
The emerging role of proton therapy for esophagus cancer
Background
Approximately 18,000 patients will be diagnosed with esophageal cancer within the United States in 2019 (1). The majority of patients will have locoregionally confined disease and therefore they are eligible for curative-intent treatment. Despite this, outcomes remain poor with an estimated 16,000 deaths annually and 5-year overall survival (OS) of approximately 20%. More effective treatment options are needed to improve these outcomes.
Surgical or endoscopic techniques are the mainstay curative treatment options for patients with resectable, early stage disease (2). Typically, endoscopic resection is used for patients with disease limited to the esophageal mucosa and select cases of patients with superficial submucosal extension. Patients with submucosal or muscularis propria involvement may be eligible for esophagectomy as the sole curative intervention. CRT has also demonstrated efficacy in the management of early stage esophageal cancer and serves as an alternative treatment strategy for patients who are deemed medically inoperable or desire non-surgical curative approaches (3-5).
Patients with locally advanced disease have a higher risk of locoregional and distant disease relapse with surgery alone and they are managed with combined modality therapy. The standard of care for patients with potentially resectable disease is “tri-modality” therapy involving pre-operative CRT followed by esophagectomy (6-10). Multiple clinical trials have demonstrated that the addition of CRT prior to surgery improves outcomes compared to surgery alone (6-10). For instance, the ChemoRadiotherapy for Esophageal cancer followed by Surgery Study (CROSS) Trial included patients with locally advanced esophageal cancer who were randomized to either esophagectomy alone or pre-operative CRT (41.4 Gy in 23 fractions with external beam photon technique) with concurrent weekly carboplatin and paclitaxel followed by esophagectomy (6). Pre-operative CRT was associated with a pathologic complete response rate of 29% [23% for adenocarcinoma and 49% for squamous cell carcinoma (SCC)] and demonstrated improvements in margin negative resection rates, lymph node clearance, locoregional control (LRC), and OS.
CRT is also a potentially curative treatment for patients with unresectable, non-metastatic disease (11-13). The Radiation Therapy Oncology Group (RTOG) trial 8501 included patients with locally advanced esophageal cancer who were randomized to either RT alone (64 Gy in 32 fractions) or CRT consisting of 50 Gy in 25 fractions with concurrent 5-fluorouracil (5FU) and cisplatin (11). This study demonstrated improvements in OS, LRC, and distant metastasis with the addition of chemotherapy to RT. Importantly, this study demonstrated long-term survivorship in approximately 20–25% of patients receiving CRT with no long-term survivors in the RT alone cohort, thus establishing CRT as the standard of care in patients not eligible for surgery.
For patients with esophageal cancer treated with CRT, a substantial proportion experience acute and/or late treatment-related AEs. In the CROSS trial, a majority of patients experienced fatigue, cytopenia, nausea, or anorexia during CRT, while approximately 20% experienced grade 3+ (serious or life-threatening) AEs (6). Additionally, a large proportion of patients experienced major post-operative complications including pulmonary complications (46%), cardiac complications (21%), anastomotic leakage (22%), or death (6%). Similarly, in RTOG trial 8501, 44% of patients experienced “severe” AEs and 20% experienced life-threatening AEs (11). While disease recurrence remains the major cause of death for esophageal cancer patients treated with CRT, a significant proportion of patients will die of non-cancer causes including cardiac, pulmonary, and/or renal failure in the first few years following completion of treatment. The high rate of treatment-related AEs with current photon-based CRT regimens and surgery has curbed enthusiasm for exploring more intensified chemotherapy or RT regimens.
Rationale for the use of proton therapy for esophageal cancer
For esophageal cancer, the RT target volume includes the esophagus and regional lymph nodes (14), which are surrounded by non-target normal organs including the lungs, heart, kidneys, liver, and bowel that are sensitive to the effects of RT. The likelihood of injury to these organs is directly correlated with the dose delivered to the organ and the volume of the organ irradiated (15-19). Several studies of patients receiving CRT for esophagus cancer demonstrate a relationship between RT dose to the lungs and heart and risk of pulmonary or cardiac complications (20-24). Additionally, a recent trial involving CRT for lung cancer demonstrated that increasing RT dose to the heart was a significant independent predictor for risk of death (25), while data from women receiving RT for breast cancer suggest that the relative risk of major coronary event increases by 7.4% for each 1 Gy increase in mean heart dose (26). These data suggest that treatment techniques which reduce the RT dose to critical organs including the heart and lungs could lead to reduced AEs, improved outcomes, and potentially provide an opportunity for safer treatment intensification.
Historically, RT for esophagus cancer was delivered with 2-dimensional (2D) planning with relatively large treatment fields to ensure target coverage, at the cost of substantial normal tissue RT exposure. Technological advances including three-dimensional conformal photon radiotherapy (3DCRT) and subsequently intensity modulated photon radiotherapy (IMRT) have offered improved dose conformality and reductions in RT exposure to normal tissues while maintaining target coverage. Chandra et al. reported that 7-field IMRT, when compared to 3DCRT, significantly reduced the percentage of lung receiving at least 10 Gy (V10 Gy) from 40% to 29% (P=0.01), V20 Gy from 19% to 14% (P=0.01), and mean lung dose (MLD) from 14.8 Gy to 11.8 Gy (P=0.01) (27). Lin et al. performed a retrospective propensity score matched comparison of patients receiving either 3DCRT or IMRT and demonstrated an association with IMRT and improved OS (P<0.001) (28). There were no differences in cancer-specific mortality or distant metastasis identified between the treatment cohorts, but there was lower cumulative incidence of cardiac death identified in those who received IMRT compared to 3DCRT (P=0.049). These data highlight that while disease recurrence remains the major cause of death, a significant proportion of patients will die of non-cancer causes possibly related to normal tissue radiation exposure which could potentially be mitigated by advanced RT techniques.
Standard external beam RT techniques use photon beams which deposit RT dose in tissue all the way along the beam path including within normal tissues. In contrast, proton beam therapy (PBT), through its unique physical properties, allows a reduction in dose proximal to the target and almost no dose distal to the target. Therefore, PBT may improve the therapeutic ratio by reducing dose to non-target normal tissues while maintaining equivalent target coverage.
Dosimetric comparisons of PBT and photon-based RT techniques
PBT has a distinct physical advantage over photon-based techniques as it is capable of delivering the same RT dose to the target volume but with significantly lower entrance and exit doses of radiation passing through surrounding normal organs. This is particularly pertinent given the central location of the esophagus and its proximity to the lungs, heart, kidneys, liver, and bowel. Table 1 summarizes select published dosimetric comparisons of proton vs. photon techniques in the treatment of esophagus cancer (29-37). These data suggest clinically meaningful reductions in the volume of heart, lung, and liver receiving low to intermediate RT doses and in the mean doses to each of these organs.

Full table
Passive scatter PBT (PS-PBT) was the initial PBT technology implemented for treatment of esophageal cancer. PS-PBT involves placing a variable degrader (called a range modulator wheel) into the path of the proton beam to produce a “spread out Bragg-peak” (SOBP) covering the depths of the tumor. The SOBP is then scattered laterally into a broad beam using thin foils, which is subsequently confined to a given (tumor conforming) shape using an aperture (typically brass). Tissue compensators are used to conform dose to the target distally. While PS-PBT does eliminate exit dose, thereby minimizing dose to normal tissues located distal to the target, the doses proximal to the target cannot be modulated and are not ideally conformal.
Zhang et al. compared dosimetry with PS-PBT versus IMRT for a cohort of 15 patients with esophageal cancer treated to a dose of 50.4 Gy in 28 fractions (29). PS-PBT offered significant reductions in radiation exposure to the heart, lung, and total body mean dose. They further compared PS-PBT delivered with a 2-field anteroposterior-posteroanterior (AP/PA) technique or a 3-field technique with right and left posterior oblique fields (RPO/LPO) combined with an AP field. The AP/PA technique offered improved lung sparing whereas the 3-field AP/RPO/LPO technique offered improved conformality and better heart sparing.
Xi et al. performed a retrospective comparison of 343 patients treated with IMRT or PS-PBT delivered with 2-field posterior and left posterior-oblique fields (34). The mean doses to the lung and heart were 10.0 and 19.9 Gy for IMRT versus 6.5 and 11.6 Gy for PBT (P<0.001), respectively. Compared with the IMRT group, the PBT group had significantly lower lung V5 Gy (48% vs. 28%, P<0.001), V10 Gy (32% vs. 23%, P<0.001), and V20 Gy (18% vs. 11%, P<0.001). The V30 Gy of the heart (19% vs. 24%, P<0.001) was also significantly lower for the PBT vs. IMRT group. Similarly, Shiraishi et al. reported on a cohort of 727 patients treated to a dose of 50.4 Gy in 28 fractions with either PBT (predominately PS-PBT) or IMRT (33). PBT was associated with improvements in heart mean dose, heart V5–40 Gy, lung mean dose, lung V5–30 Gy, liver mean dose, and liver V30 Gy.
Recent technologic advances in PBT delivery have allowed for the implementation of pencil beam scanning PBT (PBS-PBT), also referred to as intensity modulated proton therapy (IMPT). PBS-PT uses fast dipole magnets to steer or scan the proton beam “spot-by-spot” transversely (in x and y) over the tumor cross-section along with fast beam energy switching to cover given tumor depths, or tumor “layers”, thus allowing for volumetric “painting” of the tumor with proton “spots.” PBS-PBT offers improved dose conformality at proximal edges of a target volume compared to PS-PBT, which could offer further clinical benefits.
Welsh et al. evaluated three potential field arrangements for delivering PBS-PBT to a dose of 65.8 Gy in 28 fractions: 2-field AP/PA, 2-field RPO/LPO, and 3-field AP/RPO/LPO (30). The AP/PA field arrangement was best for reducing the lung dose although it did not offer any improvement in mean heart dose or heart V20–50 when compared to IMRT. The LPO/RPO technique was best for reducing the heart dose, and the AP/RPO/LPO techniques provided reductions in both lung and heart exposure.
Shiraishi et al. performed a dosimetric comparison amongst three modalities: IMRT, PS-PBT, and PBS-PBT (33). While both PBT techniques offered heart-sparing compared with IMRT, PBS-PBT offered further improvements when compared with PS-PBT, with reductions in the heart V20 Gy, V30 Gy, V40 Gy, and dose received by heart substructures including the left atrium, right atrium, left main coronary artery, and left circumflex artery. Data also suggest reductions in the lung mean dose, lung V5 Gy, and lung V20 Gy with the use of PBS-PBT when compared with PS-PBT (38).
Figures 1 and 2 demonstrate comparison PBS-PBT and IMRT treatment plans (Figure 1) and the associated dose-volume histogram analysis (Figure 2) for a patient with distal esophagus adenocarcinoma treated at Mayo Clinic.
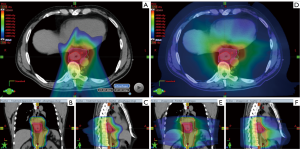
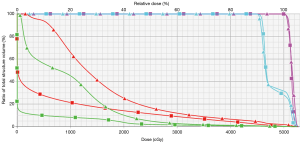
Planning considerations
While PBT offers significant dosimetric advantages over conventional photon-based techniques, there are also a number of treatment planning challenges which must be considered. Assuming homogeneous (i.e., water equivalent) tissue, the depth at which peak energy (dose) is deposited along a single proton track is primarily determined by the proton’s kinetic energy/velocity. The energy deposition is inversely proportional to the square of proton velocity and approximately proportional to the product of Z/A (atomic number to atomic mass ratio) and density of the attenuating medium. Because of this, in the context of a proton beam of given energy interacting with given material, there is a rapid peak of energy deposition, termed the Bragg Peak, just upstream of the beam's useful range. This Bragg Peak phenomenon allows the sparing of tissues both proximal and distal to the target, however, it also produces therapeutic challenges as the dose distribution of the proton beam is much more sensitive to tissue material and density changes (i.e., anatomical changes) than a conventional photon beam.
There are several important challenges in treating tumors within the thorax or upper abdomen (e.g., esophagus tumors), which are discussed in more detail by Tryggestad et al. in this issue of the journal (39). A few pertinent challenges specific for esophageal cancer are the intra-fraction anatomical changes which occur due to periodic tumor motion and the associated “interplay effect” (which is specific to PBS-PBT) (40), diaphragmatic motion, and inter-fraction variation in patient anatomy which can occur in the context of setup variability, weight loss, pleural effusions, stomach distension, or tumor changes.
To overcome these challenges, several planning solutions have been implemented to allow for robust delivery of PBT for esophageal cancer. Patients should undergo 4-dimensional (4D) CT simulation to characterize respiratory motion of the target and adjacent normal structures (29,41). Patient-specific proton beam angles should be chosen to preferentially spare the organ deemed at highest risk of injury (29,30). Also, choosing beam angles which minimize the change in water-equivalent depth or thickness (WET) throughout the respiratory cycle will offer maximum robustness (42-44). Yu et al. have suggested the most motion-robust gantry angles to be posterior, specifically angles between 180° and 220° (44). If free-breathing (i.e., non-breath hold) techniques are used, the target volume should be designed on the 4D dataset and the final plan should be evaluated on the end inspiratory and expiratory phases of the CT data set (19), which validates the plan is robust to (assumed static) WET extremes, ignoring potential periodic motion effects. For PBS-PBT specifically, several techniques have been utilized to minimize dosimetric plan degradation due to motion interplay. Repainting strategies, such as a maximum monitor-unit threshold-based isolayered repainting technique, have been commonly used to better disperse delivered proton spots across the breathing cycle and thereby dampen dosimetric heterogeneity (44-50). Beam-gating strategies have also been used, which can include free-breathing treatment with respiratory, phase- or amplitude-based gating or treatment with image-guided breath-hold (BH) techniques (44). BH for esophagus tumors can be challenging due to the potential of inter-BH variability in diaphragmatic position and shape. Abdominal compression (AC) to reduce diaphragmatic excursion has also been successfully employed in the PBT setting (51). With AC, care must be taken to assure that any WET changes introduced by the device are robustly accommodated during treatment planning.
To summarize, incorporation of motion management techniques for esophageal treatments should facilitate development of PBT plans which are appropriately robust to diaphragmatic motion and target interplay effects. However, motion management comes with costs, e.g., clinicians must anticipate the potential for introduction of additional uncertainties, increased technical and process complexity, and overall practice efficiency decreases associated with extending the treatment delivery time (46). Verification CT scans should be performed periodically during the treatment course to evaluate for inter-fraction anatomic or setup changes.
Early clinical data
Despite having considerable dosimetric data demonstrating potential advantages of PBT in the treatment of esophagus cancer, current clinical data are more limited. Table 2 summarizes the current clinical data evaluating PBT for esophagus cancer (22,33,34,52-63).
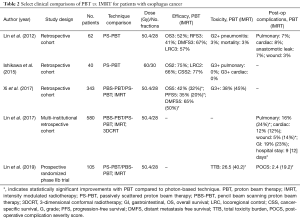
Full table
The University of Tsukuba reported some of the first experiences with PS-PBT for esophageal cancer and a recent publication updates their experience (52,53). They reported on a cohort of 51 patients with predominately locally advanced SCC who were treated with curative-intent mixed photon-proton plans (n=40) or PBT alone (n=6). Patients received an initial photon field including the tumor and elective regions to a median dose of 48 Gy followed by a PS-PBT boost to the gross disease to a median dose of 31.7 Gy (median total dose of 76 Gy) or PS-PBT alone to a median dose of 79 Gy. No patients received concurrent chemotherapy. Five-year OS was 34% and AEs were favorable as no patients required a mid-treatment break and there were no symptomatic late cardiopulmonary AEs, thus establishing early feasibility and efficacy. A more recent series included 40 patients (majority SCC) treated with PS-PBT to a dose of 60 Gy in 30 fractions with concurrent 5FU and cisplatin (55). Two-year OS and LRC were 75% and 66%, respectively. Acute AEs were favorable with grade 3 AEs including hematologic (20%), esophagitis (22%), and dermatitis (5%). No patients experienced grade 3 or higher cardiopulmonary AEs.
The MD Anderson Cancer Center was the first institution in the United States to implement and report outcomes of PS-PBT for esophageal cancer. Lin et al. reported on a cohort of 62 patients who received PS-PBT to a dose of 50.4 Gy in 28 fractions with concurrent chemotherapy (54). Twenty-nine patients (47%) underwent subsequent esophagectomy. Three-year OS and RFS were 52% and 41%, respectively. Late grade 2+ pneumonitis occurred in 3% of patients and post-operative complications included pulmonary (6.5%), cardiac (8%), anastomotic leak (6.5%), and wound complications (3.2%), thus demonstrating feasibility and promising early outcomes for the use of PBT.
Wang et al. examined the potential association between RT modality (PS-PBT vs. photon techniques) and the risk of post-operative complications in cohort of 444 patients with esophageal cancer treated with pre-operative CRT followed by esophagectomy (22). Post-operative complications in the overall cohort included pulmonary (25%), cardiac (15%), and wound (10%). PS-PBT was associated with a lower incidence of pulmonary complications, with the mean lung radiation dose being the strongest predictor of post-operative pulmonary complications.
Lin et al. further investigated the association of RT treatment technique and post-operative complications in a retrospective multi-institutional series of 580 patients with esophageal cancer who received pre-operative 3DCRT (n=214), IMRT [255], or PS-PBT [111] and concurrent chemotherapy followed by esophagectomy (57). Approximately 90% of patients had adenocarcinoma of the distal esophagus or gastroesophageal junction (GEJ) with the majority having stage III/IV (63%) disease. The median RT dose was 50.4 Gy in 28 fractions. The PS-PBT cohort, compared with the IMRT and 3DCRT cohorts, had the lowest post-operative complication rates including pulmonary (16% vs. 24% vs. 40%, P<0.001), cardiac (12% vs. 12% vs. 27%, P<0.001), and wound (5% vs. 14% vs. 15%, P=0.014), respectively. The duration of hospital stay was also shorter for patients receiving PS-PBT (9 vs. 12 vs. 13 days, P<0.001). PS-PBT and IMRT were associated with lower rates of pulmonary and cardiac complications compared to 3DCRT. When compared to IMRT, PS-PBT was associated with a lower incidence in wound complications.
More recently Xi et al. at MD Anderson Cancer Center reported on a cohort of 343 patients with esophageal adenocarcinoma (71%) or SCC (29%) treated with definitive CRT (without surgery) using either PBT (predominately PS-PBT) or IMRT (34). The majority of patients had T3-4 (89%) tumors, lymph node involvement (69%), and tumors located within the distal esophagus or GEJ. Patients were treated to a median dose of 50.4 Gy in 28 fractions with concurrent chemotherapy. No differences were identified in grade 3 or higher AEs between the PBT and IMRT cohorts (38% vs. 45%, P=0.192). On multivariate analysis, PBT was associated with better OS [hazard ratio (HR) 0.688; P=0.01)], PFS (HR 0.640; P=0.001), and locoregional failure-free survival (LRFFS) (HR 0.684; P=0.041), particularly in patients with stage III disease.
Recent data demonstrate the role of host immunity status on cancer control and the detrimental impact of treatment-related lymphopenia on patient outcomes (60,64). Several studies have demonstrated that PBT, compared with photon-based techniques, is associated with a reduction in the risk of treatment-related grade 4 lymphopenia in patients with esophageal cancer. Lymphocytes are exquisitely sensitive to RT; therefore, it has been hypothesized that the observed reduction in lymphopenia with PBT versus photon RT relates to reduction in dose to the total body, heart, lungs, and/or other putative organs at risk. Importantly, Davuluri et al. found that severe, grade 4, lymphopenia was associated with worsened OS, distant metastasis, PFS, and local recurrence for patients with esophagus cancer (60,64). In this series, the factor most strongly associated with grade 4 lymphopenia was the mean total body RT dose, a variable most strongly impacted by RT modality (PBT vs. photon-techniques) (odds ratio 1.22 per Gy, P<0.001).
Garant et al. reported a prospective registry series comparing patient-reported quality of life (QoL) for patients with esophagus cancer treated with PBS-PBT vs. photon-based CRT (61). A majority of patients had adenocarcinoma (~75%) and 60% were treated with pre-operative CRT followed by esophagectomy. PBS-PBT was most commonly delivered with 2 posterior-oblique fields, to a dose of 50–50.4 Gy in 25–28 fractions. QoL was assessed by the Functional Assessment of Cancer Therapy-Esophageal (FACT-E) questionnaire, which is a validated metric evaluating patient QoL in domains of physical well-being, social and family well-being, emotional well-being, functional well-being, in addition to specific esophageal cancer symptomatology. Baseline QoL was similar between patients treated with photon RT vs. PBT; however, treatment with photon RT, compared with PBS-PBT, was associated with greater detriments in QoL both on univariate and multivariate analysis.
These data support the feasibility, efficacy, and favorable AE profile for curative intent proton-based CRT (34,52,53,55,56,60,62,64). Additionally, they provide hypothesis-generating data suggesting PBT may be associated with improved survival potentially attributable to toxicity reduction, including cardiopulmonary sparing (34,52,53,55,56,62), or through a reduction in severe treatment-related lymphopenia which has been associated with disease recurrence and OS (60,64).
Prospective trials
While retrospective series have demonstrated the feasibility of PBT and have suggested possible improvements in outcomes when compared to photon techniques, prospective evaluation remains necessary. Multiple institutional and cooperative group trials are underway or recently completed, including a phase 2 trial from Loma Linda University (NCT01684904) (“A Phase II Trial of Proton Chemoradiotherapy for Resectable Esophageal or Esophagogastric Junction Cancer”) (65), a phase 1 dose escalation trial from University of Pennsylvania (NCT02213497) (“Dose Escalation of Neoadjuvant Proton Beam Radiotherapy With Concurrent Chemotherapy in Locally Advanced Esophageal Cancer”) (66), and a prospective observational study from Mayo Clinic (NCT02452021) (“Pencil Beam Scanning Proton Radiotherapy for Esophageal Cancer”) (67) which has completed accrual.
Lin et al. recently presented early outcomes of (NCT01512589) (“Phase IIB Randomized Trial of PBT versus IMRT for the Treatment of Esophageal Cancer”) (63). In this trial, patients were randomized 1:1 to receive either PBT (PBS-PBT or PS-PBT) or IMRT to a dose of 50.4 Gy in 28 fractions with concurrent chemotherapy. The Pocock-Simon method was used to adaptively balance cohorts for induction chemotherapy use, potential resectability, stage, histology, and age ≥65. Co-primary endpoints were PFS and total toxicity burden (TTB) within the first 12 months, which is computed as a composite score from 11 serious AEs, and among patients who undergo surgery, post-operative complications. At trial closure in March of 2019 (in anticipation of opening NRG-GI006), 145 patients had been randomized, of whom 105 (61 IMRT and 44 PBT) were evaluable. Of the 29 unevaluable patients in the PBT cohort, 22 (76%) were treated off protocol with IMRT due to insurance denial of PBT. Of the 11 unevaluable in the IMRT cohort, 8 (72%) withdrew consent and were treated with PBT. Cohorts were well balanced and approximately 50% underwent surgery. In the PBT cohort, 90% received PS-PBT. With 1-year follow-up, a greater TTB was observed with IMRT compared with PBT, which exceeded the pre-determined interim stopping boundary. IMRT had a posterior mean TTB 2.3 times higher [40.2 (95% CI: 26.5–55.2) vs. 17.2 (95% CI: 10.5–24.4)] and a mean post-operative complication severity score that was 7 times higher [19.2 (95% CI: 7.6–32.7) vs. 2.4 (95% CI: 0.34–5.02)] than PBT. PFS and OS were similar with PBT vs. IMRT, although further follow-up is needed. These data represent the first prospective randomized data evaluating PBT for patients with esophagus cancer, providing preliminary evidence that dosimetric benefits with PBT may translate into clinically meaningful reductions in AEs.
NRG-GI006 trial (NCT03801876) (“Phase III randomized trial of PBT versus IMRT for the treatment of esophageal cancer”) was opened in March of 2019. The study is planned to enroll 300 patients to further clarify if PBT improves OS and AE rates compared with IMRT (68). Clinicians are urged to support NRG-GI006 by opening and enrolling patients on this study. However, there are a few foreseeable limitations of this trial. For instance, patients thought to derive substantial dosimetric benefits with PBT may not be enrolled on clinical trial due to the perception of a relative lack of equipoise between PBT and IMRT. As demonstrated by Lin et al., insurance denials could limit or bias which patients ultimately receive PBT, while some patients randomized to IMRT may withdraw from the trial due to a desire for PBT (63). As previously discussed, PBS-PBT is superior to PS-PBT in sparing the heart and lung, although both techniques are allowed on the PBT arm of the trial (33,38). Previous studies have demonstrated an association between RT plan quality and oncologic outcomes (69). Given the complexities of PBT treatment planning and delivery, careful central review and quality assurance will be required.
Future directions
Radiotherapy dose escalation was evaluated in Intergroup trial 0123, which compared 50.4 and 64.8 Gy (2D or 3D treatment planning) with concurrent 5FU and cisplatin for patients with unresectable esophagus cancer of predominately SCC histology (13). Dose escalation did not improve OS, and LRC was only 40–50%. Welsh et al. evaluated patterns of progression after definitive CRT and found that of those with local progression, 90% occur within the original gross tumor volume (70). Therefore, it has been suggested that a repeat evaluation of dose-escalation with modern, conformal RT techniques is warranted. Recently, data were reported from a phase I/II trial of definitive CRT with a dose of 50.4 Gy in 28 fractions with a simultaneously integrated boost to the gross disease to 63 Gy (71). Patients were treated with either IMRT (n=39) or PBS-PBT (n=7). The 2-year local recurrence risk was 33% and 2-year OS was 41%, which represented an improvement in local recurrence [HR 0.49; 95% confidence interval (CI), 0.26–0.92; P=0.03] and OS (HR, 0.66; 95% CI, 0.47–0.94; P=0.02) when compared to a matched cohort treated to 50.4 Gy. These benefits were most pronounced for patients with adenocarcinoma. Despite being an underpowered subgroup comparison, there was a suggestion of improved outcomes for those receiving PBS-PBT versus IMRT. Re-evaluation of CRT dose-escalation may be warranted with a study design incorporating the use of PBS-PBT, which may offer the greatest chance of gains in oncologic efficacy and mitigation of toxicity.
Given the anticipated reduction in AEs associated with PBT, systemic treatment intensification may also be a feasible strategy with the goal of improving outcomes. The clinical trial RTOG 1010 is comparing trimodality therapy with or without trastuzumab for patients with Her2 overexpressing esophageal adenocarcinoma (72). If this study demonstrates benefit of anti-Her2 directed therapy, which has known risk of cardiac toxicity, PBT would be an attractive RT option given superior cardiac sparing, compared with photon-based RT.
Several ongoing trials are evaluating the role of immune checkpoint inhibition for esophageal cancer, including in patients with localized disease receiving CRT. While the impact of CRT-related lymphopenia on response rates to immunotherapy remains unclear (73), one could hypothesize that the preservation of lymphocyte counts with PBT may improve the efficacy of immunotherapy. Furthermore, immune checkpoint inhibitors are associated with a risk of immune-mediated pulmonary, cardiac, gastrointestinal, and hepatic toxicities, providing rationale for the use of PBT which better spares these organs compared with photon-based RT.
Conclusions
PBT has demonstrated dosimetric advantages compared with photon-based techniques, which may result in improvements in the therapeutic ratio by reducing treatment-related AEs and possibly improving efficacy. PBT offers opportunity for future investigation including re-evaluation of treatment intensification, incorporation into trials investigating the role of immunotherapy, or with the sole goal of mitigating toxicity. Clinicians should be urged to enroll patients on prospective trials investigating the role of PBT for esophagus cancer, for example, the currently open NRG-GI006 phase III randomized trial.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019;69:7-34. [Crossref] [PubMed]
- NCCN. NCCN Clinical Practice Guidelines in Oncology: Esophageal and Esophagogastric Junction Cancer. Version 1.2019. 2019. Available online: https://www.nccn.org/professionals/physician_gls/pdf/esophageal.pdf
- Jethwa KR, Deng W, Gonuguntla K, et al. Multi-Institutional Evaluation of Curative Intent Chemoradiotherapy for Patients with Clinical T1N0 Esophageal Adenocarcinoma. Int J Radiat Oncol Biol Phys 2019;105:E186-E187. [Crossref]
- Kato H, Sato A, Fukuda H, et al. A phase II trial of chemoradiotherapy for stage I esophageal squamous cell carcinoma: Japan Clinical Oncology Group Study (JCOG9708). Jpn J Clin Oncol 2009;39:638-43. [Crossref] [PubMed]
- Kato K, Igaki H, Ito Y, et al. Parallel-group controlled trial of esophagectomy versus chemoradiotherapy in patients with clinical stage I esophageal carcinoma (JCOG0502). American Society of Clinical Oncology, 2019.
- van Hagen P, Hulshof MC, van Lanschot JJ, et al. Preoperative chemoradiotherapy for esophageal or junctional cancer. N Engl J Med 2012;366:2074-84. [Crossref] [PubMed]
- Stahl M, Walz MK, Stuschke M, et al. Phase III comparison of preoperative chemotherapy compared with chemoradiotherapy in patients with locally advanced adenocarcinoma of the esophagogastric junction. J Clin Oncol 2009;27:851-6. [Crossref] [PubMed]
- Tepper J, Krasna MJ, Niedzwiecki D, et al. Phase III trial of trimodality therapy with cisplatin, fluorouracil, radiotherapy, and surgery compared with surgery alone for esophageal cancer: CALGB 9781. J Clin Oncol 2008;26:1086-92. [Crossref] [PubMed]
- Walsh TN, Noonan N, Hollywood D, et al. A comparison of multimodal therapy and surgery for esophageal adenocarcinoma. N Engl J Med 1996;335:462-7. [Crossref] [PubMed]
- Sjoquist KM, Burmeister BH, Smithers BM, et al. Survival after neoadjuvant chemotherapy or chemoradiotherapy for resectable oesophageal carcinoma: an updated meta-analysis. Lancet Oncol 2011;12:681-92. [Crossref] [PubMed]
- Herskovic A, Martz K, al-Sarraf M, et al. Combined chemotherapy and radiotherapy compared with radiotherapy alone in patients with cancer of the esophagus. N Engl J Med 1992;326:1593-8. [Crossref] [PubMed]
- Suntharalingam M, Winter K, Ilson D, et al. Effect of the Addition of Cetuximab to Paclitaxel, Cisplatin, and Radiation Therapy for Patients With Esophageal Cancer: The NRG Oncology RTOG 0436 Phase 3 Randomized Clinical Trial. JAMA Oncol 2017;3:1520-8. [Crossref] [PubMed]
- Minsky BD, Pajak TF, Ginsberg RJ, et al. INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol 2002;20:1167-74. [Crossref] [PubMed]
- Wu AJ, Bosch WR, Chang DT, et al. Expert Consensus Contouring Guidelines for Intensity Modulated Radiation Therapy in Esophageal and Gastroesophageal Junction Cancer. Int J Radiat Oncol Biol Phys 2015;92:911-20. [Crossref] [PubMed]
- Dawson LA, Kavanagh BD, Paulino AC, et al. Radiation-associated kidney injury. Int J Radiat Oncol Biol Phys 2010;76:S108-15. [Crossref] [PubMed]
- Gagliardi G, Constine LS, Moiseenko V, et al. Radiation dose-volume effects in the heart. Int J Radiat Oncol Biol Phys 2010;76:S77-85. [Crossref] [PubMed]
- Kavanagh BD, Pan CC, Dawson LA, et al. Radiation dose-volume effects in the stomach and small bowel. Int J Radiat Oncol Biol Phys 2010;76:S101-7. [Crossref] [PubMed]
- Marks LB, Bentzen SM, Deasy JO, et al. Radiation dose-volume effects in the lung. Int J Radiat Oncol Biol Phys 2010;76:S70-6. [Crossref] [PubMed]
- Pan CC, Kavanagh BD, Dawson LA, et al. Radiation-associated liver injury. Int J Radiat Oncol Biol Phys 2010;76:S94-100. [Crossref] [PubMed]
- Fukada J, Shigematsu N, Takeuchi H, et al. Symptomatic pericardial effusion after chemoradiation therapy in esophageal cancer patients. Int J Radiat Oncol Biol Phys 2013;87:487-93. [Crossref] [PubMed]
- Tucker SL, Liu HH, Wang S, et al. Dose-volume modeling of the risk of postoperative pulmonary complications among esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;66:754-61. [Crossref] [PubMed]
- Wang J, Wei C, Tucker SL, et al. Predictors of postoperative complications after trimodality therapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2013;86:885-91. [Crossref] [PubMed]
- Wang SL, Liao Z, Vaporciyan AA, et al. Investigation of clinical and dosimetric factors associated with postoperative pulmonary complications in esophageal cancer patients treated with concurrent chemoradiotherapy followed by surgery. Int J Radiat Oncol Biol Phys 2006;64:692-9. [Crossref] [PubMed]
- Wei X, Liu HH, Tucker SL, et al. Risk factors for pericardial effusion in inoperable esophageal cancer patients treated with definitive chemoradiation therapy. Int J Radiat Oncol Biol Phys 2008;70:707-14. [Crossref] [PubMed]
- Bradley JD, Paulus R, Komaki R, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol 2015;16:187-99. [Crossref] [PubMed]
- Darby SC, Ewertz M, McGale P, et al. Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 2013;368:987-98. [Crossref] [PubMed]
- Chandra A, Guerrero TM, Liu HH, et al. Feasibility of using intensity-modulated radiotherapy to improve lung sparing in treatment planning for distal esophageal cancer. Radiother Oncol 2005;77:247-53. [Crossref] [PubMed]
- Lin SH, Wang L, Myles B, et al. Propensity score-based comparison of long-term outcomes with 3-dimensional conformal radiotherapy vs intensity-modulated radiotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;84:1078-85. [Crossref] [PubMed]
- Zhang X, Zhao KL, Guerrero TM, et al. Four-dimensional computed tomography-based treatment planning for intensity-modulated radiation therapy and proton therapy for distal esophageal cancer. Int J Radiat Oncol Biol Phys 2008;72:278-87. [Crossref] [PubMed]
- Welsh J, Gomez D, Palmer MB, et al. Intensity-modulated proton therapy further reduces normal tissue exposure during definitive therapy for locally advanced distal esophageal tumors: a dosimetric study. Int J Radiat Oncol Biol Phys 2011;81:1336-42. [Crossref] [PubMed]
- Makishima H, Ishikawa H, Terunuma T, et al. Comparison of adverse effects of proton and X-ray chemoradiotherapy for esophageal cancer using an adaptive dose-volume histogram analysis. J Radiat Res 2015;56:568-76. [Crossref] [PubMed]
- Warren S, Partridge M, Bolsi A, et al. An Analysis of Plan Robustness for Esophageal Tumors: Comparing Volumetric Modulated Arc Therapy Plans and Spot Scanning Proton Planning. Int J Radiat Oncol Biol Phys 2016;95:199-207. [Crossref] [PubMed]
- Shiraishi Y, Xu C, Yang J, et al. Dosimetric comparison to the heart and cardiac substructure in a large cohort of esophageal cancer patients treated with proton beam therapy or Intensity-modulated radiation therapy. Radiother Oncol 2017;125:48-54. [Crossref] [PubMed]
- Xi M, Xu C, Liao Z, et al. Comparative Outcomes After Definitive Chemoradiotherapy Using Proton Beam Therapy Versus Intensity Modulated Radiation Therapy for Esophageal Cancer: A Retrospective, Single-Institutional Analysis. Int J Radiat Oncol Biol Phys 2017;99:667-76. [Crossref] [PubMed]
- Macomber MW, Bowen SR, Gopan O, et al. Heart Dose and Outcomes in Radiation Treatment for Esophageal Cancer. Cureus 2018;10:e2378. [PubMed]
- Hirano Y, Onozawa M, Hojo H, et al. Dosimetric comparison between proton beam therapy and photon radiation therapy for locally advanced esophageal squamous cell carcinoma. Radiat Oncol 2018;13:23. [Crossref] [PubMed]
- Liu C, Bhangoo RS, Sio TT, et al. Dosimetric comparison of distal esophageal carcinoma plans for patients treated with small-spot intensity-modulated proton versus volumetric-modulated arc therapies. J Appl Clin Med Phys 2019;20:15-27. [PubMed]
- Chuong MD, Hallemeier CL, Jabbour SK, et al. Improving Outcomes for Esophageal Cancer using Proton Beam Therapy. Int J Radiat Oncol Biol Phys 2016;95:488-97. [Crossref] [PubMed]
- Tryggestad EJ, Liu W, Pepin MD, et al. Managing treatment-related uncertainties in proton beam radiotherapy for gastrointestinal cancers. J Gastrointest Oncol 2020;11:212-24. [Crossref]
- Bert C, Grozinger SO, Rietzel E. Quantification of interplay effects of scanned particle beams and moving targets. Phys Med Biol 2008;53:2253-65. [Crossref] [PubMed]
- Yaremko BP, Guerrero TM, McAleer MF, et al. Determination of respiratory motion for distal esophagus cancer using four-dimensional computed tomography. Int J Radiat Oncol Biol Phys 2008;70:145-53. [Crossref] [PubMed]
- Mori S, Chen GT. Quantification and visualization of charged particle range variations. Int J Radiat Oncol Biol Phys 2008;72:268-77. [Crossref] [PubMed]
- Mori S, Wolfgang J, Lu HM, et al. Quantitative assessment of range fluctuations in charged particle lung irradiation. Int J Radiat Oncol Biol Phys 2008;70:253-61. [Crossref] [PubMed]
- Yu J, Zhang X, Liao L, et al. Motion-robust intensity-modulated proton therapy for distal esophageal cancer. Med Phys 2016;43:1111-8. [Crossref] [PubMed]
- Furukawa T, Inaniwa T, Sato S, et al. Moving target irradiation with fast rescanning and gating in particle therapy. Med Phys 2010;37:4874-9. [Crossref] [PubMed]
- Gelover E, Deisher AJ, Herman MG, et al. Clinical implementation of respiratory-gated spot-scanning proton therapy: An efficiency analysis of active motion management. J Appl Clin Med Phys 2019;20:99-108. [Crossref] [PubMed]
- Knopf AC, Hong TS, Lomax A. Scanned proton radiotherapy for mobile targets-the effectiveness of re-scanning in the context of different treatment planning approaches and for different motion characteristics. Phys Med Biol 2011;56:7257-71. [Crossref] [PubMed]
- Mori S, Inaniwa T, Furukawa T, et al. Amplitude-based gated phase-controlled rescanning in carbon-ion scanning beam treatment planning under irregular breathing conditions using lung and liver 4DCTs. J Radiat Res 2014;55:948-58. [Crossref] [PubMed]
- Schatti A, Zakova M, Meer D, et al. Experimental verification of motion mitigation of discrete proton spot scanning by re-scanning. Phys Med Biol 2013;58:8555-72. [Crossref] [PubMed]
- Seco J, Robertson D, Trofimov A, et al. Breathing interplay effects during proton beam scanning: simulation and statistical analysis. Phys Med Biol 2009;54:N283-94.
- Lin L, Souris K, Kang M, et al. Evaluation of motion mitigation using abdominal compression in the clinical implementation of pencil beam scanning proton therapy of liver tumors. Med Phys 2017;44:703-12. [Crossref] [PubMed]
- Sugahara S, Tokuuye K, Okumura T, et al. Clinical results of proton beam therapy for cancer of the esophagus. Int J Radiat Oncol Biol Phys 2005;61:76-84. [Crossref] [PubMed]
- Mizumoto M, Sugahara S, Nakayama H, et al. Clinical results of proton-beam therapy for locoregionally advanced esophageal cancer. Strahlenther Onkol 2010;186:482-8. [Crossref] [PubMed]
- Lin SH, Komaki R, Liao Z, et al. Proton beam therapy and concurrent chemotherapy for esophageal cancer. Int J Radiat Oncol Biol Phys 2012;83:e345-51. [Crossref] [PubMed]
- Ishikawa H, Hashimoto T, Moriwaki T, et al. Proton beam therapy combined with concurrent chemotherapy for esophageal cancer. Anticancer Res 2015;35:1757-62. [PubMed]
- Takada A, Nakamura T, Takayama K, et al. Preliminary treatment results of proton beam therapy with chemoradiotherapy for stage I-III esophageal cancer. Cancer Med 2016;5:506-15. [Crossref] [PubMed]
- Lin SH, Merrell KW, Shen J, et al. Multi-institutional analysis of radiation modality use and postoperative outcomes of neoadjuvant chemoradiation for esophageal cancer. Radiother Oncol 2017;123:376-81. [Crossref] [PubMed]
- Lester SC, Lin SH, Chuong M, et al. A Multi-institutional Analysis of Trimodality Therapy for Esophageal Cancer in Elderly Patients. Int J Radiat Oncol Biol Phys 2017;98:820-8. [Crossref] [PubMed]
- Prayongrat A, Xu C, Li H, et al. Clinical outcomes of intensity modulated proton therapy and concurrent chemotherapy in esophageal carcinoma: a single institutional experience. Adv Radiat Oncol 2017;2:301-7. [Crossref] [PubMed]
- Routman DM, Garant A, Lester SC, et al. A Comparison of Grade 4 Lymphopenia With Proton Versus Photon Radiation Therapy for Esophageal Cancer. Adv Radiat Oncol 2019;4:63-9. [Crossref] [PubMed]
- Garant A, Whitaker TJ, Spears GM, et al. A Comparison of Patient-Reported Health-Related Quality of Life During Proton Versus Photon Chemoradiation Therapy for Esophageal Cancer. Pract Radiat Oncol 2019;9:410-7. [Crossref] [PubMed]
- Ono T, Wada H, Ishikawa H, et al. Clinical Results of Proton Beam Therapy for Esophageal Cancer: Multicenter Retrospective Study in Japan. Cancers (Basel) 2019. [Crossref] [PubMed]
- Lin SH, Hobbs B, Thall P, et al. Results of a Phase II Randomized Trial of Proton Beam Therapy vs Intensity Modulated Radiation Therapy in Esophageal Cancer. Int J Radiat Oncol Biol Phys 2019;105:680-1. [Crossref]
- Davuluri R, Jiang W, Fang P, et al. Lymphocyte Nadir and Esophageal Cancer Survival Outcomes After Chemoradiation Therapy. Int J Radiat Oncol Biol Phys 2017;99:128-35. [Crossref] [PubMed]
- ClinicalTrials.gov. A Phase II Trial of Proton Chemoradiotherapy for Resectable Esophageal or Esophagogastric Junction Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT01684904
- ClinicalTrials.gov. Dose Escalation of Neoadjuvant Proton Beam Radiotherapy With Concurrent Chemotherapy in Locally Advanced Esophageal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02213497
- ClinicalTrials.gov. Pencil Beam Scanning Proton Radiotherapy for Esophageal Cancer. Available online: https://clinicaltrials.gov/ct2/show/NCT02452021
- NRG GI-006: Phase III randomized trial of proton beam therapy (PBT) versus intensity modulated photon radiotherapy (IMRT) for the treatment of esophageal cancer. Available online: https://ichgcp.net/clinical-trials-registry/NCT03801876
- Abrams RA, Winter KA, Regine WF, et al. Failure to adhere to protocol specified radiation therapy guidelines was associated with decreased survival in RTOG 9704--a phase III trial of adjuvant chemotherapy and chemoradiotherapy for patients with resected adenocarcinoma of the pancreas. Int J Radiat Oncol Biol Phys 2012;82:809-16. [Crossref] [PubMed]
- Welsh J, Settle SH, Amini A, et al. Failure patterns in patients with esophageal cancer treated with definitive chemoradiation. Cancer 2012;118:2632-40. [Crossref] [PubMed]
- Chen D, Menon H, Verma V, et al. Results of a Phase 1/2 Trial of Chemoradiotherapy With Simultaneous Integrated Boost of Radiotherapy Dose in Unresectable Locally Advanced Esophageal Cancer. JAMA Oncol 2019. [Epub ahead of print]. [Crossref] [PubMed]
- ClinicalTrials.gov. A phase III trial evaluating the addition of trastuzumab to trimodality treatment of HER2-overexpressing esophageal adenocarcinoma. Available online: https://clinicaltrials.gov/ct2/show/NCT01196390
- Diehl A, Yarchoan M, Hopkins A, et al. Relationships between lymphocyte counts and treatment-related toxicities and clinical responses in patients with solid tumors treated with PD-1 checkpoint inhibitors. Oncotarget 2017;8:114268-80. [Crossref] [PubMed]


