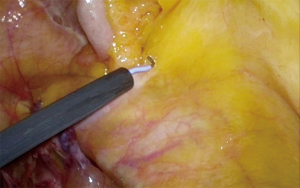
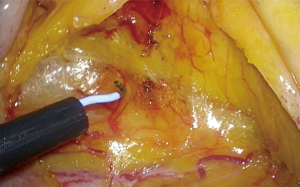
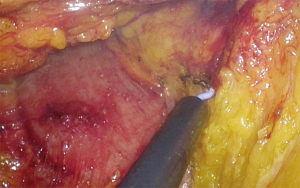
An incision over the peritoneum is made via monopolar diathermy at the interface between the right colon mesentery and the retroperitoneum (refer to Figure 2). As the dissection proceeds in the cephalad direction, the gonadal vessels and ureter (Figure 3) may be visualized beneath the retroperitoneal fascia. The duodenum and pancreatic head (Figure 4) can be located the medial area of dissection while Gerota’s fascia can be seen at the lateral aspect. It is pertinent to dissect in the avascular plane anterior to the duodenum in order to prevent any injury either to the duodenum or to the pancreatic capsule as that may result in troublesome bleeding. Continued dissection in a superior direction will lead to the plane that is posterior to the transverse colon. Superior laterally, beyond Gerota’s fascia; the dissected space is separated from the hepatorenal space (Morrison’s space) by a layer of peritoneum.
Central vessel ligation
The location of the superior mesenteric artery and vein (SMA and SMV, collectively termed surgical trunk or ST) is usually identifiable from inspection of the mesentery. Peritoneum overlying the ST is cauterized superficially for purpose of surface marking.
A point 5 cm proximal to the ileocecal valve is identified on the terminal ileum where it is dissected free from its adjoining mesentery. The terminal ileum is then transected using a laparoscopic stapler. The mesentery of the terminal ileum is then divided toward the direction of the ST using an energy device.
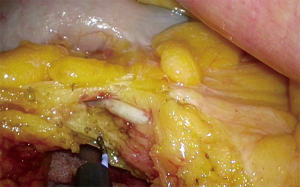
Just before reaching the ST (which was earlier surface marked using cautery), the ileal vein should be dissected free (Figure 5). The ileocolic vein usually joins the ileal vein approximately at the level of the 3rd portion of the duodenum to form the SMV. An avascular plane (Figure 6) exists immediately anterior to it. Most of the time, the SMV lies on the right of the SMA. Dissection proceeds in a cephalad direction following the avascular zone anterior to the ST; the peritoneum overlying to this avascular zone can be divided safely.

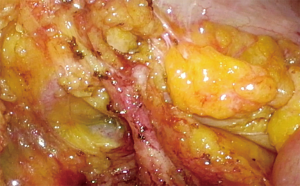
The ileocolic vein and artery (Figure 7) are dissected free and ligated at their origin using a combination of Hemolock clips (5 mm) and energy device (termed double ligation). Right colic vessels, if present, are identified and ligated. It is imperative that the vessels are dissected clearly and demonstrated to be inserting into the ST prior to ligation. This is to prevent inadvertent ligation to the ST.

Approximate location of the middle colic vessels can be located via inspection of the transverse colonic mesentery. The main middle colic vessels are dissected free (Figures 8,9) and ligated at the root (applicable for mid transverse colon tumors). Alternatively, depending on the extent of right hemicolectomy, the branch of the middle colic vessels is ligated (performed for caecum, ascending colon, hepatic flexure and proximal transverse colon tumors). The gastro colic trunk is usually identified and preserved.
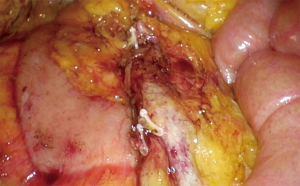
The transverse colon is then divided with a laparoscopic stapler 5 cm distal to the tumor. Prior to transection, the surgeon should inspect the transverse colon closely to ensure that no ischemic tissue is left behind. Lateral attachments of the right colon are divided till the specimen is completely free.
Anastomosis
The ileocolic anastomosis may be performed in iso or anti peristaltic fashion; this is left to the discretion of the primary surgeon. A 60 mm laparoscopic stapler is used to create the anastomosis. Refer to Figure 10. The resultant ileo-colotomy is closed using absorbable sutures in two layers. Alternatively, in cases where an anti-peristaltic anastomosis is performed, the ileo-colotomy may be sealed/closed via a firing of the stapler. Refer to Figure 11 which shows that the ileocolic anastomosis is sealed using a stapler. When performing this step, it is important the surgeon ensures that the stapler grasps and seals the full thickness of the bowel refer to Figure 12 which shows the completed anastomosis.
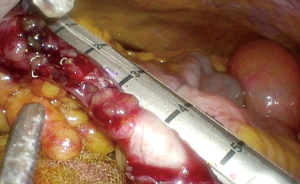
Should the transverse colon be sufficiently mobile, the bowel ends may be delivered through a midline incision and the subsequent anastomosis is performed extra corporeally.
The mesenteric window is routinely closed and a drain is sited in the right paracolic gutter. The specimen is extracted via a limited Pfannenstiel incision (wound protector routinely utilized) in the case of intra corporeal anastomosis.
Postoperative management
The nasogastric tube is removed at the end of the operation. Once awake, the patient is allowed fluids per oral. From the first postoperative day, the patient is then progressed to an oral diet. Average length of hospital stay is about 5 days after operation. The drain is removed prior to discharge from hospital.
Discussion
Historically, the treatment of rectal cancer yielded worse outcomes compared to colon cancer. After Heald et al. (1-3) demonstrated that by performing TME, the local recurrence rate for rectal cancer could be reduced to 5%, it led to a marked transformation in the prognosis of rectal cancer treatment and made TME the cornerstone of optimum rectal cancer therapy.
The main concept of TME is sharp dissection along embryological planes to remove the rectum in a fascial lined package; this is a transferrable and teachable technique. Standardization of operative techniques in colon cancer surgery has shown to lead to improvement in clinical outcomes (16). Surgical education programs were then developed in many countries to teach surgeons how to perform TME satisfactorily (17-19). Together with neoadjuvant and adjuvant treatment protocols, rectal cancer prognosis has surpassed that of colon cancer in some European countries (20,21).
The desired endpoint of CME/CVL is better local control and survival. The technique follows a similar rationale as TME but applied in the area of colon cancer surgery. It is a radical principal, hypothesizing that tumour cells metastasize along their lymphatics but within the confines of the mesocolic fascia. Through the removal of the tumour and its mesentery with an intact mesocolic fascia, the dissemination of tumour cells is limited. As the lymphatic drainage of the colon follows closely with its arterial supply, ligation of feeding vessels at their origin (CVL) maximizes the harvest of lymph nodes. Conversely, surgery performed in the non-anatomical plane results in the disruption of the mesocolic fascia and causes spillage of tumour cells, potentially increasing the risk of poorer oncological outcomes.
The application of CME/CVL for left sided colonic cancer has been widely accepted. However, the technique is not as readily applied for right sided lesions. To the authors’ knowledge, most surgeons do not undertake such extensive dissection. Some of the attributed reasons are the vascular variability of the right colon and risk of vessel injury from dissection in the immediate vicinity of the SMA/SMV.
There is, however, increasing evidence that addresses the oncological benefits of more radical surgery for right sided colonic cancers and impressive results have been reported from units that specialize in performing the procedure. In 2009, Hohenberger et al. (15) reported on 1,329 patients who underwent CME/CVL, achieving a 5-year survival rate of 89% together improving the local recurrence rate to 3.6% from 6.5%. Other centres have since reported favourable outcomes (22-29). Bertelsen et al. (24), on behalf of the Danish Colorectal Cancer Group, showed that CME for stage 3 colon cancer gave rise to better survival rates compared to patients who did not receive CME. Recently in 2019, the same author (25) reported that CME conferred an absolute risk reduction of 8.2% (CME patients 9.7% vs. non-CME patients 17.9%) in terms of local recurrence risk. Recommendation was even made in the same paper that CME should be a standard of care for right sided colon cancer.
On the other hand, readers should also note the presence of studies that do not point towards a favourable result for CME. A systemic review by Kontovounisios et al. involving more than 5,000 patients and 30 studies, that concluded that CME did not result in long term survival benefit for the patient (30). The authors opine that while there is increasing data in support of CME/CVL, outcomes from analysis of larger patient cohorts or a randomised controlled trial should be awaited before the operation can be regarded as standard of care.
CME/CVL and D3 dissection are two surgical techniques which share common characteristics. In the current medical literature, CME/CVL is frequently mentioned together with D3 dissection; in fact, it is not unusual to see these two terms used interchangeably. In Japan, surgeons perform D3 dissection as the standard procedure for cT3, cT4 and/or node positive colon cancer. The term refers to the extent of lymphadenectomy. According to the Japanese Classification of Colorectal Carcinoma, D3 dissection means that all three lymph node stations, namely pericolic, intermediate and main, are dissected (31).
Both procedures emphasize sharp dissection along embryological planes with ligation of feeding vessels at their origin. Differences include the recommended length of bowel transection—CME/CVL points of transection are usually at the terminal ileum and proximal transverse colon (or 5 cm distal to the tumour edge for mid transverse colonic tumours. On the other hand, surgeons who practise D3 dissection adhere closely to the transection of 10 cm proximal and distal from the epicentre of the tumour or 5 cm distal from a vessel that is identified intra operatively as a feeding vessel. In a comparison of surgical specimen post CME/CVL with D3 dissections, West et al. (32) reported that both techniques demonstrated similar rates of resection along CME planes. In addition, patients who underwent CME/CVL showed increased length of bowel resected as well as contained increased quantity of lymph nodes. The study, however, did not proceed to correlate these differences to clinical outcomes.
Compared to conventional surgery, both CME/CVL and D3 dissection procedures lead to a larger resected area of mesocolic lined mesentery (33-36) with the removal of a larger quantity of lymph nodes. An evaluation of specimens resected by surgeons who performed CME surgery was compared against specimens resected by surgeons who performed non-CME surgery showed that not surprisingly, in the former, a larger amount of mesocolon was removed. What was notable was that patients with CME specimens were also reported to have higher 5-year survival rates (33). In another study, West et al. (29) reported CME/CVL surgical specimens that demonstrated preserved mesocolic fascia alone is associated with a superior 5-year overall survival compared to non CME resections, with the difference in survival most marked in stage III cancer patients.
Apart from being a surrogate of a more extensive resection, increased lymph node yield is an independent marker of longer survival (37-40). It also ensures more precise tumour staging and aids in determining which patients may benefit from adjuvant chemotherapy. The incidence of metastatic apical lymph nodes ranges from 1% to 8% (41-43). Further justification for CME/CVL is that performing a radical nodal clearance maximises the chances of removal of apical lymph node (also known as D3 or main node). Also, intra operative inspection or palpation will not identify the presence of apical lymph nodes though it is more associated with T4 lesions (44).
Presently, a lymphadenectomy yield of 12 lymph nodes is regarded as a minimum standard (38). However, many other factors also affect the number of detected lymph nodes. These include patient age and gender, tumour site, use of prior radio-chemotherapy and histological examination techniques.
The landmark paper from Hohenberger reported an overall complication rate of 19% of patients who underwent CME/CVL. This encompassed an anastomotic leak rate of 2.6% with a postoperative mortality rate of 3%, which could be explained by the fact that 9.5% of this cohort of patients underwent emergency surgery as their index surgery. Several studies have gone on to demonstrate the safety profile of CME/CVL as well as D3 dissections (45,46). Bertelsen et al. opined that after standardizing technique for CME/CVL, there was no difference in complication rates when compared to conventional colonic surgery (35). In a case control study, Bernhoff et al. (46) concluded that CME surgery was not associated with an increased 90-day mortality. However, the authors note the presence of studies (27,28), that demonstrate increased risk from performing CME/ CVL. We opine that while the inherent nature of CME/CVL increases the potential of surgical complications, this should be mitigated by having the operation performed by an experienced surgeon from a high volume unit.
Major randomised controlled trials (RCTs) comparing the laparoscopic versus open colorectal surgery (5,11) showed a higher incidence of organ injuries from the former. Since then, the uptake of minimally invasive surgery for colorectal resections has increased worldwide. Many specialised units have reported performing laparoscopic CME/CVL and producing comparable quality of surgical specimens compared to open surgery (47-50). In one of the few RCTs related to the subject of radical right colectomy, Yamamoto et al compared laparoscopic and open D3 colonic resections which demonstrated lower morbidity rates in the laparoscopic group whilst also showing that patients who underwent laparoscopic D3 resections were associated with the usual benefits of minimally invasive surgery.
Conclusions
Laparoscopic CME/CVL is a procedure that should be promulgated as it can improve the prognosis of colon cancer. Guidelines should be developed that stipulate minimum quality standards of the operation. Surgeons who wish to perform the procedure should participate in a formal training program and be adequately proctored before attempting the operation.
Acknowledgments
Funding: None.
Footnote
Provenance and Peer Review: This article was commissioned by the Guest Editors (Nan Zun Teo, James Chi-Yong Ngu) for the series “Current Strategies in Colon Cancer Management” published in Journal of Gastrointestinal Oncology. The article was sent for external peer review organized by the Guest Editors and the editorial office.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/jgo.2019.11.08). The series “Current Strategies in Colon Cancer Management” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- Heald RJ, Husband EM, Ryall RDH. The mesorectum in rectal cancer surgery - the clue to pelvic recurrence? Br J Surg 1982;69:613-6. [Crossref] [PubMed]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- Schwenk W, Haase O, Neudecker J, et al. Short-term benefits for laparoscopic colorectal resection. Cochrane Database Syst Rev 2005.CD003145. [PubMed]
- MacFarlane JK, Ryall RD, Heald RJ. Mesorectal excision for rectal cancer. Lancet 1993;341:457-60. [Crossref] [PubMed]
- Hewett PJ, Allardyce RA, Rieger NA, et al. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg 2008;248:728-38. [Crossref] [PubMed]
- Stevenson ARL, Solomon MJ, Lumley JW, et al. Effect of laparoscopic assisted resection vs open resection on pathological outcomes in rectal cancer - The ALaCaRT Randomized Clinical Trial. JAMA 2015;314:1356-63. [Crossref] [PubMed]
- Nelson H, Sargent DJ, Stryker SJ, et al. Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med 2004;350:2050-9. [Crossref] [PubMed]
- Veldkamp R, Kuhry E, Bonjer HJ, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol 2005;6:477-84. [Crossref] [PubMed]
- Hazebroek EJ. Color Study Group. COLOR: a randomized clinical trial comparing laparoscopic and open resection for colon cancer. Surg Endosc 2002;16:949-53. [Crossref] [PubMed]
- van der Pas MH, Hagline E, Cuesta MA, et al. Colorectal cancer laparoscopic or open resection II (COLOR II) study group el al. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol 2013;14:210-8. [Crossref] [PubMed]
- Guillou PJ, Quirke P, Thorpe H, et al. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet 2005;365:1718-26. [Crossref] [PubMed]
- Green BL, Marshall HC, Collinson F, et al. Long term follow up of the Medical Research Council CLASICC trial of conventional versus laparoscopically assisted resection in colorectal cancer. Br J Surg 2013;100:75-82. [Crossref] [PubMed]
- Kang SB, Park JW, Jeong SY, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol 2010;11:637-45. [Crossref] [PubMed]
- Jeong SY, Park JW, Nam BH, et al. Open versus laparoscopic surgery for mid-rectal or low-rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open-label, non-inferiority, randomised controlled trial. Lancet Oncol 2014;15:767-74. [Crossref] [PubMed]
- Hohenberger W, Weber K, Matzel K, et al. Standardized surgery for colonic cancer: complete mesocolic excision and central ligation- technical notes and outcome. Colorectal Dis 2009;11:354-64. [Crossref] [PubMed]
- Bokey EL, Chapuis PH, Dent OF, et al. Surgical technique and survival in patients having a curative resection for colon cancer. Dis Colon Rectum 2003;46:860-866. [Crossref] [PubMed]
- Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg 2002;89:1142-9. [Crossref] [PubMed]
- Martling AL, Holm T, Rutqvist LE, et al. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm: Stockholm Colorectal Cancer Study Group, Basingstoke Bowel Cancer Res Project. Lancet 2000;356:93-6. [Crossref] [PubMed]
- Wibe A, Møller B, Norstein J, et al. A national strategic change in treatment policy for rectal cancer: Implementation of total mesorectal excision as routine treatment in Norway—A national audit. Dis Colon Rectum 2002;45:857-66. [Crossref] [PubMed]
- Birgisson H, Talbäck M, Gunnarsson U, et al. Improved survival in cancer of the colon and rectum in Sweden. Eur J Surg Oncol 2005;31:845-53. [Crossref] [PubMed]
- Iversen LH, Nørgaard M, Jepsen P, et al. Trends in colorectal cancer survival in northern Denmark: 1985-2004. Colorectal Dis 2007;9:210-7. [Crossref] [PubMed]
- Storli KE, Lygre KB, Iversen KB, et al. Laparoscopic complete mesocolic excisions for colonic cancer in the last decade: Five-year survival in a single centre. World J Gastrointest Surg 2017;9:215-23. [Crossref] [PubMed]
- Merkel S, Weber K, Hohenberger W, et al. Prognosis of patients with colonic carcinoma before, during and after implementation of complete mesocolic excision. Br J Surg 2016;103:1220-9. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Jansen JE, et al. Disease-free survival after complete mesocolic excision compared with conventional colon cancer surgery: a retrospective, population-based study. Lancet Oncol 2015;16:161-8. [Crossref] [PubMed]
- Bertelsen CA, Neuenschwander AU, Kleif J, et al. 5-year outcome after complete mesocolic excision for right sided colon cancer: a population based cohort study. Lancet Oncol 2019;20:1556-65. [Crossref] [PubMed]
- Siani LM, Pulica C. Laparoscopic Complete Mesocolic Excision with Central Vascular Ligation in right colon cancer: long-term oncologic outcome between mesocolic and nonmesocolic planes of surgery. Scand J Surg 2015;104:219-26. [Crossref] [PubMed]
- Galizia G, Lieto E, De Vita F, et al. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int J Colorectal Dis 2014;29:89-97. [Crossref] [PubMed]
- Zurleni T, Cassiano A, Zurleni F, et al. Surgical and oncological outcomes after complete mesocolic excision in right sided colon cancer compared with conventional surgery: a retrospective, single institution study. Int J Colorectal Dis 2018;33:1-8. [Crossref] [PubMed]
- West NP, Morris EJA, Quirke P, et al. Pathology grading of colon cancer surgical resection and its association with survival: a retrospective observational study Lancet Oncol 2008;9:857-65. [Crossref] [PubMed]
- Kontovounisios C, Kinross J, Tekkis P, et al. Complete mesocolic excision in colorectal cancer: a systematic review. Colorectal Dis 2015;17:7-16. [Crossref] [PubMed]
- Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2019 for treatment of colorectal cancer. Int J Clin Oncol 2020;25:1-42. [Crossref] [PubMed]
- West NP, Kobayashi H, Quirke P, et al. Understanding optimal colonic cancer surgery: comparison of Japanese D3 resection and European complete mesocolic excision with central vascular ligation. J Clin Oncol 2012;30:1763-9. [Crossref] [PubMed]
- West NP, Hohenberger W, Quirke P, et al. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J Clin Oncol 2010;28:272-8. [Crossref] [PubMed]
- Wang J, Hassett JM, Dayton MT, et al. Lymph node ratio: role in the staging of node-positive colon cancer. Ann Surg Oncol 2008;15:1600-8. [Crossref] [PubMed]
- Bertelsen CA, Bols B, Vilandt J, et al. Can the quality of colonic surgery be improved by standardization of surgical technique with complete mesocolic excision? Colorectal Dis 2011;13:1123-9. [Crossref] [PubMed]
- Kobayashi H, West NP, Hohenberger W, et al. Quality of surgery for stage III colon cancer: comparison between England, Germany, and Japan. Ann Surg Oncol 2014;21:S398-S404. [Crossref] [PubMed]
- Nelson H, Petrelli N, Sargent D, et al. Guidelines 2000 for colon and rectal cancer surgery. J Natl Cancer Inst 2001;93:583-96. [Crossref] [PubMed]
- Le Voyer TE, Sigurdson ER, Catalano PJ, et al. Colon Cancer survival is associated with increasing number of lymph nodes analyzed: a secondary survey of intergroup trial INT-0089. J Clin Oncol 2003;21:2912-9. [Crossref] [PubMed]
- Joseph NE, Sigurdson ER, Hanlon AL, et al. Accuracy of determining nodal negativity in colorectal cancer on the basis of the number of nodes retrieved on resection. Ann Surg Oncol 2003;10:213-8. [Crossref] [PubMed]
- Chang GJ, Rodriguez-Bigas MA, Moyer VA, et al. Lymph node evaluation and survival after curative resection of colon cancer: systematic review. J Natl Cancer Inst 2007;99:433-41. [Crossref] [PubMed]
- Paquette IM, Madoff RD, Sigurdson ER, et al. Impact of proximal vascular ligation on survival of patients with colon cancer. Ann Surg Oncol 2018;25:38-45. [Crossref] [PubMed]
- Kanemitsu Y, Komori K, Kimura K, et al. D3 lymph node dissection in right hemicolectomy with a no-touch isolation technique in patients with colon cancer. Dis Colon Rectum 2013;56:815-24. [Crossref] [PubMed]
- Tan KY, Kawamura YJ, Konishi F, et al. Distribution of the first metastatic lymph node in colon cancer and its clinical significance. Colorectal Dis 2010;12:44-7. [Crossref] [PubMed]
- Hashiguchi Y, Hase K, Yamamoto J, et al. Optimal margins and lymphadenectomy in colonic cancer surgery. Br J Surg 2011;98:1171-8. [Crossref] [PubMed]
- Yamamoto S, Inomata M, Kitano S, et al. Short term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg 2014;260:23-30. [Crossref] [PubMed]
- Bernhoff R, Sjövall A, Buchli C, et al. Complete mesocolic excision in right-sided colon cancer does not increase severe short-term postoperative adverse events. Colorectal Dis 2018;20:383-9. [Crossref] [PubMed]
- Galizia G, Lieto E, Mabilia A, et al. Is complete mesocolic excision with central vascular ligation safe and effective in the surgical treatment of right-sided colon cancers? A prospective study. Int J Colorectal Dis 2014;29:89-97. [Crossref] [PubMed]
- Subbiah R, Bansal S, Palanivelu PR, et al. Initial retrocolic endoscopic tunnel approach (IRETA) for complete mesocolic excision (CME) with ventral vascular ligation (CVL) for right colonic cancers: technique and pathological radicality. Int J Colorectal Dis 2016;31:227-33. [Crossref] [PubMed]
- West NP, Kennedy RH, Jenkins JT, et al. Morphometric analysis and lymph node yield in laparoscopic complete mesocolic excision performed by supervised trainees. Br J Surg 2014;101:1460-7. [Crossref] [PubMed]
- Melich G, Jeong DH, Kim NK, et al. Laparoscopic right hemicolectomy with complete mesocolic excision provides acceptable perioperative outcomes but is lengthy - analysis of learning curves for a novice minimally invasive surgeon. Can J Surg 2014;57:331-6. [Crossref] [PubMed]