Trends of incidence and survival in patients with gastroenteropancreatic signet ring cell carcinoma: an analysis from the Surveillance, Epidemiology, and End Results program
Introduction
Signet ring cell carcinoma (SRC) is a rare and distinct entity of adenocarcinoma whose morphological appearance is characterized by abundant intracytoplasmic mucin pushing nucleus to the periphery at least 50% of the tumor volume according to World Health Organization (WHO) (1). Generally, biological behavior of SRC has been considered more aggressively than other histological subtypes of adenocarcinoma (2-4). However, different primary site of SRC showed inconsistent clinical features and prognosis (4-7). The comprehensive understanding of SRC in gastrointestinal tract and pancreas is not well illustrated, particular for site-specific differences in incidence, prevalence and long-term survival. There are still uncertainties if the prognostic impact of different anatomic sites on clinical features and survival outcomes (8,9). Therefore, it is of great importance to clarify the distribution and prognosis of SRC in different sites of gastrointestinal tract and pancreas in a large population.
A retrospective site-stratified analysis was performed using the Surveillance, Epidemiology and End Results (SEER) database included only primary gastroenteropancreatic SRC patients who were diagnosed between 2000 and 2014. The purpose of our study was to investigate the impact of different primary tumor sites on clinical features and median overall survival (OS) outcomes in patients with gastroenteropancreatic SRC. Finding in this large population-based study may help to provide a comprehensive epidemiologic picture on the prevalence and prognosis of gastroenteropancreatic SRC.
Methods
Data collection
The National Cancer Institute’s SEER database was investigated in this study; this database was released in November 2016 and included cancer registries covering 28% of the US population. The SEER Quality Improvement Programme established strict quality control of cancer registries and maintained them through continual monitoring, assessment, and education. The data released by the SEER database do not require informed patient consent since cancer was a reportable disease in the United States. We obtained the permission to access the SEER database with the ID number 10947-Nov2016 via Internet access method. Cases of invasive gastroenteropancreatic (esophagus, stomach, small intestine, appendix, colon, rectum and pancreas) SRC [International Classification of Diseases for Oncology (ICD-O)-3 8,490/3] reported to the SEER program from January 2000 to December 2014 were included in this study.
Classification of gastroenteropancreatic SRC
According to version 3 of the ICD-O, the pathological characteristics included grade (Grade I: well-differentiated; Grade II: moderately differentiated; Grade III: poorly differentiated; Grade IV: undifferentiated or anaplastic). Due to the number was limited; Grade I and Grade II were combined into just one category for the analyses.
Statistical analysis
The differences in continuous data were detected by One-way ANOVA with the Student-Newman-Keuls post hoc test. The differences in categorical data were compared by the Chi-square test. The annual incidence rates, which were age adjusted to the 1970 standard million US population, expressed as cases per 1,000,000 persons. The incidences and OS rates in the period of 2000–2014 were calculated by SEER 18 databases to maximize the representativeness of this study. The time of follow-up for all analyses was from the deadline of study, date of diagnosis until death, or date of last contact.
Multivariable survival analyses of the SEER 18 database were conducted to evaluate the most recent trends in survival from January 2000 to December 2014. The total SEER 18 gastroenteropancreatic SRC cohort, which comprised all patients with gastroenteropancreatic SRC in SEER 18 were identified for multivariable survival analyses. Multivariate Cox hazards model were used to evaluate the predictors associated with prognosis of gastroenteropancreatic SRC, including time interval from diagnosis, site, stage, grade, gender, age, and race.
SEER*Stat software, version 8.3.4 (Surveillance Research Program, National Cancer Institute) was used to calculate the incidence (including annual percentage change). After fitting a least squares regression to the natural logarithm of the rates in this software, annual percentage change was calculated. The calendar year was computed as a variable and age-adjusted incidence rates were weighted by proportions of corresponding age groups in the 2000 US standard population. Data analysis was performed using IBM SPSS software for Windows, version 19.0 (IBM Corporation, Armonk, New York, USA). Comparative differences were considered significant at P<0.05.
Results
Demographic characteristics
A total of 24,613 SRC were collected in the SEER program from January 2000 to December 2014, 11,318 (46.0%) were women and 13,295 (54.0%) were men. White patients accounted for 76.2%, whereas 11.7% were Asian/Pacific islander, 10.9% were African American, and 0.7% were American Indian/Alaskan native. The baseline characteristics in this study were showed in Table 1. The mean age at diagnosis of gastroenteropancreatic SRC was 63.9 years. The mean age at diagnosis varied significantly among the tumor types. People diagnosed with appendix SRC were significantly younger (mean age, 58.8 years) than people diagnosed with SRC located in any of the other site (P<0.01 for all comparisons). A higher proportion of SRC located in appendix, colon and rectum were female compared to SRC located in esophagus, stomach, small intestine and pancreas (P<0.01). Irrespective of site, most of the SRC was Grade III/IV tumor. Tumor extension at the time of diagnosis varied by location of disease. Distant metastasis of patients with colorectal SRC was less common than the disease with other locations (P<0.01).
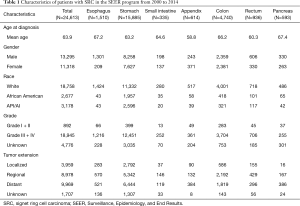
Full table
Annual incidence
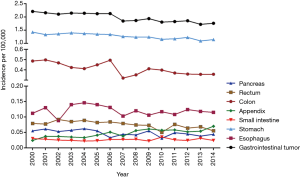
The incidence of gastroenteropancreatic SRC accounted for approximately 3.2–3.4% in all gastroenteropancreatic carcinoma. The proportion varied by tumor location (2.4–2.8% in the esophagus, 15.9–17% in the stomach, 1.1–1.8% in the small intestine, 5.1–6.3% in the appendix, 1.2–1.3% in the colon, 0.6–0.7% in the rectum, and 0.4–0.5% in the pancreas). The annual age-adjusted incidence of gastroenteropancreatic SRC was 2.209 per 100,000 persons in 2000 and dropped to 1.759 per 100,000 persons by 2014 as shown in Figure 1. Age-specific incidence rates were calculated for 3 age groups: 65 years or older, 50 to 64 years, and younger than 50 years. The most dramatic drop in incidence was notable in patients 65 years or older with 32.1% drop to 6.847 per 100,000 persons and, in those 50 to 64 years, to 3.254 per 100,000 persons. The incidence rate in those younger than 50 years remained stable. The annual percentage changed for age-adjusted incidence from 2000 to 2014 in SEER 18 was −1.79 per 100,000 persons (P<0.001).
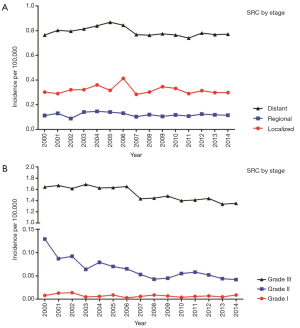
The decrease in the incidence of gastroenteropancreatic signet ring carcinoma in the period of 2000 to 2014 appeared across all stages, grades and all site apart from appendix (rise 2.9 times) and esophagus (remained stable) (Figure 1). The declines in incidence for various sites ranged from 30.4% in the colon to 19.8% in the stomach. In subgroups stratified by stage, all groups showed small decline, the overall trend was not evidence (Figure 2A). Among grade groups, incidence decreased the most in Grade II gastroenteropancreatic SRC, from 0.129 per 100,000 persons in 2000 to 0.042 per 100,000 persons in 2014 (P<0.001) (Figure 2B). In SEER 18 [2000–2014], the highest incidences were 1.273 per 100,000 persons in the stomach, 0.41 per 100,000 persons in the colon, 0.12 per 100,000 persons in the esophagus, 0.075 per 100,000 persons in the rectum, 0.048 per 100,000 persons in the pancreas, 0.047 per 100,000 persons in the appendix, and 0.026 per 100,000 persons in the small intestine (Figure 1).
Survival
The median OS time for all patients was approximately 11.1 months. Patients with localized gastroenteropancreatic SRC showed better median OS (55.6 months) in comparison with regional gastroenteropancreatic SRC (19.4 months) and distant gastroenteropancreatic SRC (5.6 months) (P<0.001). Of those with known grades, the median OS of Grade I/II gastroenteropancreatic SRC was 21.4 months, and Grade III/IV SRC had a worse median OS (12 months) (P<0.001). SRC in the appendix (26.1 months) had the best median OS among site groups, while SRC in the pancreas (3.4 months) had the worst median OS. As a whole, irrespective of site, patients with SRC had poor survival. All of these differences in median OS were significant (P<0.001).
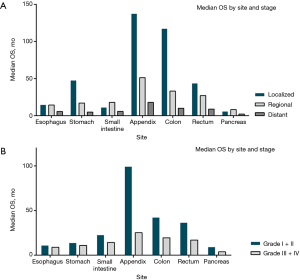
Survival patterns in the localized, regional and distant disease were evaluated according to different site and stage. In localized gastroenteropancreatic SRC, median OS was observed ranged from 5.8 months in the pancreas to 137.4 in the appendix. In regional gastroenteropancreatic SRC, median OS ranged from 8.2 months for SRC in the pancreas to 51.3 months in the appendix. Irrespective of site, patients with distant SRC had poor survival, median OS ranging from 2.1 months in the pancreas to 17.9 months in the appendix. The median OS differences in gastrointestinal tract and pancreas were observed to be significant between them (P<0.001 for log-rank test) (Figure 3A).
Furthermore, median OS were evaluated according to site and grade. Patients with Grade I/II appendix SRC had the longest median OS (99.1 months). Irrespective of site, patients with Grade III/IV SRC showed poor survival, median OS ranging from 3.8 months in the pancreas to 25.2 months in the appendix. All of the median OS differences were observed to be significant between them (P<0.001 for log-rank test) (Figure 3B).
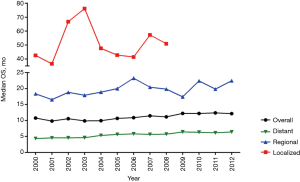
Eventually, the SEER 18 cohort [2000–2012] was focused on to evaluate the latest trends in OS for gastroenteropancreatic SRC. Between 2000–2012, median OS of gastroenteropancreatic SRC was improving slightly (Figure 4). The improvement in survival over the same time intervals was more pronounced in the subgroup with localized and regional SRC. Among grade groups, the improvement in survival over the same time intervals was more pronounced in the subgroup with Grade I/II SRC. The survival of Grade III/IV SRC was not improved significantly.
Multivariable Cox analysis of OS
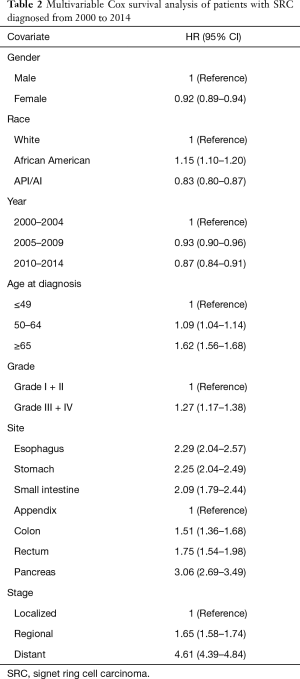
Multivariable Cox analysis was performed with hazard ratios (HRs). We found that patients with Grade III/IV SRC (HR, 1.27; 95% CI, 1.17–1.38) had worse OS than did those with Grade I/II SRC. Age, race, gender, stage, and site were all found to have significant correlation with survival. OS was worse in regional gastroenteropancreatic SRC (HR, 1.65; 95% CI, 1.58–1.74) and distant SRC (HR, 4.61; 95% CI, 4.39–4.84) than in localized gastroenteropancreatic SRC after adjustment for other covariates. SRC in the pancreas had the worst OS (HR, 3.06; 95% CI, 2.69–3.49) and SRC in the esophagus (HR, 2.29; 95% CI, 2.04–2.57) and stomach had the second worst OS (HR, 2.25; 95% CI, 2.04–2.49) compared with SRC in the appendix (Table 2).
Full table
We then focused on the SEER 18 cohort to evaluate the most recent trends in OS over 3 time periods: 2000–2004, 2005–2009, and 2009–2014. In the overall SEER 18 cohort, compared with 2000–2004, patients who received the gastroenteropancreatic SRC diagnosis between 2005 and 2009 had a 7% lower risk of death (HR, 0.93; 95% CI, 0.90–0.96) and those diagnosed in 2010–2014 had a 13% lower risk of death (HR, 0.87; 95% CI, 0.84–0.91). In these 2 sub-cohorts we also found better survival in recent years compared with previous years. All of the above comparisons were significant at P<0.001.
Discussion
SRC is a rare histological subtype of adenocarcinoma and current knowledge is mostly derived from single institution studies based on small population size, to gain comprehensive insight into this rare malignancy, we utilized the SEER database to ensure a large sample size, including a total of 24,613 patients with gastroenteropancreatic SRC. We clarified the US epidemiological changes of gastroenteropancreatic SRC from 2000 to 2014. Meanwhile, findings in this study could shed new light on the site-specific differences in long-term survival.
In this population-based study, a slightly decreasing trend in the overall incidence of gastroenteropancreatic SRC was observed, except for appendix and esophagus. Specifically, our analysis revealed that SRC increased more than 2.9 times in the appendix and maintained stable in esophagus, which might partially be attributed to enhanced recognition of this disease entity. It had been known that a declining incidence of gastroenteropancreatic adenocarcinoma was observed in most recent years (10). Its similar evolving epidemiology with conventional adenocarcinomas is possible the reason why the incidence of SRC seems to be decreasing steadily from 2000 to 2014. With regard to the proportion of SRC in primary gastroenteropancreatic neoplasm, the stomach and appendix were the most common primary sites, followed by the colorectum, small intestine and pancreas. This finding was in line with previous study (9) showed that SRC was less frequently found in pancreas and lower gastrointestinal tract. Similarly, the gender distribution of SRC in appendix and colorectum was female predominated, whereas other sites of gastroenteropancreatic SRC were higher in men than women. Additionally, appendiceal SRC tended to onset in younger age at presentation, which manifested 5.9 years earlier than the average age of gastroenteropancreatic SRC. Specifically, in our analysis on the age distribution, the declined incidence of SRC was mainly associated with the drop in older than 50 years people, especially for aged 65 years and the older group. Interestingly, there was almost no significant change in younger and 50 years old group, a finding consistent with other reports (11,12). The possible reasons for this was an increasing number of screening endoscopies and imaging technology performed in age older than 50 years people, in whom first cancer screening was most likely to occur. Furthermore, gastroenterological endoscope techniques could perform detection and resection early, which was also useful to prevent tumor progression at an early stage (13,14).
With convincing data in our series that gastroenteropancreatic SRC tends to carry the poor prognosis with a high proportion of metastasis at diagnosis. After adjustment for other covariates, multivariate Cox proportional model analysis suggests median OS of patients with SRC was the worst in pancreas, accompanied with SRC in the esophagus and stomach had the second worst OS. The more aggressive behavior of SRC in pancreas and the upper gastrointestinal tract, rather than colorectal SRC, may reflect the underlying different molecular profiles, although the carcinogenesis of SRC in the different sites of GI tract and pancreas is largely unknown. Interestingly, our finding showed appendiceal SRC had the best prognosis, followed by the colon and rectum. Similarly, synchronous distant metastasis was less commonly found in patients with colorectal SRC, ultimately implying a better outcome for these patients, than in those with the remaining gastroenteropancreatic SRC.
Meanwhile, this finding agreed with other studies (12,15-18) showed that SRC tumors were more frequently poorly differentiated and undifferentiated pathological grade as well as advanced stage with more distant metastasis involvement. Based on this observation, the majority of cases presented in stage III and IV. Similarly, the grade distribution at diagnosis was skewed to poorly differentiated and undifferentiated pathological grade. This finding did not align with a previous study in Asian that patients reported with a more frequent presentation in early-stage (19,20). The possible reasons for this is largely due to patients with SRC in US population have distinct features from the Asian population, whose SRC located in esophagogastric junction and upper stomach were less common in Asian (21). Specifically, in our data analysis on different races, disparities are seen in survival rates between Asian Americans and white people. Asian American had better prognosis than African American, whereas white people lay in between them, although we did not investigate the cause of this racial disparity.
It has been known that patients of early gastric carcinoma with SRC conferred a more favorable prognosis after standardized surgical procedure than advanced stage SRC (22,23).Our study was in line with the reported by Bamboat et al. (24), which, despite its small sample size, suggested that the prognostic impact of signet ring gastric cancer may be dependent on tumor stage. Interestingly, our notable finding was that appendiceal SRC had more favorable prognosis than remaining gastroenteropancreatic SRC in the early stage. However, advanced stage of appendiceal SRC was still should be considered as an unfavorable high risk of this disease. Researchers have previously reported that SRC of the stomach does not necessarily portend a worse prognosis when adjusted for stage (25). Consequently, we believed that stage-adjusted analysis was important in clarifying the prognosis of SRC.
The analysis regarding the pathological grade revealed that SRC was the more frequent presentation of poor differentiated and undifferentiated grade at diagnosis. Subgroup analysis was conducted in gastroenteropancreatic SRC of Grade I/II and Grade III/IV, suggesting that poor prognosis of SRC was also associated with the poor differentiated and undifferentiated pathological grade (26). Grade III/IV was another critical factor leading to the poor prognosis of gastroenteropancreatic SRC. Therefore, the poor prognosis of SRC was mainly caused by high tumor stages and poor differentiation at the time of diagnosis. US population had a small number of patients in early-stage and Grade I/II group may explain the reason why we reported gastroenteropancreatic SRC had a worse prognosis than the Asian countries (20).
Survival for overall gastroenteropancreatic SRC had just improved slightly over a period of 15 years, reflecting few therapeutic advances on the survival of patients with SRC in gastrointestinal tract and pancreas. Upon further analysis, the improvement of survival was mainly associated with the enhancement of localized and regional stage people, especially for SRC in pathological Grade I/II, whereas survival rates were more static for distant SRC and Grade III/IV SRC. The poor survival outcome of gastroenteropancreatic SRC was another reason why more attention should be paid to early detection and assessment of SRC. This observation highlighted the importance of SRC screening with routine endoscopy and radiologic imaging techniques to identify and treat this entity disease at early stage. Thus, a widely accepted early screening detection program should be recommended. Furthermore, an increasing number of screening endoscopies also could help to earlier detection of these lesions and to a speedier definite treatment.
In addition to stage, grade and primary site, multivariate Cox proportional model analysis demonstrated that the gender, age and race were other independent risk factors for the prognosis of gastroenteropancreatic SRC, which was helpful for risk stratification and therapeutic management of this entity disease. Therefore, these prognostic implications should be taken into consideration for therapeutic guideline development and treatment decision making of the disease. Surgical resection was still the mainstay treatment for the disease, followed by adjuvant chemotherapy and radiotherapy. Although advances had been made in the treatment of patients with gastroenteropancreatic adenocarcinoma, therapies response of patients with SRC in the advanced stage or poor differentiation grade was still unfavorable. It is necessary to explore new therapeutic modalities to achieve further improvements in the clinical outcome for this subset patient (27,28).
Limitation and strength
Due to a lack of relevant clinical information regarding adjuvant chemotherapy or radiotherapy strategies, we acknowledged several limitations existed in this study. Additionally, several known predictive factors were not captured by the SEER database, such as surgical strategies, distant metastasis site, and recurrence-free survival, if available, which would provide an insight into analysis of incidence and survival in patients with gastroenteropancreatic SRC. Such drawbacks were inherent to all studies that relied on retrospective data. However, SEER database is one of the largest registries allowing comparative analysis for gastroenteropancreatic SRC. To our knowledge, this is the most comprehensive and up-to-data population-based study to evaluate the demographic characteristics, incidence and long-term survival outcomes of gastroenteropancreatic SRC according to the gender, age, race, stage, grade and primary tumor site.
Conclusions
The present study provided a comprehensive epidemiology and prognosis picture of gastroenteropancreatic SRC. Majority of heterogeneous SRC tended to carry the poor prognosis with a high proportion of distant metastasis. The poor prognosis of SRC in US population was mainly caused by high tumor stages and poor differentiation at diagnosis.
Acknowledgments
The authors acknowledge the efforts of the SEER Program tumor registries in the creation of the SEER database.
Funding: The study was supported by The National Natural Science Foundation of China (Grant No. 81860433 and No. 81860466). Guangdong Provincial Science and Technology Plan, Guangzhou Science and Technology Plan (Grant No. 2017A020215036 and No. 201806020036).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. It was determined to be a retrospective analysis of publicly available, de-identified data and was determined to be exempt from requiring written informed consent.
References
- Bosman FT, Carneiro F, Hruban RH, et al. editors. WHO classification of tumours of the digestive system. 4th edition. WHO press, 2010.
- Nitsche U, Zimmermann A, Späth C, et al. Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 2013;258:775-82; discussion 782-3. [Crossref] [PubMed]
- Gopalan V, Smith RA, Ho YH, et al. Signet-ring cell carcinoma of colorectum--current perspectives and molecular biology. Int J Colorectal Dis 2011;26:127-33. [Crossref] [PubMed]
- Nafteux PR, Lerut TE, Villeneuve PJ, et al. Signet ring cells in esophageal and gastroesophageal junction carcinomas have a more aggressive biological behavior. Ann Surg 2014;260:1023-9. [Crossref] [PubMed]
- Liu K, Wan J, Bei Y, et al. Prognostic Impact of Different Histological Types on Gastric Adenocarcinoma: a Surveillance, Epidemiology, and End Results Database Analysis. Pathol Oncol Res 2017;23:881-7. [Crossref] [PubMed]
- Belli S, Aytac HO, Karagulle E, et al. Outcomes of surgical treatment of primary signet ring cell carcinoma of the colon and rectum: 22 cases reviewed with literature. Int Surg 2014;99:691-8. [Crossref] [PubMed]
- Chen JS, Hsieh PS, Hung SY, et al. Clinical significance of signet ring cell rectal carcinoma. Int J Colorectal Dis 2004;19:102-7. [Crossref] [PubMed]
- Chu PG, Weiss LM. Immunohistochemical characterization of signet-ring cell carcinomas of the stomach, breast, and colon. Am J Clin Pathol 2004;121:884-92. [Crossref] [PubMed]
- Goldstein NS, Long A, Kuan SF, et al. Colon signet ring cell adenocarcinoma: immunohistochemical characterization and comparison with gastric and typical colon adenocarcinomas. Appl Immunohistochem Mol Morphol 2000;8:183-8. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin 2017;67:7-30. [Crossref] [PubMed]
- Huang B, Ni M, Chen C, et al. Younger Age Is Associated with Poorer Survival in Patients with Signet-Ring Cell Carcinoma of the Colon without Distant Metastasis. Gastroenterol Res Pract 2016;2016:2913493.
- Lu M, Yang Z, Feng Q, et al. The characteristics and prognostic value of signet ring cell histology in gastric cancer: A retrospective cohort study of 2199 consecutive patients. Medicine (Baltimore) 2016;95:e4052. [Crossref] [PubMed]
- Brenner H, Altenhofen L, Stock C, et al. Prevention, Early Detection, and Overdiagnosis of Colorectal Cancer Within 10 Years of Screening Colonoscopy in Germany. Clin Gastroenterol Hepatol 2015;13:717-23. [Crossref] [PubMed]
- Veitch AM, Uedo N, Yao K, et al. Optimizing early upper gastrointestinal cancer detection at endoscopy. Nat Rev Gastroenterol Hepatol 2015;12:660-7. [Crossref] [PubMed]
- Nitsche U, Friess H, Agha A, et al. Prognosis of mucinous and signet-ring cell colorectal cancer in a population-based cohort. J Cancer Res Clin Oncol 2016;142:2357-66. [Crossref] [PubMed]
- Hyngstrom JR, Hu CY, Xing Y, et al. Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base. Ann Surg Oncol 2012;19:2814-21. [Crossref] [PubMed]
- Pande R, Sunga A, Levea C, et al. Significance of signet-ring cells in patients with colorectal cancer. Dis Colon Rectum 2008;51:50-5. [Crossref] [PubMed]
- Bittorf B, Merkel S, Matzel KE, et al. Primary signet-ring cell carcinoma of the colorectum. Langenbecks Arch Surg 2004;389:178-83. [Crossref] [PubMed]
- Chon HJ, Hyung WJ, Kim C, et al. Differential Prognostic Implications of Gastric Signet Ring Cell Carcinoma: Stage Adjusted Analysis From a Single High-volume Center in Asia. Ann Surg 2017;265:946-53. [Crossref] [PubMed]
- Kim DY, Park YK, Joo JK, et al. Clinicopathological characteristics of signet ring cell carcinoma of the stomach. ANZ J Surg 2004;74:1060-4. [Crossref] [PubMed]
- Kwon KJ, Shim KN, Song EM, et al. Clinicopathological characteristics and prognosis of signet ring cell carcinoma of the stomach. Gastric Cancer 2014;17:43-53. [Crossref] [PubMed]
- Imamura T, Komatsu S, Ichikawa D, et al. Early signet ring cell carcinoma of the stomach is related to favorable prognosis and low incidence of lymph node metastasis. J Surg Oncol 2016;114:607-12. [Crossref] [PubMed]
- Hyung WJ, Noh SH, Lee JH, et al. Early gastric carcinoma with signet ring cell histology. Cancer 2002;94:78-83. [Crossref] [PubMed]
- Bamboat ZM, Tang LH, Vinuela E, et al. Stage-stratified prognosis of signet ring cell histology in patients undergoing curative resection for gastric adenocarcinoma. Ann Surg Oncol 2014;21:1678-85. [Crossref] [PubMed]
- Taghavi S, Jayarajan SN, Davey A, et al. Prognostic significance of signet ring gastric cancer. J Clin Oncol 2012;30:3493-8. [Crossref] [PubMed]
- Barresi V, Reggiani Bonetti L, Domati F, et al. Prognostic relevance of histopathological features in signet ring cell carcinoma of the colorectum. Virchows Arch 2016;469:267-75. [Crossref] [PubMed]
- Chen L, Liu X, Gao L, et al. The clinicopathological features and prognosis of signet ring cell carcinoma of the esophagus: A 10-year retrospective study in China. PLoS One 2017;12:e0176637. [Crossref] [PubMed]
- Tamhankar AS, Ingle P, Engineer R, et al. Signet ring colorectal carcinoma: Do we need to improve the treatment algorithm? World J Gastrointest Oncol 2016;8:819-25. [Crossref] [PubMed]