Bidirectional chemotherapy in patients with gastric cancer and peritoneal metastasis
Introduction
Gastric cancer remains a global health burden with nearly 1 million new diagnoses annually. Of those diagnosed nearly three-quarters will die of their disease (1). As with most solid organ tumors surgical resection offers the greatest chance for cure, yet overall survival remains dismal with only 33% alive at 5 years (2). A major barrier to curative resection for gastric cancer is the propensity for early peritoneal dissemination. An estimated 25% of patients with gastric cancer are not eligible for surgery because of metastatic disease at diagnosis (3). Surgical intervention in the setting of metastatic disease remains investigational and median survival estimates approximate 12 months with best available therapy.
Peritoneal carcinomatosis is found in 10–20% of cases in which curative gastrectomy is intended and is the primary site of recurrence in up to 50% of patients with gastric adenocarcinoma (4). In order to address the frequent problem of peritoneal metastasis in gastric cancer, cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) have been evaluated in a variety of studies attempting to establish this therapy as a viable treatment strategy. In studies where regional therapy was associated with improved survival it was generally in patients with a low disease burden and when complete cytoreduction was achieved (5). Given these data, for many patients in which peritoneal disease burden is too great and attempts at cytoreduction are most likely incomplete, systemic chemotherapy remains front line treatment.
Patients with advanced gastric cancer are often treated with a systemic chemotherapy regimen consisting of a fluoropyrimidine and a platinum agent. Dual agent therapy marginally improves survival when compared to single agent therapy (3). Thus, in patients with good performance status and limited comorbidities, three-drug regimens that include a taxane have demonstrated increased time to progression and an improvement in overall survival (6).
Unfortunately, modest improvements in overall survival are often countered by increased toxicity. When gastric cancer progresses after first line therapy, effective second line treatment options are limited, with none resulting in a median progression free survival (PFS) more than five months (7).
A unique subset of patients with gastric adenocarcinoma exhibit metastases limited to the peritoneum in the absence of solid organ metastases. Given the diminished value of second line systemic therapy, and the limited efficacy of cytoreductive surgery and HIPEC, patients with carcinomatosis require palliative treatment options with limited toxicity. One such therapy shown to be effective in other peritoneal surface malignancies is the administration of intraperitoneal chemotherapy in combination with systemic therapy, also referred to as bidirectional chemotherapy (8). For this therapy taxanes represent ideal agents given their high molecular weight and hydrophobic properties that allow for prolonged intraperitoneal retention (9). The long dwell times promote direct penetration of the tumor by the agent and associated microvascular destruction. Repeated dosing through a peritoneal access port is thought to increase the depth of tumor penetration, which is necessary to maximize the efficacy of the drug (9). Previous studies using intraperitoneal taxanes have shown good tolerance and limited systemic adverse events, even with concomitant intravenous administration (8).
Our study aim is to use intraperitoneal taxane therapy as an adjunct to systemic chemotherapy in the treatment of patients with unresectable, peritoneal-only, metastatic gastric cancer. We hypothesize that the combination of systemic and regional therapy will result in greater tumor control and improved PFS.
Methods
A clinical trial of intraperitoneal paclitaxel in combination with intravenous paclitaxel and oral capecitabine was approved by the Institutional Review Board of the National Cancer Institute, National Institutes of Health (NIH), Bethesda, Maryland, USA (NCT04034251).
Design
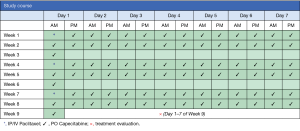
This is a single institution, phase II study to determine the efficacy of intraperitoneal paclitaxel in combination with intravenous paclitaxel and oral capecitabine in patients with gastric cancer and advanced peritoneal metastasis (Figure 1).
Eligibility
Patients must have histologically or cytologically confirmed gastric adenocarcinoma with peritoneal only metastases. Patient must be physically able to undergo laparoscopy with subcutaneous port placement and have adequate renal, hematologic, and liver function.
- Histologically or cytologically confirmed gastric adenocarcinoma, including Siewert III gastroesophageal junction adenocarcinoma;
- Radiographic evidence of peritoneal carcinomatosis and/or subradiographic evidence of peritoneal carcinomatosis found at staging laparoscopy;
- Patients may be treatment naïve or have received systemic chemotherapy prior to enrollment;
- Age >18 years;
- Performance status (ECOG) <2;
- Normal organ and marrow function;
- Physiologically able to undergo laparoscopy and systemic chemotherapy.
Exclusion
Patients with disseminated extra-peritoneal or solid organ metastasis are ineligible for this study. In addition, patients who have had progression of disease while receiving paclitaxel or have received any other regional therapy are not eligible to participate.
Intervention
Imaging studies for accurate staging will be completed and, after confirmation of eligibility, patients will be enrolled on the treatment protocol. Patients will undergo initial laparoscopy to evaluate the extent of peritoneal carcinomatosis and assigned a peritoneal cancer index (PCI) score. During the procedure a titanium implantable port with peritoneal catheter (BardPort®, Bard Access System, Salt Lake City, UT, USA) will be placed. Within post-operative days 1–3, as dictated by clinical status, patients will begin intraperitoneal paclitaxel and intravenous paclitaxel (day 1) followed by oral capecitabine. Patients will receive capecitabine twice-daily for 14 days, followed by a 7-day treatment free interval, in each 3-week cycle (Figure 1). One course will comprise 3 cycles. Treatment evaluation will take place after 3 cycles of treatment are completed (+/− 7 days) with repeat imaging (CT and/or PET) and laparoscopy to assess for treatment response. Patients with an objective response or stable disease (detailed below) will continue to a subsequent course of treatment. Patients with progressive disease will be taken off study. Patients may also elect to stop protocol-related therapy at any time.
Definition of response
An objective response will be declared if a patient has a reduction in PCI of greater than or equal to 4 points from baseline as determined by laparoscopy, histopathologic evidence of tumor treatment effect in re-staging laparoscopy biopsy, and/or resolution of small volume ascites. Stable disease is defined as absence of new intra- or extra-peritoneal disease or a PCI within 2 points from original baseline measurement. Progressive disease is defined as PCI increase greater than 4 points from baseline, new ascites or malignant bowel obstruction, or new extra-peritoneal disease. Standard response evaluation criteria in solid tumors (RECIST) will be used in instances where measurable disease is present on imaging.
Study endpoints and correlative studies
The primary endpoint is to determine PFS in patients with peritoneal metastases from gastric cancer after repeated intraperitoneal and systemic paclitaxel administration with concurrent oral capecitabine therapy. Secondary endpoints include intraperitoneal PFS, extra-peritoneal disease-free survival, and frequency of objective histopathologic response. In addition, we will describe the morbidity associated with this treatment strategy and determine overall survival. We will study peritoneal metastasis through basic research utilizing a novel ex vivo human tissue platform for observation of peritoneal metastasis, real-time cellular imaging, and manipulation of tumor-microenvironment interactions. We intend to correlate response to therapy using proteogenomic subtyping of primary tumor and metastases. Moreover, we will explore whether tumor responsiveness to paclitaxel in ex vivo tumor tissue models is associated with clinical treatment response in patients.
Statistical considerations
The goal is to determine if the IV/IP and oral treatment described could be associated with a 9-month median PFS compared to a maximum 5 months median PFS based on historical controls (10). Given that patients may have received prior therapy, there will be two arms of the study: patients who are treatment naïve and patients who have received prior therapy. In each of these two cohorts, the target accrual of 32 patients provides 80% power to detect a 4-month improvement in median PFS with a one-sided 0.10 alpha level test.
Conclusions
Systemic therapy for metastatic gastric cancer remains largely ineffective. Although studies have demonstrated potential clinical benefit with bidirectional chemotherapy, this technique has not been evaluated prospectively in North America. We believe intraperitoneal chemotherapy administration in combination with systemic therapy may delay progression of gastric carcinomatosis. In addition, our translational and basic science efforts have the potential to expand our knowledge of gastric cancer metastasis using novel tumor model systems. Gastric cancer with peritoneal carcinomatosis is a rare and deadly disease in the United States, therefore patients and physicians should consider enrollment in a clinical trial.
Acknowledgments
The study is supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394-424. [Crossref] [PubMed]
- Cunningham SC, Kamangar F, Kim MP, et al. Survival after gastric adenocarcinoma resection: eighteen-year experience at a single institution. J Gastrointest Surg 2005;9:718-25. [Crossref] [PubMed]
- Wagner AD, Syn NL, Moehler M, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2017;8:CD004064. [PubMed]
- Montori G, Coccolini F, Ceresoli M, et al. The treatment of peritoneal carcinomatosis in advanced gastric cancer: state of the art. Int J Surg Oncol 2014;2014:912418.
- Rudloff U, Langan RC, Mullinax JE, et al. Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 2014;110:275-84. [Crossref] [PubMed]
- Van Cutsem E, Moiseyenko VM, Tjulandin S, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol 2006;24:4991-7. [Crossref] [PubMed]
- Mahipal A, Choi M, Kim R. Second-Line Treatment of Advanced Gastric Cancer: Where Do We Stand? J Natl Compr Canc Netw 2015;13:1281-91. [Crossref] [PubMed]
- Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal Cisplatin and Paclitaxel in Ovarian Cancer. N Engl J Med 2006;354:34-43. [Crossref] [PubMed]
- Yamaguchi H, Kitayama J, Ishigami H, et al. Breakthrough therapy for peritoneal carcinomatosis of gastric cancer: Intraperitoneal chemotherapy with taxanes. World J Gastrointest Oncol 2015;7:285-91. [Crossref] [PubMed]
- Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol 2009;20:666-73. [Crossref] [PubMed]