
Resection of non-hepatic colorectal cancer metastasis
Introduction
Colorectal cancer (CRC) continues to be one of the leading causes of significant health problems and cancer-related death in the world. Each year about one million people are diagnosed with CRC worldwide with an estimated 140,000 individuals being diagnosed in the United States (1). About one-half of patients will either present with colorectal liver metastasis (CLM) or develop them during the course of their disease (2,3). While roughly 20% of all patients will present with synchronous liver metastasis, another 25-30% will present with metachronous disease (4-7). Common sites of extra-hepatic metastatic disease include the lung, hilar/peri-hepatic lymph nodes, as well as the peritoneum (8-12). While systemic chemotherapy remains the cornerstone of therapy for patients with stage IV colorectal disease, some patients are optimally managed with the addition of surgical therapy (13-17).
Previous data on patients with extrahepatic disease (EHD) and CLM have suggested that these patients have a poor prognosis (18-20). As such, in most instances, EHD was traditionally considered a strong relative or absolute contraindication to surgical resection. However, more recently our group, as well as others, have reported long-term survival after resection of EHD (9-12,21-25). As a consequence, resection of EHD from a colorectal primary has increasingly become accepted over the last decade. We herein review the management of patients with EHD metastatic disease from a colorectal primary tumor. Specifically, we highlight the data on the surgical management of patients with metastatic disease at the most common EHD sites (e.g. lung, hilar/peri-hepatic lymph nodes, peritoneum), as well as define general oncological principles for treating this challenging cohort of patients.
CRC Metastasis: Implication of Number and Anatomic Site
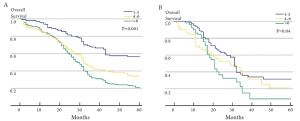
There has been controversy regarding the relative importance of total number of EHD metastatic tumors versus location of the specific metastatic site (23,24,26). Some investigators have suggested that the total number of metastatic lesions is the dominant factor that predicts outcome following surgical resection (24,26). In a provocative paper by Elias et al., the authors argued that the site of the metastatic disease did not matter - only the number of metastatic lesions (26). In this study, the total number of tumors impacted survival, but the location of the metastatic disease did not. However, data from this study were difficult to interpret due to the small number of patients included in each subset analysis. More recently, our group published a large, international series looking at resection of extra-hepatic CRC metastases (8). In this study, both the total number of metastases and the location of the metastatic disease were associated with prognosis. Survival was strongly associated with overall tumor burden (Figure 1). We noted, however, that among patients with a large tumor burden (>6 metastatic lesions) the relative prognostic impact of anatomic location was less (Figure 2). Of note, among patients with a lower burden of disease, anatomic location of the metastatic disease had a strong influence on survival (Table 1). As such, both total number of EHD metastases and the location of the metastases should be considered when assessing patients for surgery.
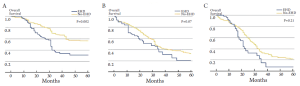
Full table
Pulmonary Metastasis
The lung is one of the most common metastatic sites for colorectal carcinoma. About 2-7% of patients with primary CRC will present with isolated lung metastasis, while about 10% of patients will have synchronous pulmonary and CLM. The lung is the most accepted EHD site managed with surgical resection. In 1944, Blalock reported the first successful resection of pulmonary metastasis from colorectal carcinoma. Subsequently, Thomford in 1965 defined specific criteria for resection of metastatic colorectal disease to the lung (27). Today, resection of pulmonary metastasis is well-established, although the evidence for the effectiveness of metastasectomy largely comes from retrospective studies (28-35). Similar to surgical management of all patients with metastatic disease, patient selection is critical in identifying the best candidates for resection.
Clinical practice guidelines for the management of patients with pulmonary metastasis have been established (36). Specifically, general recommendations for the surgical resection of pulmonary metastasis include: (I) metastasis are technically resectable with microscopically negative (R0) margins (II) general and functional risks are tolerable (III) primary tumor is controlled, and (IV) no extra-thoracic lesions are present (with the exception of hepatic lesions in which complete removal of both hepatic and pulmonary metastasis is feasible) (Table 2). The presence of concomitant clinically positive disease in the mediastinal or hilar lymph nodes is a strong contraindication to pulmonary metastasectomy, as this is an ominous prognostic factor associated with prohibitively poor long-term survival (31-34,37,38).

Full table
Surgical resection for pulmonary metastasis is associated with a reported 5-year survival ranging from 20% to 60% (28-30,32,39). Several factors have been associated with prognosis following surgical resection of pulmonary CRC metastasis. Specifically, high preoperative carcinoembryonic antigen (CEA) has been shown to be an independent factor associated with worse long-term survival (40-43). The number of pulmonary lesions is also associated with long-term outcome. Multiple studies have noted that tumor number is an important independent predictor of long-term outcome (43,44). In one of the largest registry studies examining long-term results of lung metastasectomy among 5206 cases, the reported 5-year survival was 43% for patients with single lesions versus 27% for patients with four or more lesions (45). Another factor that impacts outcome is whether the patient presents with synchronous or metachronous disease, as well as the disease-free interval between resection of the primary tumor and the pulmonary metastasis. Several studies have noted that a disease-free interval of greater than 1 year between the time of the diagnosis of the primary tumor and the pulmonary metastasis was associated with improved outcomes (37,45).
Patients whose disease pattern includes both pulmonary and hepatic metastases may benefit from either simultaneous or sequential lung and liver resections (39,46-50). In general, patients who present with synchronous pulmonary and CLM can undergo either simultaneous or staged resections. Among patients who need an extensive liver resection, a staged approach may be preferable. In other circumstances where the disease is more limited a simultaneous approach can be performed with low morbidity and perioperative mortality (51). When undertaking a staged approach, outcome appears comparable regardless of whether the lung or liver resection is undertaken first (51). As such, the approach should be individualized. For patients with metachronous metastasis, a longer time interval between the detection of the lung and liver metastasis has been associated with a better prognosis (46-50).
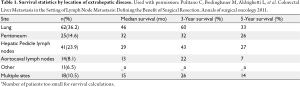
After pulmonary metastasectomy, 50-75% of patients will recur, both with pulmonary as well as other EHD sites (35). Local or intra-pulmonary recurrence can be due to an incomplete resection, lymphangitic spread, or “floating” cancer cells (52,53). Despite the relatively high incidence of recurrence, the overall survival associated with pulmonary metastasectomy ranges from 48-60% (Figure 3) (37,39-50,54). In a meta-analysis incorporating 14 studies and 1684 patients, most of whom underwent a unilateral wedge resection for limited disease(53%), the overall 5-year survival was 48% (54). Of note, 5-year survival was only 17% among patients with peri-bronchial/hilar lymph nodes and no patient with mediastinal lymphadenopathy survived to 5 years (38). In contrast, patients who had no nodal disease had a 5-year survival of 60%. The authors noted a median survival of 29 months overall; however, among those patients with a disease-free interval of 3 years or more between the primary tumor treatment and the diagnosis of the pulmonary metastasis median overall survival was 49 months (45).
Given the relative high incidence of recurrence following pulmonary metastasectomy, there has been interest in repeat pulmonary resection (Table 3). Park et al. reported a 79.3% 5-year survival after second metastasectomy and a 5-year survival of 77.8% after a third resection (50). Other studies have shown similar results with 5-year survival ranging from 42-61%, suggesting that second and third resection of recurrences are viable options for patients with recurrent disease and can lead to long-term survival in a subset of patients (53,55,56).
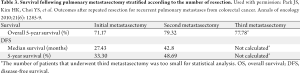
Full table
Extrahepatic Lymph-Node Metastases
In addition to loco-regional nodal basins, CRC may metastasize to peri-hepatic, hilar, or para-aortic lymph nodes. Nodal metastasis outside the regional CRC basin may represent “metastasis from metastasis” as a subset of patients who do not have regional lymph node metastasis can subsequently be found to have peri-hepatic or hilar lymph node metastasis (57-59). While the overall incidence of lymph node metastasis in the setting of CLM is hard to define, most studies have reported a range of 1-10% (21,60-63). Traditionally, metastatic disease in the hilar or para-aortic lymph nodes has been considered a strong relative contraindication to surgery due to poor long-term survival among this group of patients. With more effective chemotherapy, as well as the recent publication on improved outcomes for patients with non-regional lymph node metastasis, the role of resection of CLM in the setting of lymph node metastasis has been reconsidered (8,11,22,64,65).
Several studies have examined the impact of lymph node metastasis through the use of empiric routine lymphadenectomy at the time of liver surgery (21,59,63,66). In a study by Elias et al., lymph node dissection of the hepatic pedicle was undertaken in 100 consecutive patients undergoing curative hepatectomy for CLM in whom lymph node involvement of the hepatic pedicle was not macroscopically detectable (63). Microscopic lymph node involvement was found in 14 patients. In a separate study by the Strasbourg group, among 160 patients who had routine lymphadenectomy, the authors reported an 11% incidence of microscopic disease in the lymph nodes (59). Laurent et al. reported an incidence of 15% for microscopic disease in the peri-hepatic/hilar lymph nodes (21). Early reports from these centers noted a poor survival among patients with microscopic lymph node metastasis, with 5-year survival in the range of 5-18%. More recently other groups reported more favorable long-term survival, noting that the specific site of the metastatic lymph node disease is important in stratifying patients with regards to prognosis (11,22,67).
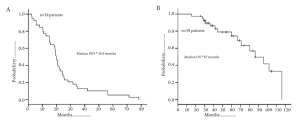
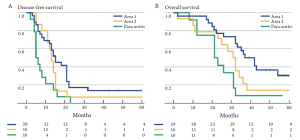
Among patients with CLM, the location of the lymph node metastasis may dictate the relative survival benefit of surgical intervention. Specifically, Jaeck et al. note that liver resection did not offer a survival benefit among patients with lymph node metastasis along the common hepatic artery and celiac axis (area 2), but was beneficial for those patients with lymph node disease restricted to the hepatoduodenal ligament and retro-pancreatic location (area 1) (59). Adam et al. similarly noted a difference in outcome when comparing survival of patients with lymph node metastasis in area 1 versus area 2 (22). In a separate multi-institutional international study from our own group, we found that - while long-term survival may be possible in patients with lymph node metastasis in area 2 - surgery held the most benefit for patients with lymph node disease relegated to area 1 (14% versus 30%) (Figure 4). In contrast to areas 1 and 2, the outcome of surgery for patients with para-aortic lymph node metastasis is particularly dismal. Adam et al. reported a median survival of only 17 months for this group of patients and every patient experienced a recurrence. In the report by Pulitano et al., these investigators similarly noted no long-term survivors among patients operated on in the setting of para-aortic lymph node disease (67). Taken together, these data strongly suggest that lymph node location should be taken into consideration when deliberating about whether surgical resection should be undertaken. While overall survival in the setting of lymph node disease outside the CRC lymph node basin is only in the range of 18-20%, certain subsets of patients such as those with disease restricted to the hepatoduodenal ligament (area 1) may have a 5-year survival up to 30%.
In addition to the location of the lymph node disease, the presence of clinically “positive” macroscopic disease is also a critical factor in outcome. Unlike patients with sub-clinical microscopic disease, patients with clinically evident macroscopic disease almost universally have a poor outcome. As such, most clinicians have concluded that resection of macroscopic lymph node metastasis should be a contraindication to hepatic resection (15,19,20,68). A review by Rodgers and McCall of 15 studies in the literature describing liver resection for CLM reported on 145 patients with macroscopic lymph node involvement, of whom only 5 were alive at 5 years (61). In several separate studies that reported on patients with macroscopic nodal involvement, the authors noted that virtually all patients were dead within 5 years of surgery (62,69,70). As such, patients with clinical macroscopically evident lymph node metastasis should be treated in a multi-modality setting with preoperative chemotherapy with only a well-selected subset considered for eventual surgery.
Peritoneal Carcinomatosis
Peritoneal carcinomatosis is a form of disease progression that affects 30% to 40% of patients with CRC (71,72). Traditionally, peritoneal carcinomatosis has been associated with a median survival of only 6 to 9 months (72-74). Peritoneal carcinomatosis is thought to result from peritoneal spread of cancer cells or seeding of the peritoneum during surgery (75,76). While many consider peritoneal carcinomatosis to be a form of disseminated disease portending an extremely poor outcome, Sugarbaker and colleagues have challenged this concept (76). Specifically, some clinicians have argued that peritoneal carcinomatosis is more akin to loco-regional recurrence/metastasis for which a more aggressive approach may be appropriate in select circumstances (76,77).
Some centers have reported on their experience with cytoreduction surgery (CRS) and intraperitoneal chemotherapy for peritoneal CRC metastasis. Surgery for peritoneal disease usually involves complete CRS with removal of all gross disease in combination with hyperthermic intraperitoneal chemotherapy, usually consisting of the installation of mitomycin C or oxaliplatin for 30-90 minutes after CRS is completed. Using this approach, median survival exceeding 60 months has been reported in a well-selected subset of patients (78). The approach to patients with peritoneal metastasis from CRC, however, still remains highly controversial. Such therapy remains not the standard of care and is not indicated for most patients, especially those with disseminated carcinomatosis (3). Among those patients with peritoneal disease, patient selection is critical to achieving acceptable outcomes.
A consensus statement published by a consortium of cytoreduction centers noted eight clinical and radiographic variables to select patients. Specifically, an Eastern Cooperative Oncology Group (ECOG) performance status ≤2, no extra-abdominal disease, up to three small resectable hepatic parenchymal metastases, no biliary or ureteral obstruction, less than one site of small bowel obstruction, small volume mesenteric disease, and minimal disease in the gastro-hepatic ligament (79). The authors noted that these guidelines should allow for improved selection of patients for complete CRS, in turn giving patients a better chance for survival. Complete CRS is perhaps the most critical factor associated with survival, and therefore only patients with low volume peritoneal CRC disease should be considered for resection (77,79,80).
Verwaal et al. reported a randomized trial examining patients treated with systemic chemotherapy (5-Fluoro-uracil and leucovorin) versus operative cytoreduction with intra-peritoneal therapy (81). In this study, cytoreduction with intraperitoneal chemotherapy was shown to be associated with a survival benefit (median survival: systemic chemotherapy, 12.6 months versus cytoreduction and intraperitoneal chemotherapy, 22.4 months). The study is difficult to interpret, however, in light of currently available more efficacious systemic chemotherapy. An update of the trial with a median follow-up of almost 8 years reported a 5-year survival of 43% among patients with no gross residual disease, but no patient who had gross residual disease left at the time of CRS survived to 5 years (82). In more contemporary retrospective studies, other investigators have similarly noted the feasibility of long-term survival in a select group of patients. For example, Glehen et al. reported on 506 patients undergoing CRS and hyperthermic intraperitoneal chemotherapy at 28 institutions. The overall medial survival was 19.2 months for hyperthermic intraperitoneal chemotherapy with a 5-year survival of 19% (12). Elias et al reported that cytoreduction and hyperthermic intraperitoneal chemotherapy was able to achieve a 5-year survival of 51% among patients with isolated, resectable peritoneal disease (77). More recently Shen et al. reported that complete CRS plus hyperthermic intraperitoneal chemotherapy for limited peritoneal CRC disease had a comparable survival to patients undergoing hepatic resection for CLM (83). Specifically, the 1-, 3-, and 5-year overall survival for a complete CRS was 91%, 48%, and 26% versus 87%, 59%, and 34% for patients undergoing resection of CLM. The study has been criticized, however, for the relatively low 5-year survival reported among patients with resected hepatic metastasis - making any true comparison difficult. In a meta-analysis by Cao et al. the authors reported a general trend toward a survival benefit for CRS and hyperthermic intraperitoneal chemotherapy versus the control groups (84). While such results are encouraging and provocative, patients with peritoneal CRC disease should still be considered at very high risk of disseminated disease. As such, surgery for this group of patients needs to be extremely selective and done within a multi-disciplinary approach.
Conclusion
It is important to note that in a large series of over 1,600 patients with CLM only 10% underwent resection of non-hepatic CRC metastasis (8). Despite the very select nature of this cohort, the 5-year survival was only 26%. Therefore, based on the high risk of disseminated disease, most patients with non-hepatic metastatic CRC cancer should initially be treated with systemic chemotherapy. While this general approach is particularly warranted for patients with macroscopic lymph nodes or peritoneal disease, some patients - such as those with isolated, solitary pulmonary metastasis - may be appropriate for “up-front” surgical resection. For the majority of patients with non-hepatic CRC metastasis who receive systemic chemotherapy, continued and iterative reassessment with cross-sectional imaging is required. Patients who progress on therapy should receive additional chemotherapy and, in general, not be considered candidates for resection. Patients with responsive or stable disease on systemic therapy should be considered for surgery if a complete resection (R0) of the disease sites is feasible. Both the number and the site of metastatic disease needs to factor into the decision to offer surgery. Specifically, patients with a large burden of disease (6 or more lesions/disseminated peritoneal disease) and those with certain anatomic sites of disease (para-aortic lymph nodes, peritoneal disease) have a very guarded prognosis. As such, surgery should only be undertaken in a very select subset of these patients who have clearly demonstrated responsive or quiescent disease for a prolonged period of time. Patients should be discussed in the context of a multidisciplinary team. While non-hepatic CRC metastasis should no longer be an absolute contra-indication to surgery, careful selection is tantamount to ensuring maximal benefit of surgical resection.
Footnote
No potential conflict of interest.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin 2011;61:69-90. [PubMed]
- Steele G Jr, Ravikumar TS. Resection of hepatic metastases from colorectal cancer. Biologic perspective. Ann Surg 1989;210:127-138. [PubMed]
- Pawlik TM, Schulick RD, Choti MA. Expanding criteria for resectability of colorectal liver metastases. Oncologist 2008;13:51-64. [PubMed]
- Cook AD, Single R, McCahill LE. Surgical resection of primary tumors in patients who present with stage IV colorectal cancer: an analysis of surveillance, epidemiology, and end results data, 1988 to 2000. Ann Surg Oncol 2005;12:637-645. [PubMed]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-318, discussion 318-321. [PubMed]
- Finlay IG, McArdle CS. Occult hepatic metastases in colorectal carcinoma. Br J Surg 1986;73:732-735. [PubMed]
- Altendorf-Hofmann A, Scheele J. A critical review of the major indicators of prognosis after resection of hepatic metastases from colorectal carcinoma. Surg Oncol Clin N Am 2003;12:165-192. xi.. [PubMed]
- Pulitanò C, Bodingbauer M, Aldrighetti L, et al. Liver resection for colorectal metastases in presence of extrahepatic disease: results from an international multi-institutional analysis. Ann Surg Oncol 2011;18:1380-1388. [PubMed]
- Elias D, Sideris L, Pocard M, et al. Results of R0 resection for colorectal liver metastases associated with extrahepatic disease. Ann Surg Oncol 2004;11:274-280. [PubMed]
- Inoue M, Ohta M, Iuchi K, et al. Benefits of surgery for patients with pulmonary metastases from colorectal carcinoma. Ann Thorac Surg 2004;78:238-244. [PubMed]
- Jaeck D. The significance of hepatic pedicle lymph nodes metastases in surgical management of colorectal liver metastases and of other liver malignancies. Ann Surg Oncol 2003;10:1007-1011. [PubMed]
- Glehen O, Kwiatkowski F, Sugarbaker PH, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol 2004;22:3284-3292. [PubMed]
- Galizia G, Lieto E, Orditura M, et al. First-line chemotherapy vs bowel tumor resection plus chemotherapy for patients with unresectable synchronous colorectal hepatic metastases. Arch Surg 2008;143:352-358, discussion 358. [PubMed]
- Nash GM, Saltz LB, Kemeny NE, et al. Radical resection of rectal cancer primary tumor provides effective local therapy in patients with stage IV disease. Ann Surg Oncol 2002;9:954-960. [PubMed]
- Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992;216:493-504, discussion 504-505. [PubMed]
- Ruo L, Gougoutas C, Paty PB, Guillem JG, Cohen AM, Wong WD. Elective bowel resection for incurable stage IV colorectal cancer: prognostic variables for asymptomatic patients. J Am Coll Surg 2003;196:722-728. [PubMed]
- Stillwell AP, Buettner PG, Ho YH. Meta-analysis of survival of patients with stage IV colorectal cancer managed with surgical resection versus chemotherapy alone. World J Surg 2010;34:797-807. [PubMed]
- Ekberg H, Tranberg KG, Andersson R, et al. Determinants of survival in liver resection for colorectal secondaries. Br J Surg 1986;73:727-731. [PubMed]
- Hughes KS, Simon R, Songhorabodi S, et al. Resection of the liver for colorectal carcinoma metastases: a multi-institutional study of patterns of recurrence. Surgery 1986;100:278-284. [PubMed]
- Adson MA, van Heerden JA, Adson MH, Wagner JS, Ilstrup DM. Resection of hepatic metastases from colorectal cancer. Arch Surg 1984;119:647-651. [PubMed]
- Laurent C, Sa Cunha A, Rullier E, Smith D, Rullier A, Saric J. Impact of microscopic hepatic lymph node involvement on survival after resection of colorectal liver metastasis. J Am Coll Surg 2004;198:884-891. [PubMed]
- Adam R, de Haas RJ, Wicherts DA, et al. Is hepatic resection justified after chemotherapy in patients with colorectal liver metastases and lymph node involvement? J Clin Oncol 2008;26:3672-3680. [PubMed]
- Elias D, Ouellet JF, Bellon N, Pignon JP, Pocard M, Lasser P. Extrahepatic disease does not contraindicate hepatectomy for colorectal liver metastases. Br J Surg 2003;90:567-574. [PubMed]
- Carpizo DR, Are C, Jarnagin W, et al. Liver resection for metastatic colorectal cancer in patients with concurrent extrahepatic disease: results in 127 patients treated at a single center. Ann Surg Oncol 2009;16:2138-2146. [PubMed]
- Aoki T, Umekita N, Tanaka S, Noda K, Warabi M, Kitamura M. Prognostic value of concomitant resection of extrahepatic disease in patients with liver metastases of colorectal origin. Surgery 2008;143:706-714. [PubMed]
- Elias D, Liberale G, Vernerey D, et al. Hepatic and extrahepatic colorectal metastases: when resectable, their localization does not matter, but their total number has a prognostic effect. Ann Surg Oncol 2005;12:900-909. [PubMed]
- Thomford NR, Woolner LB, Clagett OT. The surgical treatment of metastatic tumors in the lungs. J Thorac Cardiovasc Surg 1965;49:357-363. [PubMed]
- Fiorentino F, Hunt I, Teoh K, Treasure T, Utley M. Pulmonary metastasectomy in colorectal cancer: a systematic review and quantitative synthesis. J R Soc Med 2010;103:60-66. [PubMed]
- Rotolo N, De Monte L, Imperatori A, Dominioni L. Pulmonary resections of single metastases from colorectal cancer. Surg Oncol 2007;16:S141-S144. [PubMed]
- Tan KK, Lopes Gde L Jr, Sim R. How uncommon are isolated lung metastases in colorectal cancer? A review from database of 754 patients over 4 years. J Gastrointest Surg 2009;13:642-648. [PubMed]
- Saito Y, Omiya H, Kohno K, et al. Pulmonary metastasectomy for 165 patients with colorectal carcinoma: A prognostic assessment. J Thorac Cardiovasc Surg 2002;124:1007-1013. [PubMed]
- Welter S, Jacobs J, Krbek T, Poettgen C, Stamatis G. cPrognostic impact of lymph node involvement in pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg 2007;31:167-172. [PubMed]
- Koga R, Yamamoto J, Saiura A, Yamaguchi T, Hata E, Sakamoto M. Surgical resection of pulmonary metastases from colorectal cancer: Four favourable prognostic factors. Jpn J Clin Oncol 2006;36:643-648. [PubMed]
- Iizasa T, Suzuki M, Yoshida S, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg 2006;82:254-260. [PubMed]
- Internullo E, Cassivi SD, Van Raemdonck D, Friedel G, Treasure TESTS Pulmonary Metastasectomy Working Group. Pulmonary metastasectomy: a survey of current practice amongst members of the European Society of Thoracic Surgeons. J Thorac Oncol 2008;3:1257-1266. [PubMed]
- Network NCC. NCCN Colon Cancer Panel Practice Guidelines in Oncology. Colon Cancer. Fort Washington: PA;2009.
- Pfannschmidt J, Klode J, Muley T, Dienemann H, Hoffmann H. Nodal involvement at the time of pulmonary metastasectomy: experiences in 245 patients. Ann Thorac Surg 2006;81:448-454. [PubMed]
- Veronesi G, Petrella F, Leo F, et al. Prognostic role of lymph node involvement in lung metastasectomy. J Thorac Cardiovasc Surg 2007;133:967-972. [PubMed]
- Shah SA, Haddad R, Al-Sukhni W, et al. Surgical resection of hepatic and pulmonary metastases from colorectal carcinoma. J Am Coll Surg 2006;202:468-475. [PubMed]
- Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol 2009;16:1026-1032. [PubMed]
- Watanabe K, Nagai K, Kobayashi A, Sugito M, Saito N. Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg 2009;96:1058-1065. [PubMed]
- Takahashi S, Nagai K, Saito N, et al. Multiple resections for hepatic and pulmonary metastases of colorectal carcinoma. Jpn J Clin Oncol 2007;37:186-192. [PubMed]
- Kanemitsu Y, Kato T, Hirai T, Yasui K. Preoperative probability model for predicting overall survival after resection of pulmonary metastases from colorectal cancer. Br J Surg 2004;91:112-120. [PubMed]
- Onaitis MW, Petersen RP, Haney JC, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg 2009;87:1684-1688. [PubMed]
- Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. The International Registry of Lung Metastases. J Thorac Cardiovasc Surg 1997;113:37-49. [PubMed]
- Jarabo JR, Fernández E, Calatayud J, et al. More than one pulmonary resections or combined lung-liver resection in 79 patients with metastatic colorectal carcinoma. J Surg Oncol 2011;104:781-786. [PubMed]
- Zabaleta J, Aguinagalde B, Fuentes MG, et al. Survival after lung metastasectomy for colorectal cancer: importance of previous liver metastasis as a prognostic factor. Eur J Surg Oncol 2011;37:786-790. [PubMed]
- Yang YY, Fleshman JW, Strasberg SM. Detection and management of extrahepatic colorectal cancer in patients with resectable liver metastases. J Gastrointest Surg 2007;11:929-944. [PubMed]
- Kobayashi K, Kawamura M, Ishihara T. Surgical treatment for both pulmonary and hepatic metastases from colorectal cancer. J Thorac Cardiovasc Surg 1999;118:1090-1096. [PubMed]
- Park JS, Kim HK, Choi YS, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol 2010;21:1285-1289. [PubMed]
- Mineo TC, Ambrogi V, Tonini G, et al. Longterm results after resection of simultaneous and sequential lung and liver metastases from colorectal carcinoma. J Am Coll Surg 2003;197:386-391. [PubMed]
- Shiono S, Ishii G, Nagai K, et al. Histopathologic prognostic factors in resected colorectal lung metastases. Ann Thorac Surg 2005;79:278-282, discussion 283. [PubMed]
- Kanzaki R, Higashiyama M, Oda K, et al. Outcome of surgical resection for recurrent pulmonary metastasis from colorectal carcinoma. Am J Surg 2011;202:419-426. [PubMed]
- Pfannschmidt J, Dienemann H, Hoffmann H. Surgical resection of pulmonary metastases from colorectal cancer: a systematic review of published series. Ann Thorac Surg 2007;84:324-338. [PubMed]
- Chen F, Sakai H, Miyahara R, Bando T, Okubo K, Date H. Repeat resection of pulmonary metastasis is beneficial for patients with colorectal carcinoma. cWorld. J Surg 2010;34:2373-2378.
- Welter S, Jacobs J, Krbek T, Krebs B, Stamatis G. Long-term survival after repeated resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg 2007;84:203-210. [PubMed]
- August DA, Sugarbaker PH, Schneider PD. Lymphatic dissemination of hepatic metastases. Implications for the follow-up and treatment of patients with colorectal cancer. Cancer 1985;55:1490-1494. [PubMed]
- Dworkin MJ, Earlam S, Fordy C, Allen-Mersh TG. Importance of hepatic artery node involvement in patients with colorectal liver metastases. J Clin Pathol 1995;48:270-272. [PubMed]
- Jaeck D, Nakano H, Bachellier P, et al. Significance of hepatic pedicle lymph node involvement in patients with colorectal liver metastases: a prospective study. Ann Surg Oncol 2002;9:430-438. [PubMed]
- Scheele J, Stangl R, Altendorf-Hofmann A, Gall FP. Indicators of prognosis after hepatic resection for colorectal secondaries. Surgery 1991;110:13-29. [PubMed]
- Rodgers MS, McCall JL. Surgery for colorectal liver metastases with hepatic lymph node involvement: a systematic review. Br J Surg 2000;87:1142-1155. [PubMed]
- Kokudo N, Sato T, Seki M, et al. Hepatic lymph node involvement in resected cases of liver metastases from colorectal cancer. Dis Colon Rectum 1999;42:1285-1290, discussion 1290-1291. [PubMed]
- Elias D, Saric J, Jaeck D, et al. Prospective study of microscopic lymph node involvement of the hepatic pedicle during curative hepatectomy for colorectal metastases. Br J Surg 1996;83:942-945. [PubMed]
- Nakamura S, Suzuki S, Konno H. Resection of hepatic metastases of colorectal carcinoma: 20 years’ experience. J Hepatobiliary Pancreat Surg 1999;6:16-22. [PubMed]
- Oussoultzoglou E, Romain B, Panaro F, et al. Long-term survival after liver resection for colorectal liver metastases in patients with hepatic pedicle lymph nodes involvement in the era of new chemotherapy regimens. Ann Surg 2009;249:879-886. [PubMed]
- Elias DM, Ouellet JF. Incidence, distribution, and significance of hilar lymph node metastases in hepatic colorectal metastases. Surg Oncol Clin N Am 2003;12:221-229. [PubMed]
- Pulitanò C, Bodingbauer M, Aldrighetti L, et al. Colorectal Liver Metastasis in the Setting of Lymph Node Metastasis: Defining the Benefit of Surgical Resection. Ann Surg Oncol 2012;19:435-442. [PubMed]
- Cady B, Stone MD, McDermott WV Jr, et al. Technical and biological factors in disease-free survival after hepatic resection for colorectal cancer metastases. Arch Surg 1992;127:561-568, discussion 568-569. [PubMed]
- Beckurts KT, Hölscher AH, Thorban S, Bollschweiler E, Siewert JR. Significance of lymph node involvement at the hepatic hilum in the resection of colorectal liver metastases. Br J Surg 1997;84:1081-1084. [PubMed]
- Nakamura S, Yokoi Y, Suzuki S, Baba S, Muro H. Results of extensive surgery for liver metastases in colorectal carcinoma. Br J Surg 1992;79:35-38. [PubMed]
- Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer 1989;63:364-367. [PubMed]
- Sadeghi B, Arvieux C, Glehen O, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 2000;88:358-363. [PubMed]
- Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg 2002;89:1545-1550. [PubMed]
- Confuorto G, Giuliano ME, Grimaldi A, Viviano C. Peritoneal carcinomatosis from colorectal cancer: HIPEC? Surg Oncol 2007;16:S149-S152. [PubMed]
- Kusamura S, Baratti D, Zaffaroni N, et al. Pathophysiology and biology of peritoneal carcinomatosis. World J Gastrointest Oncol 2010;2:12-18. [PubMed]
- Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res 1996;82:79-100. [PubMed]
- Elias D, Lefevre JH, Chevalier J, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol 2009;27:681-685. [PubMed]
- Sugarbaker PH, Chang D, Koslowe P. Prognostic features for peritoneal carcinomatosis in colorectal and appendiceal cancer patients when treated by cytoreductive surgery and intraperitoneal chemotherapy. Cancer Treat Res 1996;81:89-104. [PubMed]
- Esquivel J, Sticca R, Sugarbaker P, et al. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of peritoneal surface malignancies of colonic origin: a consensus statement. Society of Surgical Oncology. Ann Surg Oncol 2007;14:128-133. [PubMed]
- Elias D, Blot F, El Otmany A, et al. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 2001;92:71-76. [PubMed]
- Verwaal VJ, van Ruth S, de Bree E, et al. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol 2003;21:3737-3743. [PubMed]
- Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2005;12:65-71. [PubMed]
- Shen P, Hawksworth J, Lovato J, et al. Cytoreductive surgery and intraperitoneal hyperthermic chemotherapy with mitomycin C for peritoneal carcinomatosis from nonappendiceal colorectal carcinoma. Ann Surg Oncol 2004;11:178-186. [PubMed]
- Cao C, Yan TD, Black D, Morris DL. A systematic review and meta-analysis of cytoreductive surgery with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol 2009;16:2152-2165. [PubMed]


