
Telomerase activity in the differential diagnosis of pancreatic mass
Introduction
Currently, there are a large number of tumor markers specific to different types of malignant tumors (1).
The highly specific tumor markers of pancreatic malignancies indicate the presence of telomerase activity in the studied cells. Samples of these malignancies are obtained via puncture under the ultrasound control (2,3). Telomerase is an enzyme that consists of an RNA component and a protein part capable of increasing the length of telomeric DNA sequences.
In 1971, Olovnikov first described the specialized enzyme system that controls and maintains the telomere length of DNA and proposed the hypothesis of marginotomy (4). Greider and Blackburn identified telomerase within a protozoan Tetrahymena culture (5).
Cancer cells divide uncontrollably and continuously. The activation of telomerase in them leads to the stabilization of telomere length that gives the tumor cell an unlimited replicative potential, as well as the ability to grow unrestrained. This process is the basis for the diagnosis of malignant tumors (6).
Difficulties of differential diagnosis of pancreatic formations and choice of treatment tactics determine the surgical treatment required. The early detection of telomerase activity in tumor tissues obtained by puncture under ultrasound control is necessary (7).
The purpose of the research is to determine the activity of telomerase (AT) in the tumor tissues of the pancreas to improve the differential diagnosis of pancreatic malignancies.
Methods
Eighty-eight patients with pancreatic mass were observed from 2015 to 2019 at the Department of Surgery No.1 of University Clinical Hospital No.1 of the Sechenov University. The study involved 38 men and 50 women. The mean age of the patients was 53 (±7) years.
All 88 patients underwent ultrasound examination and computed tomography (CT) of the abdominal organs, determination of the concentration of CA 19-9 tumor marker in blood plasma, percutaneous puncture of the pancreatic tumor under ultrasound control, followed by the histological examination of the obtained material. In addition, telomerase activity was detected in the tumor tissue obtained by biopsy. The puncture was performed with a biopsy needle QuickCore 18 G, 20 cm. Abdominal ultrasounds were performed on an AcusonSequoia 512 apparatus (USA) according to the standard procedure. Computed tomography with intravenous contrast was performed on a ToshibaAquilonOne 320 (Japan).
Tissue samples obtained by puncture biopsy were studied to detect the activity of telomerase (AT) telomeric repeat amplification protocol (TRAP) with non-isotopic detection of the reaction products. The samples were then painted with a gel with SYBR Gold dye (Molecular Probes, USA), visualized on a UV transilluminator (X =300 nm), and digital images were produced using a Kodak DC-200 camera (8,9). While assessing the ‘positive-negative’ grade (qualitative assessment), the presence or absence of a specific ladder of TRAP products in the gel was determined. The result was deemed positive if several bands were clearly defined in the gel. The result was considered negative if only the first band was visible or there were gaps on a course of the bands (nonspecific pattern), or in the case of the complete absence of reaction products (10). The semi-quantitative assessment of AT was also performed, followed by the measurement of protein in the cell extract К562. High telomerase activity (+++) corresponded to the amount of luminescence of the extract of 0.04 µg protein К562. At the average activity of the enzyme (++), the luminescence level was between 0.04 and 0.004 µg of protein extract. The level of luminescence corresponding to the extract with less than 0.004 µg of protein K562 has been taken as low AT (+).
All patients were divided into two groups in accordance with laboratory and instrumental methods (ultrasound, computed tomography, and determination of the concentration of CA 19-9 tumor marker in blood plasma) (Table 1).

Full table
The first group of 20 out of the 88 patients had diagnosed signs of pancreatic cancer and elevated plasma levels of the CA 19-9 tumor marker (normal value is up to 0.35 u/mL), according to an ultrasound and CT scan. The second group of 68 patients (out of 88) had no clear signs confirming the presence of cancer, according to an ultrasound and CT scan, CA 19-9 corresponded to the norm.
The puncture of the pancreatic tumors under ultrasound control allowed the differentiation of malignant cells and the determination of telomerase activity. The results of the histological studies of the pancreatic mass tissues and the detection of telomerase activity within determined the necessity to separate the 68 patients of the second group into three smaller groups. Thirty-two patients of the first group were diagnosed with cancer. Twenty-two patients of the second group had no evidence of pancreatic cancer. However, their telomerase activity was moderate. Fourteen patients of the third group had no malignant cells, and telomerase activity was low.
This study has been conducted in accordance with the conforms to the provisions of in accordance with the Helsinki Declaration as revised in 2013. This study was approved by the Ethics Committee of the University Clinical Hospital No.1 of the Sechenov University. Each patient gave written informed consent.
Nonparametric methods were used to analyze the statistical significance of the differences between the groups as well as features in the groups, taking into consideration the number of objects for each feature. The methods used include the Mann-Whitney and Chi-square (χ2) tests with Yates correction, and Fisher’s exact criterion. Differences were considered statistically significant at P<0.05.
Results
There is no significant difference between ultrasound and computed tomography in determining whether a tumor is malignant or benign (P≥0.05). According to the CT scans and ultrasounds, tumors were localized in the head and body of the pancreas in 13 out of 20 patients in the first group, and others had tumors in the tail of the pancreas. The masses had indistinct torose contours and heterogeneous contents with signs of high and low densities.
The tumors of all patients had pathological vascularization. Twelve out of the 20 patients had a tumor spread to the upper mesenteric artery. Two patients had the tumor spread to the stomach wall. The values of the CA 19-9 tumor marker in blood plasma increased in all patients of the first group. The histological analysis of the pancreatic mass punctures has shown that 6 out of the 20 patients had adenosquamous carcinoma, 9 had ductal adenocarcinoma, 2 had low-differentiated adenocarcinoma, and 3 had high-grade differentiated adenocarcinoma. The telomerase activity of these 20 patients was high (+++), (P<0.05). Six out of the 20 patients underwent major surgery. The other 14 patients had an unresectable tumor and underwent systematic chemotherapy at their place of residence. Thereafter, all patients underwent case follow-ups.
The results of the instrumental research of the patients in the second group were not indicative of a malignant process in the pancreas. The mass of 54 out of the 68 patients had indistinct contours and heterogeneous structures. The remaining 14 patients had clear contours of the mass and the homogeneous structure, according to ultrasound and CT data. The tumors sprouted into the celiac trunk in 24 out of the 68 patients. Evidence of CDK tumors was revealed in 17 out of the 68 patients. Thirty-seven out of the 68 patients had a partial peripheral contrast, whereas the remaining 14 patients had no signs of such. The mass was localized in the head of the pancreas in 19 out of 68 cases, in the body in 29 cases, and in the tail area of the pancreas in 20 cases. Sixteen patients with tumors in the head of the pancreas were diagnosed with extension of Wirsung and the common bile duct without clinical signs of acute pancreatitis. These patients were jaundiced (icteric staining of the skin and sclera, darkening of urine and lightening of the stool).
According to the histological study of pancreatic punctures and the determination of telomerase activity within, patients of the second group were divided into three subgroups. The first subgroup included 32 patients; the second included 22 patients, and the third included 14 patients (Table 2).
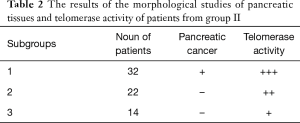
Full table
The first subgroup was diagnosed with a high-grade differentiated adenocarcinoma in once patient, a signet ring cell carcinoma in two patients moderately differentiated adenocarcinoma in 13 patients, and low-differentiated adenocarcinoma in 16 patients. High (+++) telomerase activity in all patients of the first subgroup corresponded to the malignant process (P<0.05). Analysis of the data of the first subgroup revealed the malignant nature of pancreatic tumors, and eight of the 32 patients underwent major surgery. The tumors sprouted into the celiac trunk in the remaining 24 patients, which impeded major surgery. For further treatment, inoperable patients were referred to an oncologist at their place of residence.
Patients of the second group had neither clear instrumental nor histological evidence of the malignant process, though telomerase activity was average (++). Therefore, these patients required dynamic observation and repeated biopsies with histological research of the punctures and the determination of telomerase activity. All 22 patients were diagnosed with an elevated level of telomerase activity from average (++) to high (+++) over six months (P<0.05), while 15 out of the 22 patients had histological examinations that revealed high-grade differentiated adenocarcinoma. Four patients had signet ring cell carcinoma, and three had moderately differentiated adenocarcinoma, according to the results of the histological study. All patients required surgery. Patients of the third subgroup did not have evidence for the malignant process, and telomerase activity was low (+), according to instrumental and histological studies (P<0.05). No negative dynamics were found from the dynamic survey over six and twelve months. CT data, ultrasound data, normal values of the CA 19-9 tumor marker, and the absence of increased telomerase activity testified to the benign nature of the disease. In the course of the histological research of the punctures, ten out of 14 patients were diagnosed with pseudo-tumor-like pancreatitis and four with benign tumors of the pancreas. Every patient received conservative therapy and was examined by gastroenterologists. All underwent follow-ups.
Discussion
Telomerase activity is a component of the cell that favors its unlimited proliferation. This component is known to be absent in a normal cell for which the number of divisions is limited (11). Most tumor markers are important only in combination with other methods that confirm the presence of cancer, as their levels can increase in other non-cancer diseases (12).
Currently, scientists have found that telomerase activity increases in the tumor tissues of 90% of patients suffering from stomach, prostate, and thyroid gland cancer (13-15).
High telomerase activity (+++) in the tumor mass tissues of the pancreas accurately determined the presence of cancer. One-hundred-per-cent of patients with pancreatic cancer had high telomerase activity (+++), which was confirmed histologically (P<0.05). The data obtained are consistent with the results of Nakashima et al., 2009 and Uehara et al., 1999 and 2008, who showed that 11 out of 13 patients with pancreatic adenocarcinoma displayed increased telomerase activity in their pancreatic juices (16-18). Telomerase activity was observed by Inoue et al., 2001 in 88% of patients suffering from mucinous pancreatic cysts; 22% of them had cystadenocarcinoma of the pancreas, as confirmed anatomically. The sensitivity and specificity of this method are 92% and 88%, respectively (19).
The average telomerase activity (++) in pancreatic tumors gives an early indication of pancreatic cancer and requires dynamic observation for six to 12 months (P<0.05). Low (+) or negative telomerase activity is a sign of the benign nature of the disease.
Similar results were obtained by Firsova et al., 2016, who showed the absence of telomerase activity in acute pancreatitis (20).
Conclusions
The comparison of laboratory instrumental methods of research with the detection of telomerase activity in pancreatic masses allows the determination of the malignant process at an early stage, allowing the performance of major surgery promptly. The identification of the average degree of telomerase activity in a pancreatic mass requires a dynamic laboratory and instrumental examination of the patient. This method will improve the quality of diagnosis and treatment for patients with pancreatic tumors.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Ethics Committee of the University Clinical Hospital No.1 of the Sechenov University (05-09). Each patient gave written informed consent.
References
- Cummins G, Yung DE, Cox BF, et al. Luminally expressed gastrointestinal biomarkers. Expert Rev Gastroenterol Hepatol 2017;11:1119-34. [Crossref] [PubMed]
- Chernousov AF, Musayev GKh, Khorobrikh TV, et al. Telomerasa as the universal marker of malignans of the pancreas. Her Surg Gastr 2011;1:4-9.
- Takihana Y, Tsuchida T, Fukasawa M, et al. Real-time quantitative analysis for human telomerase reverse transcriptase mRNA and human telomerase RNA component mRNA expressions as markers for clinicopathologic parameters in urinary bladder cancer. Int J Urol 2006;13:401-8. [Crossref] [PubMed]
- Olovnikov AM. A theory of marginotomy. The incomplete copying of template margin in enzymic synthesis of polynucleotides and biological significance of the phenomenon. J Theor Biol 1973;41:181-90. [Crossref] [PubMed]
- Greider CW, Blackburn EH. Identification of a specific telomere terminal transferase activity in Tetrahymena extracts. Cell 1985;43:405-13. [Crossref] [PubMed]
- Kelland L. Targeting the limitless replicative potential of cancer: the telomerase/telomere pathway. Clin Cancer Res 2007;13:4960-3. [Crossref] [PubMed]
- Barvanyan GM. Improvement of Surgery of Pancreatic Head Masses. Sovrem Tehnol Med 2017;9:155-61. [Crossref]
- Glukhov AI, Zimnik OV, Gordeev SA, et al. Inhibition of telomerase activity of melanoma cells in vitro by antisense oligonucleotides. Biochem Biophys Res Commun 1998;248:368-71. [Crossref] [PubMed]
- Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science 1994;266:2011-5. [Crossref] [PubMed]
- Nicol WK, Monaghan AJ. Detection of Telomerase Enzyme Activity by TRAP Assay. In: Roulston JE, Bartlett JMS. editors. Molecular Diagnosis of Cancer. Totowa: Humana Press, 2004:311-22.
- Saldanha SN, Andrews LG, Tollefsbol TO. Analysis of telomerase activity and detection of its catalytic subunit, hTERT. Anal Biochem 2003;315:1-21. [Crossref] [PubMed]
- Erlykina EI, Obukhova LM, Alyasova AV, et al. Elemental homeostasis of blood plasma in malignant tumors of blood plasma in malignant tumors of epithelial tissues. Trace Elem Med (Moscow) 2015;16:28-35. [Crossref]
- Svinareva LV, Glukhov AI, Zimnik OV, et al. The study of telomerase activity in gastric cancer. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry 2011;5:188-92.
- Vicentini C, Gravina GL, Angelucci A, et al. Detection of telomerase activity in prostate massage samples improves differentiating prostate cancer from benign prostatic hyperplasia. J Cancer Res Clin Oncol 2004;130:217-21. [Crossref] [PubMed]
- Meid FH, Gygi CM, Leisinger HJ, et al. The use of telomerase activity for the detection of prostatic cancer cells after prostatic massage. J Urol 2001;165:1802-5. [Crossref] [PubMed]
- Nakashima A, Murakami Y, Uemura K, et al. Usefulness of human telomerase reverse transcriptase in pancreatic juice as a biomarker of pancreatic malignancy. Pancreas 2009;38:527-33. [Crossref] [PubMed]
- Uehara H, Nakaizumi A, Tatsuta M, et al. Diagnosis of pancreatic cancer by detecting telomerase activity in pancreatic juice: comparison with K-ras mutations. Am J Gastroenterol 1999;94:2513-8. [Crossref] [PubMed]
- Uehara H, Nakaizumi A, Iishi H, et al. In situ telomerase activity in pancreatic juice may discriminate pancreatic cancer from other pancreatic diseases. Pancreas 2008;36:236-40. [Crossref] [PubMed]
- Inoue H, Tsuchida A, Kawasaki Y, et al. Preoperative diagnosis of intraductal papillary-mucinous tumors of the pancreas with attention to telomerase activity. Cancer 2001;91:35-41. [Crossref] [PubMed]
- Firsova VG, Parshikov VV, Gradusov VP. Tactical Issues of Treatment of Fluid Collections According to the Revised Atlanta Classification of Local Complications of Acute Pancreatitis. J Exp Clin Surg 2016;9:114-23. [Crossref]


