Perioperative management of hepatic resection
Introduction
Colorectal cancer (CRC) represents the third most common malignancy in the United States, and almost half the affected patients will develop hepatic metastases during the course of their disease (1-3). Resection of CRC liver metastases remains the best option for potential cure for selected patients (4,5); however, hepatic resection is not without its inherent risks to the patient. Intraoperatively patients may be subjected to major hemorrhage and hypotension, while postoperatively, issues may include ongoing hemorrhage, coagulopathy, renal failure, cardiac, and pulmonary disturbances in addition to the inherent complications of hepatic resection such a biliary fistula and liver failure. After the initial steps of proper patient selection, management decisions made in the perioperative setting can have lasting implications for surgical recovery and patient survival.
Many of the maneuvers aimed at preparing the patient with colorectal cancer liver metastases for the operating room are geared towards reducing blood loss during surgery, as acute blood loss anemia requiring blood product transfusion remains a challenge in liver surgery (6,7). Transfusion may be associated with poor surgical outcomes, early cancer recurrence, and reduced survival for this subset of patients (8-18). Prior reports have examined the role of transfusion for cancer patients in the perioperative period, and while the precise mechanism is unclear, the generalized immune dysregulation from transfusion has shown to potentially enhance tumor growth, hasten recurrence, and decrease cancer-specific survival (19,20). In the colorectal cancer patient with liver metastasis undergoing hepatectomy, the risk of blood transfusion has been found to be particularly concerning (8,21).
Improvements in surgical technology and technique and perioperative management have resulted in marked reductions in mortality and morbidity over time (6,22). Despite this progress, considerable room remains for further improvement. This chapter will review established and innovative measures to manage CRC liver metastases patients in the perioperative setting. Attention will be given to anesthesia and analgesia, blood conservation and transfusion. Particular attention will be paid to perioperative strategies designed to decrease the need for blood transfusion.
Anesthesia, analgesia, and fluid administration
Optimizing hemodynamics and fluid administration is crucial in patients undergoing major hepatic resection. As with all surgical patients, fluid administration is necessary during operation; however, the balance between providing adequate resuscitation to ensure proper end organ perfusion while maintaining a low patient central venous pressure (0-5 mm Hg) during the parenchymal transection phase to minimize hepatic venous back bleeding is unique to liver surgery. Furthermore, following the acute reduction of hepatic function patients are thrown into some degree of liver failure and may develop substantial ascites and edema. This can precipitate other complications, such as wound breakdown, liver failure and death. The follow section covers the use of invasive monitoring, fluid administration, and epidural anesthesia/analgesia and how to negotiate these techniques and concepts.
Central venous pressure and fluid administration
Communication regarding surgical manipulation and management of hemodynamics between the anesthesiology and surgical staff is a critical component of optimal outcomes. Unless there is a preoperative expectation of extensive vascular involvement, plans for vascular occlusion, or underlying cardiac dysfunction, we do not monitor liver resection patients with Swan Ganz catheters. In our practice, the majority of patients undergoing major hepatic resection are monitored with continuous central venous pressure (CVP). Invasive monitoring is sometimes forgone in the young, thin, healthy individual with tumors away from the major vessels.
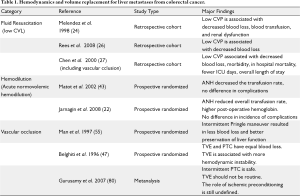
Proper CVP management is crucial to successful liver surgery, and requires open communication between surgeons, anesthesiologists, and physician extenders prior to, during and following surgery. Due to concern of substantial blood loss, some hepatic surgeons advocate for preoperative volume loading to establish a euvolemic or hypervolemic state in anticipation of ensuing intraoperative blood loss (23,24). Other groups, including ours, feel that this distends the central veins and increases the difficulty in controlling blood loss during resection from hepatic veins during parenchymal transaction (24,25). As such, others have supported performing hepatectomies under low CVP (0-5 mm Hg) (see Table 1) (24,26,27). In one study, comparing low CVP strategies to the standard CVP cohort, there was a correlation between blood loss and transfusion with CVP; patients with low CVP had a median blood loss of 200 mL versus 1000 mL, and 2% versus 48% required transfusions (32). This target CVP should be discussed prior to surgery.
Full table
By performing hepatectomies under low CVP, both the blood flow and size of the IVC and other vessels are decreased compared to patients with higher CVPs (24). Mobilization of the liver and dissection of the hepatic veins is facilitated by less distended outflow (24). Further, during parenchymal dissection, hepatic venous bleeding is minimized as a result of the reduced venous distention. In the event that there is inadvertent venous injury during the dissection, the low CVP provides for an operative environment that is more conducive to controlling hemorrhage. Because of these unique physiologic differences compared to matched controls in patients with higher CVPs, multiple groups have demonstrated improved outcomes with low CVP hepatectomy, and have advocated for its universal adoption (26,27). Melendez et al. showed that using low CVP techniques had fewer patients with renal compromise (3% versus 13%). Chen et al. found similar results, with decreased blood loss (725 mL versus 2300 mL, P<0.001) and a reduction in postoperative morbidity (10.3% versus 22.2%, P=0.04) (See Table 1).
Importantly, proper CVP management begins in the preoperative setting, and not only after the patient is intubated. There are several areas where efforts to maintain low intraoperative CVP can be sabotaged inadvertently. Some examples include the preoperative holding area or at induction, where fluids are typically administered at a higher rate to prevent hypotension. It is valuable to communicate with the anesthesia team especially if they are not experienced with hepatic resection in this regard. Any patient who spends a night in the hospital prior to hepatic resection is at risk of overhydration, as fluids are typically administered to patients that are kept NPO. Identifying this risk requires attention to detail prior to surgery.
Rehydration to a euvolemic, physiologic state following hepatic resection while still in the operating room is critical to restoring hepatic and renal perfusion. This process requires strong communication between the operating surgeon, the anesthesia team and the individuals managing the postoperative care, as starting CVP, extent of resection, method of analgesia, and other comorbid factors must be considered when rehydrating to avoid over hydration, which may precipitate development of ascites and an overloaded state. This is a dynamic process, which depends on titration of fluids to blood pressure, urine output, and body weight.
Analgesia
The complexities of hemodynamic management are heightened with the use of different methods of analgesia – one such technique is the use of epidural analgesia. While there is an established utility of epidural analgesia in the cardiothoracic literature (33-36), other groups and ours have shown the benefits of epidural anesthesia in hepatectomy may not be as straightforward, and may predispose risk to transfusion (37-40). In a review of 367 patients, our group found that those who underwent hepatic elective partial hepatectomies with epidural analgesia were independently associated with increased risk of transfusions (OR 3.64, P<0.001) (41).
This relative risk for transfusion may be based on the sympathectometic effect which relaxes vascular smooth muscle and increases venous capacitance, which can result in relative hypotension. If a patient is undergoing low CVP surgery, this relative hypotension caused by the epidural anesthetic may inappropriately lower the threshold for transfusion, particularly at the time of induction and especially if the epidural has already started running. With this mechanistic hypothesis, we and others have further shown that patients with epidurals are predisposed to only to transfusion, but have equivocal pain control when compared with patients without epidurals (41,42). Recognizing the need for larger scale analysis of this issue, others have attempted using the NSQIP database. Unfortunately, the categorization of anesthesia type in the NSQIP does not differentiate general anesthesia from epidural anesthesia, and outcomes for hepatectomy in this dataset are not helpful, but should be examined in future analyses (43). Because of the concerns for epidural analgesia in the hepatectomy patient, Koea et al. compared single dose intrathecal morphine with epidural analgesia and found increased mobilization at post operative day (POD) 1 (P=0.01) and decreased ileus (P=0.03) (44).
Due to the potential fluid shifts associated with epidural analgesia, our group does not advocate for use of epidural anesthesia during hepatectomy. We prefer patient controlled analgesia (PCA) in the post-operative period, with close attention to the patient’s comorbid factors and remnant liver function. Other adjuncts to improve pain control that do not interfere with patient volume status include the use of local anesthetic at time of surgery, and regional pain pumps that infuse local anesthetic to the incision for several days after operation. We are also proponents of icing the wound for the first post-surgical day and placement of lidocaine patches near the wound.
Acute normovolemic hemodilution
In addition to using low CVP techniques and anesthetic modes that target central venous capacitance, other strategies can be used to minimize the loss of red blood cells (RBC). Acute normovolemic hemodilution (ANH) shares the goal of minimizing blood loss and reducing the risk of transfusion with the low CVP approach, but ANH is based on a different paradigm; instead of preventing blood loss, the volume lost is hemodiluted at the start of the case. ANH is performed by withdrawing blood while maintaining euvolemia. By diluting the blood, the blood lost during surgery contains fewer RBCs. Ironically, acute normovolemic hemodilution is not effective in preventing transfusions unless a “goal” blood loss is reached, making it particularly relevant to hepatic surgery (45,46).
This perioperative strategy aimed at dilution begins prior to the start of surgery, when patients are bled to a target hemoglobin of 8.0 g/dL (maximum of 3 L removed) and infused with a combination of albumin and crystalloid to restore isovolemia. Prospective randomized controlled studies demonstrate that it is safe and that ANH protected against allogeneic transfusions (22,28). As compared with standard volume management, Jarnagin et al. demonstrated that ANH resulted in fewer intraoperative transfusions (1.6% versus 10.4%, P=0.04). While interesting in concept, ANH is not routinely used in many centers at this time. We have not adopted this strategy yet in our own practice.
Blood loss-limiting surgical techniques
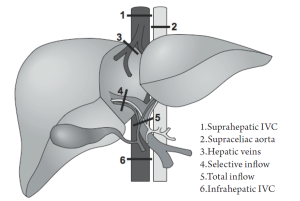
Surgeons can take measures during hepatic parenchymal transection to further limit hemorrhage. These include temporary hepatic inflow occlusion (Pringle maneuver) and total vascular exclusion (TVE). These techniques are designed to isolate hepatic circulation (inflow and/or outflow) from the systemic circulation and minimize blood loss during dissection and transection of the hepatic parenchyma (Figure 1). A central tenet to the success of vascular exclusion is based on the premise that the liver (and patient) is more tolerant to warm ischemia with reperfusion than to bleeding and the consequences of bleeding (e.g. transfusions.).
Pringle maneuver
Originally performed for hepatic trauma, the Pringle maneuver is a straightforward way to minimizing blood loss during hepatectomy (47). A noncrushing clamp or a rumel tourniquet is placed around the structures in the porta hepatis to occlude hepatic venous and arterial inflow during parenchymal transection. This can be performed in an intermittent or continuous manner with similar outcomes. It is recommended that the occlusion time be limited to an hour or less, as the ischemic insult will ultimately result in further hepatic parenchymal loss.
After hepatic pedicle clamping with the Pringle, there is a 10% decrease in the cardiac index with a 40% increase in SVR and a 40% increase in mean arterial pressure (48-51). As compared with the previously mentioned occlusion techniques, the Pringle maneuver is relatively well-tolerated, but the anesthesiology staff should be continuously informed when it is applied because of the possibility of cardiac dysfunction and of air embolism, particularly if the hepatectomy is being done under low CVP. The potential sequela of air emboli, in the patient with a low CVP who may have an open hepatic vein, can be minimized by placing the patient in 15 degree Trendelenberg (24,25,52).
The Pringle maneuver can be applied in a continuous or intermittent fashion. Many retrospective studies and prospective clinical trials have been performed examining the role and type of the Pringle maneuver, and its relationship to blood loss and reperfusion injury. Belghiti et al. compared continuous Pringle to intermittent, and found no difference in total blood loss or blood transfused (1.2 L versus 1.3 L, P=0.42), despite increased blood loss during parenchymal dissection (0.3 L versus 0.5L, P<0.01) (30). Similarly, Man et al. compared intermittent Pringle control with no vascular control showed that using the Pringle, there was less total blood loss (1.3 L vs. 2.0 L, P<0.01), fewer transfusions (0-8.6 L versus 0-12.9 L, P=0.02), and shorter liver transection time per square cm (2.0 min versus 2.8 min, P=0.02) (29). While there is a growing body of literature supporting the use of the Pringle maneuver (continuous or intermittent) in the context of decreasing blood loss and risk of transfusion, there are associated risks of reperfusion injury (53-55). Man et al. examined this concern and found that the Pringle maneuver compared with no vascular control improved post operative liver function based on arterial ketone body ratio and serum bilirubin (P<0.05 for both) (29). This protective effect is a result of both improved hemodynamics because of the Pringle and retrograde flow from the hepatic veins (56). Therefore we recommend the use of the Pringle maneuver when there is concern for blood loss potentially necessitating eventual transfusion.
We prefer the intermittent technique of a period of occlusion of five to 10 minutes followed by several minutes of reperfusion prior to reapplication of the tourniquet. Again, close communication with the anesthesia team is imperative during this period of the operation, as the Pringle maneuver may induce hypotension, especially in a patient where the CVP is kept low intentionally.
Total hepatic vascular exclusion and other methods
Other vascular occlusion techniques have evolved from the Pringle maneuver, including exclusion of the hepatic veins, occlusion of the inferior vena cava (IVC) above and below the liver, and supraceliac aortic control (48). Variations on these techniques can be summarized in the following manner (57):
- Inflow and outflow vascular occlusion
- Total hepatic vascular exclusion
- Inflow occlusion with extraparenchymal control of hepatic veins
- Inflow vascular occlusion
- Hepatic pedicle occlusion (Pringle maneuver)
- Continuous
- Intermittent
- Selective inflow occlusion
- Hemihepatic vascular clamping
- Segmental vascular clamping
- Hepatic pedicle occlusion (Pringle maneuver)
The most complete means of obtaining vascular control prior to parenchymal transection is with total vascular exclusion (TVE). With this technique the Pringle maneuver is performed, followed by a clamp across the infrahepatic IVC above the renal veins, followed by a clamp across the suprahepatic IVC (see Figure 1). After completing the hepatectomy the clamps are removed. This technique requires volume loading to prevent profound hypotension and potential cardiac arrest. Obvious communication between anesthesiology staff should be made throughout TVE, as hemodynamic instability is likely and potentially profound with venous return decreasing 50% and systemic vascular resistance increasing 80% (7,30). In our experience this technique is seldom used or necessary, but other groups are more aggressive with this approach.
Inflow occlusion with extraparenchymal control of hepatic veins is similar to TVE, but does not disrupt caval flow, thereby decreasing the likelihood of hemodynamic instability (57). In order to gain access to the hepatic veins, full mobilization of the liver is required with ligation of all short hepatic veins and liver ligaments. The remaining main hepatic veins are then dissected and looped. The Pringle is then applied in coordination with occlusion of the major hepatic veins. The Pringle maneuver can be done intermittently or continuously (but if intermittent, the hepatic veins must be unclamped as well in coordination with the Pringle). This modality has particular utility for patients with more centrally located metastases who may potentially benefit from TVE, but cannot tolerate the associated hemodynamic shifts because of underlying comorbid cardiac dysfunction or renal disease, or for patients that cannot tolerate low CVP surgery (57,58).
Selective inflow occlusion is technically more demanding and typically performed in higher risk patients with cirrhosis. In hemihepatic vascular clamping, selective occlusion of portal and arterial inflow is achieved on the side of the resection at the hilar level, preserving inflow and avoiding reperfusion to the unaffected side. Simultaneous occlusion of the major ipsilateral hepatic vein may also be performed. Segmental occlusion is an even more precise means of gaining vascular control and decreasing blood loss. This is achieved by occluding the hepatic artery inflow to that segment after hilar dissection. The portal vein branch is identified by ultrasound and a wire is threaded into the designated portal branch. A balloon is threaded over the branch and inflated, occluding the portal inflow. Dye can be injected into the portal catheter to tattoo the segment. Similar to selective inflow occlusion, this modality can be employed with cirrhotic patients with metastases isolated to periphery (59,60).
Considerations specific to colorectal cancer metastasis
In addition to the critical communication with the anesthesiology and surgery teams in the immediate preoperative and intraoperative period relating to CVP, vascular occlusion, hemodilution, and pain management, a similar didactic is necessary between medical oncologists and surgeons as it relates to adjuvant therapy, liver parenchyma, and indications and timing of hepatectomy. While we have earlier described data and progress in the hepatectomy technique grossly in terms of all hepatic disease, there is growing body of literature specific to adjuvant therapy for hepatectomies from colorectal metastases.
The mainstay neoadjuvant systemic chemotherapy for colorectal metastases has been 5-Fluorouracil (5-FU) with leucovorin and oxaliplatin (FOLFOX), or 5-FU and irinotecan (FOLFIRI). These treatment modalities have been adopted for patients in attempts to minimize the area to be resected, to create a resectable lesion, and to potentially improve oncologic outcomes (61). However, these benefits are taken against the risks of chemotherapy induced parenchymal damage including steatosis, steatohepatitis, and sinusoidal obstruction (SOS).
Steatosis (fatty liver disease) is most recognized in alcoholic hepatitis and nonalcoholic fatty liver disease (NAFLD). This pathology is represented macroscopically as a yellow liver, and histologically by retained lipid in micro and macrovesicles, altering the normal architecture of hepatocytes and their associated function (62). Steatohepatitis represents progression of steatosis, presumably from oxidative stress which causes lipid peroxidation and the development of necrotizing inflammation and unregulated hepatocellular apoptosis (63-65). Sinusoidal obstruction syndrome (SOS) represents the endpoint of progression of chemotherapy toxicity. Microscopically this condition is represented by edema of central zone hepatocytes and fibrosis and congestion of the sinusoids (66-68).
5-Fluorouracil (5-FU) alone has been reported to induce steatosis in 40-47% of patients (69-71). Addition of the platinum based agents like oxaliplatin or the topoisomerase inhibitor irinotecan has also shown to have hepatic toxicity with oxaliplatin being independently associated with steatohepatitis and irinotecan with SOS (65). The addition of the anti-VEGF antibody bevacizumab has increasing adoption as a chemotherapeutic and it is found to have a protective effect against oxaliplatin induced SOS (72).
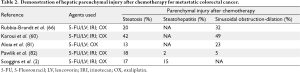
Taking into account neoadjuvant chemotherapy toxicities, multiple groups have examined perioperative outcomes as they relate to steatosis, steatohepatitis, and SOS (Table 2). Patients with steatosis after chemotherapy and eventual hepatectomy are predisposed to increased post-operative complications, but without increased mortality (75-77). For patients with steatohepatitis, there is a more significant effect on post operative liver function and patient survival following resection (65). Fewer studies have directly examined SOS as a perioperative risk factor, but as described earlier, the venous congestion in this condition predisposes to risk of transfusion, and likely the detrimental effects of transfusions are consequently involved (78).
Full table
While many groups have examined these histopathologies as they relate to perioperative outcomes, there is little consensus on the time interval between neoadjuvant therapy and hepatectomy and duration of chemotherapy. Welsh et al. showed that patients with a history of neoadjuvant chemotherapy had increased post-operative complications, with a duration of greater than five weeks protecting against complications (79). Karoui et al. came to similar conclusions that increased cycles of chemotherapy predisposed to increased complications compared to patients with fewer cycles (61).
The role of portal vein embolization (PVE) for colorectal metastasis is also expanding as it can increase the future liver remnant (FLR) by hypertrophy. By incorporating PVE, the recognized FLR of 20% of the native liver or 2 contiguous segments can be achieved when initial imaging of the metastatic lesion may preclude resection. While there is no study to date, for patients with underlying hepatic pathology after chemotherapy, there may be increased utility for PVE to increase the FLR to a larger threshold in order avoid the more established complications of patients with steatosis, steatohepatitis, and SOS (80).
Just as PVE should be considered as an adjunctive preoperative therapy for patients with underlying parenchymal pathologies, the methods of intraoperative vascular occlusion described above should also be examined. Experimental rodent models have expectedly shown that damaged livers with steatosis do not tolerate warm ischemia, potentially indicating that the pretreated liver with parenchymal damage may need special consideration to warranting ischemic preconditioning and less aggressive vascular occlusion techniques (81,82).
Conclusion
While hepatectomy for colorectal metastasis has the potential for significant blood loss requiring transfusions, a multifaceted paradigm in the perioperative period can be used to minimize blood loss. By minimizing blood loss and subsequent transfusions, the nonspecific immunosuppressive effects of allotransplantation of blood can be avoided and both perioperative and oncologic outcomes will be optimized. Coordinated efforts with medical oncologists, anesthesiologists, and the surgical teams are crucial in order to reach this goal.
Acknowledgements
The authors thank Dr. Eugenia Page, General Surgery Resident for her illustration.
Footnote
No potential conflict of interest.
References
- Sahajpal A, Vollmer CM Jr, Dixon E, et al. Chemotherapy for colorectal cancer prior to liver resection for colorectal cancer hepatic metastases does not adversely affect peri-operative outcomes. J Surg Oncol 2007;95:22-27. [PubMed]
- Scoggins CR, Campbell ML, Landry CS, et al. Preoperative chemotherapy does not increase morbidity or mortality of hepatic resection for colorectal cancer metastases. Ann Surg Oncol 2009;16:35-41. [PubMed]
- Khatri VP, Petrelli NJ, Belghiti J. Extending the frontiers of surgical therapy for hepatic colorectal metastases: is there a limit? J Clin Oncol 2005;23:8490-8499. [PubMed]
- Fong Y, Fortner J, Sun RL, Brennan MF, Blumgart LH. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 1999;230:309-318, discussion 318-321. [PubMed]
- Jamison RL, Donohue JH, Nagorney DM, Rosen CB, Harmsen WS, Ilstrup DM. Hepatic resection for metastatic colorectal cancer results in cure for some patients. Arch Surg 1997;132:505-510, discussion 511. [PubMed]
- Agrawal S, Belghiti J. Oncologic resection for malignant tumors of the liver. Ann Surg 2011;253:656-665. [PubMed]
- Emre S, Schwartz ME, Katz E, Miller CM. Liver resection under total vascular isolation. Variations on a theme. Ann Surg 1993;217:15-19. [PubMed]
- Kooby DA, Stockman J, Ben-Porat L, et al. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 2003;237:860-869, discussion 869-870. [PubMed]
- Gozzetti G, Mazziotti A, Grazi GL, et al. Liver resection without blood transfusion. Br J Surg 1995;82:1105-1110. [PubMed]
- Rosen CB, Nagorney DM, Taswell HF, et al. Perioperative blood transfusion and determinants of survival after liver resection for metastatic colorectal carcinoma. Ann Surg 1992;216:493-504, discussion 504-505. [PubMed]
- Asahara T, Katayama K, Itamoto T, et al. Perioperative blood transfusion as a prognostic indicator in patients with hepatocellular carcinoma. World J Surg 1999;23:676-680. [PubMed]
- Tartter PI, Burrows L, Kirschner P. Perioperative blood transfusion adversely affects prognosis after resection of Stage I (subset N0) non-oat cell lung cancer. J Thorac Cardiovasc Surg 1984;88:659-662. [PubMed]
- Kaneda M, Horimi T, Ninomiya M, et al. Adverse affect of blood transfusions on survival of patients with gastric cancer. Transfusion 1987;27:375-377. [PubMed]
- Foster RS Jr, Foster JC, Costanza MC. Blood transfusions and survival after surgery for breast cancer. Arch Surg 1984;119:1138-1140. [PubMed]
- Hyman NH, Foster RS Jr, DeMeules JE, Costanza MC. Blood transfusions and survival after lung cancer resection. Am J Surg 1985;149:502-507. [PubMed]
- Fong Y, Karpeh M, Mayer K, Brennan MF. Association of perioperative transfusions with poor outcome in resection of gastric adenocarcinoma. Am J Surg 1994;167:256-260. [PubMed]
- Yeh JJ, Gonen M, Tomlinson JS, Idrees K, Brennan MF, Fong Y. Effect of blood transfusion on outcome after pancreaticoduodenectomy for exocrine tumour of the pancreas. Br J Surg 2007;94:466-472. [PubMed]
- Yamamoto J, Kosuge T, Takayama T, et al. Perioperative blood transfusion promotes recurrence of hepatocellular carcinoma after hepatectomy. Surgery 1994;115:303-309. [PubMed]
- Blumberg N, Heal JM. Transfusion-induced immunomodulation and its possible role in cancer recurrence and perioperative bacterial infection. Yale J Biol Med 1990;63:429-433. [PubMed]
- Schriemer PA, Longnecker DE, Mintz PD. The possible immunosuppressive effects of perioperative blood transfusion in cancer patients. Anesthesiology 1988;68:422-428. [PubMed]
- Park JO, Gonen M, D’Angelica MI, et al. Autologous versus allogeneic transfusions: no difference in perioperative outcome after partial hepatectomy. Autologous transfusion on hepatectomy outcome. J Gastrointest Surg 2007;11:1286-1293. [PubMed]
- Jarnagin WR, Gonen M, Maithel SK, et al. A prospective randomized trial of acute normovolemic hemodilution compared to standard intraoperative management in patients undergoing major hepatic resection. Ann Surg 2008;248:360-369. [PubMed]
- Thompson HH, Tompkins RK, Longmire WP Jr. Major hepatic resection. A 25-year experience. Ann Surg 1983;197:375-388. [PubMed]
- Melendez JA, Arslan V, Fischer ME, et al. Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 1998;187:620-625. [PubMed]
- Cunningham JD, Fong Y, Shriver C, Melendez J, Marx WL, Blumgart LH. One hundred consecutive hepatic resections. Blood loss, transfusion, and operative technique. Arch Surg 1994;129:1050-1056. [PubMed]
- Rees M, Tekkis PP, Welsh FK, O’Rourke T, John TG. Evaluation of long-term survival after hepatic resection for metastatic colorectal cancer: a multifactorial model of 929 patients. Ann Surg 2008;247:125-135. [PubMed]
- Chen H, Merchant NB, Didolkar MS. Hepatic resection using intermittent vascular inflow occlusion and low central venous pressure anesthesia improves morbidity and mortality. J Gastrointest Surg 2000;4:162-167. [PubMed]
- Matot I, Scheinin O, Jurim O, Eid A. Effectiveness of acute normovolemic hemodilution to minimize allogeneic blood transfusion in major liver resections. Anesthesiology 2002;97:794-800. [PubMed]
- Man K, Fan ST, Ng IO, Lo CM, Liu CL, Wong J. Prospective evaluation of Pringle maneuver in hepatectomy for liver tumors by a randomized study. Ann Surg 1997;226:704-711, discussion 711-713. [PubMed]
- Belghiti J, Noun R, Zante E, Ballet T, Sauvanet A. Portal triad clamping or hepatic vascular exclusion for major liver resection. A controlled study. Ann Surg 1996;224:155-161. [PubMed]
- Gurusamy KS, Sheth H, Kumar Y, Sharma D, Davidson BR. Methods of vascular occlusion for elective liver resections. Cochrane Database Syst Rev 2009;CD007632. [PubMed]
- Jones RM, Moulton CE, Hardy KJ. Central venous pressure and its effect on blood loss during liver resection. Br J Surg 1998;85:1058-1060. [PubMed]
- Rigg JR, Jamrozik K, Myles PS, et al. Epidural anaesthesia and analgesia and outcome of major surgery: a randomised trial. Lancet 2002;359:1276-1282. [PubMed]
- Scott NB, Turfrey DJ, Ray DA, et al. A prospective randomized study of the potential benefits of thoracic epidural anesthesia and analgesia in patients undergoing coronary artery bypass grafting. Anesth Analg 2001;93:528-535. [PubMed]
- Block BM, Liu SS, Rowlingson AJ, Cowan AR, Cowan JA Jr, Wu CL. Efficacy of postoperative epidural analgesia: a meta-analysis. JAMA 2003;290:2455-2463. [PubMed]
- Pöpping DM, Elia N, Marret E, Remy C, Tramèr MR. Protective effects of epidural analgesia on pulmonary complications after abdominal and thoracic surgery: a meta-analysis. Arch Surg 2008;143:990-999, discussion 1000. [PubMed]
- Bracco D, Hemmerling T. Epidural analgesia in cardiac surgery: an updated risk assessment. Heart Surg Forum 2007;10:E334-E337. [PubMed]
- Jaigirdar MJ, Ahmed S, Conlon N. Should limited term intraoperative epidural analgesia be used in patients undergoing hepatic resection? Anesth Analg 2011;112:993-994, author reply 994. [PubMed]
- Lundstrøm LH, Nygård E, Hviid LB, et al. The effect of thoracic epidural analgesia on the occurrence of late postoperative hypoxemia in patients undergoing elective coronary bypass surgery: a randomized controlled trial. Chest 2005;128:1564-1570. [PubMed]
- Nierich AP, Passier M, Kalkman CJ, et al. Epidural analgesia for cardiac surgery. Cochrane Libr 2009;
- Page A, Rostad B, Staley CA, et al. Epidural analgesia in hepatic resection. J Am Coll Surg 2008;206:1184-1192. [PubMed]
- Matot I, Scheinin O, Eid A, Jurim O. Epidural anesthesia and analgesia in liver resection. Anesth Analg 2002;95:1179-1181. table of contents.. [PubMed]
- Aloia TA, Fahy BN, Fischer CP, et al. Predicting poor outcome following hepatectomy: analysis of 2313 hepatectomies in the NSQIP database. HPB (Oxford) 2009;11:510-515. [PubMed]
- Koea JB, Young Y, Gunn K. Fast track liver resection: the effect of a comprehensive care package and analgesia with single dose intrathecal morphine with gabapentin or continuous epidural analgesia. HPB Surg 2009;2009:271986.
- Weiskopf RB. Efficacy of acute normovolemic hemodilution assessed as a function of fraction of blood volume lost. Anesthesiology 2001;94:439-446. [PubMed]
- Weiskopf RB. Hemodilution and candles. Anesthesiology 2002;97:773-775. [PubMed]
- Pringle JH. V. Notes on the Arrest of Hepatic Hemorrhage Due to Trauma. Ann Surg 1908;48:541-549. [PubMed]
- Heaney JP, Stanton WK, Halbert DS, Seidel J, Vice T. An improved technic for vascular isolation of the liver: experimental study and case reports. Ann Surg 1966;163:237-241. [PubMed]
- Delva E, Camus Y, Nordlinger B, et al. Vascular occlusions for liver resections. Operative management and tolerance to hepatic ischemia: 142 cases. Ann Surg 1989;209:211-218. [PubMed]
- Clavien PA, Selzner M, Rüdiger HA, et al. A prospective randomized study in 100 consecutive patients undergoing major liver resection with versus without ischemic preconditioning. Ann Surg 2003;238:843-850, discussion 851-852. [PubMed]
- Belghiti J, Noun R, Malafosse R, et al. Continuous versus intermittent portal triad clamping for liver resection: a controlled study. Ann Surg 1999;229:369-375. [PubMed]
- Hatano Y, Murakawa M, Segawa H, Nishida Y, Mori K. Venous air embolism during hepatic resection. Anesthesiology 1990;73:1282-1285. [PubMed]
- Kim YI, Chung HJ, Song KE, et al. Evaluation of a protease inhibitor in the prevention of ischemia and reperfusion injury in hepatectomy under intermittent Pringle maneuver. Am J Surg 2006;191:72-76. [PubMed]
- Clavien PA, Yadav S, Sindram D, Bentley RC. Protective effects of ischemic preconditioning for liver resection performed under inflow occlusion in humans. Ann Surg 2000;232:155-162. [PubMed]
- Lentsch AB, Kato A, Yoshidome H, McMasters KM, Edwards MJ. Inflammatory mechanisms and therapeutic strategies for warm hepatic ischemia/reperfusion injury. Hepatology 2000;32:169-173. [PubMed]
- Kim YI, Ishii T, Aramaki M, Nakashima K, Yoshida T, Kobayashi M. The Pringle maneuver induces only partial ischemia of the liver. Hepatogastroenterology 1995;42:169-171. [PubMed]
- Smyrniotis V, Farantos C, Kostopanagiotou G, Arkadopoulos N. Vascular control during hepatectomy: review of methods and results. World J Surg 2005;29:1384-1396. [PubMed]
- Bismuth H, Castaing D, Garden OJ. Major hepatic resection under total vascular exclusion. Ann Surg 1989;210:13-19. [PubMed]
- Goseki N, Kato S, Takamatsu S, et al. Hepatic resection under the intermittent selective portal branch occlusion by balloon catheter. J Am Coll Surg 1994;179:673-678. [PubMed]
- Castaing D, Garden OJ, Bismuth H. Segmental liver resection using ultrasound-guided selective portal venous occlusion. Ann Surg 1989;210:20-23. [PubMed]
- Karoui M, Penna C, Amin-Hashem M, et al. Influence of preoperative chemotherapy on the risk of major hepatectomy for colorectal liver metastases. Ann Surg 2006;243:1-7. [PubMed]
- Hübscher SG. Histological assessment of non-alcoholic fatty liver disease. Histopathology 2006;49:450-465. [PubMed]
- Day CP. Pathogenesis of steatohepatitis. Best Pract Res Clin Gastroenterol 2002;16:663-678. [PubMed]
- Fong Y, Bentrem DJ. CASH (Chemotherapy-Associated Steatohepatitis) costs. Ann Surg 2006;243:8-9. [PubMed]
- Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol 2006;24:2065-2072. [PubMed]
- Rubbia-Brandt L, Audard V, Sartoretti P, et al. Severe hepatic sinusoidal obstruction associated with oxaliplatin-based chemotherapy in patients with metastatic colorectal cancer. Ann Oncol 2004;15:460-466. [PubMed]
- Rubbia-Brandt L, Mentha G, Terris B. Sinusoidal obstruction syndrome is a major feature of hepatic lesions associated with oxaliplatin neoadjuvant chemotherapy for liver colorectal metastases. J Am Coll Surg 2006;202:199-200. [PubMed]
- DeLeve LD, Shulman HM, McDonald GB. Toxic injury to hepatic sinusoids: sinusoidal obstruction syndrome (veno-occlusive disease). Semin Liver Dis 2002;22:27-42. [PubMed]
- Peppercorn PD, Reznek RH, Wilson P, Slevin ML, Gupta RK. Demonstration of hepatic steatosis by computerized tomography in patients receiving 5-fluorouracil-based therapy for advanced colorectal cancer. Br J Cancer 1998;77:2008-2011. [PubMed]
- Zeiss J, Merrick HW, Savolaine ER, Woldenberg LS, Kim K, Schlembach PJ. Fatty liver change as a result of hepatic artery infusion chemotherapy. Am J Clin Oncol 1990;13:156-160. [PubMed]
- Sørensen P, Edal AL, Madsen EL, Fenger C, Poulsen MR, Petersen OF. Reversible hepatic steatosis in patients treated with interferon alfa-2a and 5-fluorouracil. Cancer 1995;75:2592-2596. [PubMed]
- Ribero D, Wang H, Donadon M, et al. Bevacizumab improves pathologic response and protects against hepatic injury in patients treated with oxaliplatin-based chemotherapy for colorectal liver metastases. Cancer 2007;110:2761-2767. [PubMed]
- Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol 2006;24:4983-4990. [PubMed]
- Pawlik TM, Olino K, Gleisner AL, Torbenson M, Schulick R, Choti MA. Preoperative chemotherapy for colorectal liver metastases: impact on hepatic histology and postoperative outcome. J Gastrointest Surg 2007;11:860-868. [PubMed]
- McCormack L, Petrowsky H, Jochum W, Furrer K, Clavien PA. Hepatic steatosis is a risk factor for postoperative complications after major hepatectomy: a matched case-control study. Ann Surg 2007;245:923-930. [PubMed]
- Kooby D, Fong Y, Gonen M, et al. Hepatic steatosis is associated with increased complications following major liver resection for cancer but does not impact survival. Gastroenterology 2003;124:A794.
- Kooby DA, Fong Y, Suriawinata A, et al. Impact of steatosis on perioperative outcome following hepatic resection. J Gastrointest Surg 2003;7:1034-1044. [PubMed]
- Nakano H, Oussoultzoglou E, Rosso E, et al. Sinusoidal injury increases morbidity after major hepatectomy in patients with colorectal liver metastases receiving preoperative chemotherapy. Ann Surg 2008;247:118-124. [PubMed]
- Welsh FK, Tilney HS, Tekkis PP, John TG, Rees M. Safe liver resection following chemotherapy for colorectal metastases is a matter of timing. Br J Cancer 2007;96:1037-1042. [PubMed]
- Kneuertz PJ, Maithel SK, Staley CA, Kooby DA. Chemotherapy-associated liver injury: impact on surgical management of colorectal cancer liver metastases. Ann Surg Oncol 2011;18:181-190. [PubMed]
- Fiorini RN, Donovan JL, Rodwell D, et al. Short-term administration of (-)-epigallocatechin gallate reduces hepatic steatosis and protects against warm hepatic ischemia/reperfusion injury in steatotic mice. Liver Transpl 2005;11:298-308. [PubMed]
- Sun CK, Zhang XY, Zimmermann A, Davis G, Wheatley AM. Effect of ischemia-reperfusion injury on the microcirculation of the steatotic liver of the Zucker rat. Transplantation 2001;72:1625-1631. [PubMed]