
Making sense of adjuvant chemotherapy in colorectal cancer
Introduction
At diagnosis, surgical resection remains the mainstay of treatment for stage II and III colon cancers, with a 5-year overall survival (OS) of 80% and 60%, respectively. The goal of adjuvant chemotherapy is to eradicate micro-metastatic disease and improve survival. Over the last three decades, there have been multiple adjuvant chemotherapy trials conducted with the aim of improving survival. In this review, we discuss the evolution of adjuvant chemotherapy in the treatment of colorectal cancer and how the evidence impacts our clinical management today.
Colon cancer
Adjuvant chemotherapy in stage III colon cancer
In stage III colon cancer, 2- and 3-year disease-free survival (DFS) are accepted endpoints for clinical trials looking at the benefit of adjuvant therapy. This is based on the Adjuvant Colon Cancer End Points (ACCENT) collaborative group analysis which showed that 2- and 3-year DFS correlated with 5- and 6-year OS in colon cancer patients who received 5-fluorouracil (5-FU) based adjuvant chemotherapy (1). An update of this analysis showed that most relapses occur early (i.e., within 2 years after surgery) and that recurrence rates were less than 1.5% per year after five years (2).
With regards to timing of adjuvant chemotherapy, the increased delay to adjuvant chemotherapy was associated with worse survival among patients with resected colorectal cancer. Meta-analyses have reported that a 4-week increase in time to adjuvant chemotherapy and a delay of adjuvant chemotherapy beyond 8 weeks after surgery were associated with decrease in OS (3). Therefore, it is recommended that adjuvant chemotherapy is initiated as soon as the patient is medically able, where feasible within 12 weeks.
Benefit of specific regimens
5-fluourouracil (5-FU)-based chemotherapy
In 1990, the benefits of adjuvant chemotherapy were first demonstrated in a pivotal trial by Moertel et al. The study demonstrated improved survival and decreased recurrence from adjuvant chemotherapy with 5-FU and levamisole administered for 12 months after surgery over surgery alone (4). Subsequent trials demonstrated that 5-FU combination with leucovorin (LV) was superior (5-7), with 6 months of therapy adequate to achieve similar OS benefits compared to 12 months (8). Survival advantages have been demonstrated both with Mayo Clinic regimen (daily 5-FU bolus for five days in a 28-day cycle, for 6 months) or the Roswell Park regimen (weekly 5-FU bolus for 6 weeks in an 8-week cycle, for 6 months). Toxicity profile differed however, with more stomatitis and neutropenia with the former, and diarrhea with the latter. Infusional 5-FU regimens have also been evaluated and found to be as efficacious as bolus regimens with less toxicities (9). In the X-ACT trial, oral capecitabine was established to be as convenient, safe and equally effective alternative to the Mayo Clinic regimen for stage III colon cancer (10). The oral fluoropyrimidine capecitabine generates 5-FU preferentially in tumor tissue by way of a three-step enzymatic cascade. The final conversion of 5-FU is catalyzed by thymidine phosphorylase, which is more active in tumor than healthy tissue.
Oxaliplatin-based therapies
Oxaliplatin was approved as part of adjuvant treatment for stage III colon cancer in 2004 based on a 3-year DFS endpoint. The benefit of adding oxaliplatin to 5-FU or capecitabine carries a 20% relative risk reduction for DFS, which translates into similar improvements in overall survival (11-13). In the landmark MOSAIC trial, the addition of oxaliplatin to 5-FU based chemotherapy contributed to an absolute improvement of 5-year DFS rates and 6-year OS rates by 5.9% and 2.5% respectively (13). The XELOXA trial compared adjuvant capecitabine and oxaliplatin combination (XELOX) with bolus 5-FU/LV in stage III colon cancer, with a similar magnitude of benefit of DFS seen (11).
Regimens not recommended
Although irinotecan and oxaliplatin based regimens are thought to be equally effective in the metastatic setting, the incorporation of irinotecan to 5-FU based chemotherapy in multiple randomized, phase 3 studies was not superior to 5-FU alone in the adjuvant setting. The current evidence does not support the use of irinotecan-containing regimens in the adjuvant setting (14-17). Lack of benefit has also been observed with both anti-VEGF and anti-EGFR therapeutic antibodies in the adjuvant setting. In contrast to the trials with irinotecan-based chemotherapy, some of these trials even show worsened patient outcomes. Two randomized phase 3 trials compared a standard fluorouracil-oxaliplatin regimen to the same regimen plus bevacizumab during chemotherapy and continued for 1 year. In the AVANT study, addition of bevacizumab had no benefit, and even showed a significant reduction in OS (18). Similarly, the PETACC-8 and N0147 trials randomized patients with KRAS wild type tumors to receive FOLFOX with or without cetuximab (19,20). Both trials showed no survival benefit with the addition of cetuximab but potential harm in the cetuximab arm in N0147. Therefore, irinotecan-based chemotherapy, anti-VEGF and anti-EGFR therapies are not recommended as adjuvant treatment.
Duration of adjuvant chemotherapy
As discussed earlier, six months of oxaliplatin-based chemotherapy had been accepted as the standard for the last 15 years. However, cumulative doses of oxaliplatin increases the risk of long-term sensory neuropathy which can be debilitating. This led to the investigation of whether a shorter duration of adjuvant chemotherapy for colon cancer would lessen toxicities but provide similar survival benefit. The International Duration Evaluation of Adjuvant Therapy (IDEA) collaboration enrolled 12,834 patients with stage III colon cancer in six phase 3 trials in 12 countries (21). All patients were randomly assigned to either three or six months of adjuvant chemotherapy with either FOLFOX or XELOX (CAPOX). The primary endpoint of 3-year DFS did not meet the prespecified cut-off for noninferiority in the overall population. However, non-inferiority was observed in certain subgroups. In the pre-specified subgroup analysis according to treatment (XELOX vs. FOLFOX), six months of adjuvant FOLFOX was superior to three months, with a difference in DFS rate of 2.4% (73.6% vs. 76.0%). Among patients who received XELOX, three months of XELOX was non-inferior to six months for all stages combined. Specifically, in the low- risk (T1-3, N1) subgroup, the DFS for three months of XELOX was noninferior to six months of XELOX (HR, 0.85; 95% CI, 0.71–1.01). However, in the high-risk (T4 and/or N2) subgroup, DFS for three months of FOLFOX was inferior to six months of FOLFOX (HR, 1.20; 95% CI, 1.07–1.35) (21). The study also showed that shorter duration of chemotherapy was associated with lower rates of adverse events and peripheral neuropathy compared to longer duration of chemotherapy.
The results of the IDEA collaboration have led to a change in practice whereby in low-risk stage III disease (T1-3, N1), the duration of adjuvant oxaliplatin-based chemotherapy may be reduced to three months. In high-risk stage III disease (T4 and/or N2), six months of adjuvant chemotherapy with an oxaliplatin-based doublet chemotherapy remains the standard (22). A shared-decision making approach and discussion about the risks and benefits of adjuvant chemotherapy with the patient is encouraged.
Application in clinical practice
Adjuvant chemotherapy is recommended in stage III colon cancer. It should be initiated within 12 weeks from surgery, earlier if medically able. In low-risk stage III disease (T1-3, N1), the length of adjuvant chemotherapy can be three months (particularly, if XELOX is used) or six months, after discussion with the patient about the risks and benefits of adjuvant chemotherapy and its duration. In high-risk stage III disease (T4 and/or N2), six months of adjuvant chemotherapy with an oxaliplatin-based doublet chemotherapy is still recommended.
Adjuvant chemotherapy in stage II colon cancer
As compared to stage III disease where adjuvant chemotherapy is universally recommended, the decision for adjuvant chemotherapy for patients with stage II disease remains challenging. Surgery alone offers excellent outcomes and the small benefit for adjuvant chemotherapy in unselected patients, when weighed against toxicities, inconvenience and cost makes it difficult to justify this recommendation for all patients (23).
Clinicopathological markers of prediction
The analysis of survival outcomes by American Joint Committee on Cancer (AJCC) shows that patients with stage IIB or IIC (T4 disease) are inferior in outcomes compared to stage IIIA (T3 disease) (24). Thus, the recommendation for consideration of adjuvant chemotherapy despite the lack of direct evidence from randomised controlled trials is based on extrapolating the relative benefits of adjuvant therapy in stage III disease (25). Currently, there is uniformity in what many international clinical guidelines recognise as high-risk clinicopathologic features in selecting patients with stage II colon cancer for adjuvant treatment. These features include less than 12 lymph nodes (LNs) identified at surgery, T4 tumor, poorly differentiated tumor [excluding microsatellite instability-high (MSI-H) tumors], presence of bowel obstruction or perforation, and presence of lymphovascular invasion. Definitions of “high-risk” stage II colon cancer from expert groups are outlined in Table 1. Although these factors are associated with a poorer prognosis, they are not predictive of chemotherapy response.
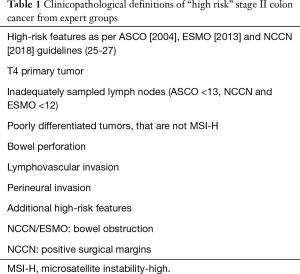
Full table
5-FU-based chemotherapy in stage II colon cancer
Many trials have investigated the benefit of adjuvant chemotherapy in stage II colon cancer. A meta-analysis of five studies with 1,016 patients from the IMPACT B2 investigators, a pooled analysis of seven trials with 3,302 patients, and an analysis of the Surveillance, Epidemiology, and End Results (SEER) database of 3,151 stage II patients have shown no benefit with adjuvant therapy in stage II disease (28-30). Contrary to these studies is the Quick and Simple and Reliable (QUASAR) study, which showed a modest (∼4%) absolute benefit despite a 20% relative reduction in the risk of recurrence and death (23). In this study, patients with stage II colon cancer were randomized to receive 5-FU chemotherapy or observation after surgery. It has been shown in previous trials that a minimum of 13 lymph nodes should be sampled to stage a colon cancer as node negative (31), and one major criticism of the study is that more than 60% of patients had less than 12 lymph nodes resected.
Oxaliplatin-based chemotherapy
The utility of oxaliplatin-based chemotherapy in stage II disease is based on a subgroup analysis from the MOSAIC study. In patients with stage II disease, the DFS benefit for FOLFOX compared to 5-FU/LV alone was approximately 3.5%. This benefit exceeded 5% in patients with stage II tumors with clinical high-risk features (undifferentiated tumors, T4, perforation, obstruction, fewer than 10 lymph nodes identified and lymphovascular invasion) (32). Further updates of the study demonstrated a significant 6-year OS benefit for patients with stage III, but not for stage II disease when an oxaliplatin-based regimen was used as adjuvant therapy (13).
Molecular biomarkers of prediction
MMR genes (MLH1, MSH2, MSH6 and PMS2) are required for the correction of nucleotide base mispairings that occur during DNA replication (33). This may be due to a consequence of a germline mutation (as in Lynch Syndrome), or more commonly an epigenetic silencing of MMR in sporadic colorectal cancers (34). MMR deficient cells accumulate errors during DNA replication, which lead to formation of abnormalities in short sequences of nucleotide bases called microsatellites (35). MMR status can be identified with immunohistochemistry through loss of expression of MMR proteins, and is highly concordant (>95%) with MSI testing using polymerase chain reaction (PCR), in which dMMR corresponds to high-degree MSI (MSI-H) (36). MSI-H or dMMR status are used as prognostic and predictive molecular biomarkers in stage II disease. Approximately 20% of stage II colon cancers have dMMR phenotype, and this is a prognostic marker of a more favourable outcome (37). These patients receive no benefit from fluoropyrimidine-based adjuvant therapy (38,39). Routine testing of MSI status should be performed for all patients with stage II colon cancer to select patients with excellent prognosis and spare them from adjuvant 5-FU chemotherapy (26).
The uptake of gene expression signatures to guide adjuvant treatment decisions to avoid overtreatment is considered mainstream in breast cancer and there have been efforts to do the same in colon cancer. Of these multi-gene assays (40), Oncotype DX colon have been studied the most extensively—while these assays can identify a subset of stage II patients with increased risk of disease recurrence, they have a limited role in predicting the true benefit of adjuvant therapy (41-43).
Of the biomarkers being studied for risk stratification of disease recurrence, circulating tumour DNA (ctDNA) holds the most promise. Seventy-nine per cent of patients with detectable ctDNA after oncological resection experienced disease recurrence, compared to only 9.8% of those without detectable ctDNA, independent of known clinical high-risk features (44). If validated in larger phase III clinical trials, ctDNA may allow for a non-invasive approach of determining disease recurrence and identifying patients who are most likely to benefit from adjuvant therapy.
There is growing importance of using immune score (Immunoscore) as a prognostic factor for early-stage colon cancer. Immunoscore is the calculation of the mean of the four percentiles obtained for CD3+ and CD8+ T cell counts at either the tumor center or invasive margin. From an analysis of 3,539 patients with early stage colorectal cancer, Immunoscore was validated as a prognostic assay for recurrence-free survival, DFS and OS. It identified a subgroup of high-risk patients as the risk of recurrence at 3-years was significantly reduced in patients with a low Immunoscore compared with those who had a high Immunoscore (45). Its applicability in practice to guide clinical decisions will need to be evaluated in prospective studies.
Application in clinical practice
For patients with stage II colon cancer, 5-FU-based chemotherapy is recommended for 6 months if the tumor has high-risk clinicopathological features. There is minimum benefit with the addition of oxaliplatin. MSI or MMR status should be checked in all stage II tumors to select patients (with MSI-high or dMMR status) who may not benefit from adjuvant chemotherapy. Currently, there is insufficient evidence to recommend the routine use of multigene assays in colon cancer, ctDNA or Immunoscore to determine the need of adjuvant therapy. A shared decision-making approach should be used for patients with stage II colorectal cancer, weighing the risks versus benefits of treatment for each individual.
Adjuvant chemotherapy in the elderly
The median age of patients at diagnosis of colon cancer is 70 and there is a need to comprehensively evaluate elderly patients to estimate individual risk/benefit ratios for adjuvant treatment. Many clinical trials enrol younger patients and conclusions drawn from treating elderly patients are dependent on subgroup analyses. A constraint of the pooled data is selection bias, in that presumably more fit elderly were enrolled on the individual clinical trials, and the fact that less than 1% of the trial participants were in their 80s.
Stage III colon cancer
Multiple population studies have shown that adjuvant therapy is beneficial in older patients. The SEER-Medicare Databases included more than 7,000 patients above 65-year- old with stage 3 colon cancer and found a survival benefit for the use of 5-FU/LV (HR 0.70; P<0.001) (46). SEER-Medicare and NCCN Outcomes Databases showed a survival benefit for adjuvant chemotherapy even in patients 75 years and older (HR 0.60; 95% CI, 0.53–0.68) (47).
The ACCENT database reported a reduced benefit to the addition of oxaliplatin to 5-FU in the adjuvant setting in patients ≥70 years of age (48). Using the National Cancer Database with more than 100,000 patients with stage III colon cancer, Margalit and colleagues found that low-risk patients older than 72 years (defined by IDEA collaborators as T3 and N1) and high-risk patients (T4 or N2 disease) older than 85 years did not benefit from doublet chemotherapy; suggesting that omission of oxaliplatin can be considered in IDEA low-risk patients older than 72 years old (49). Subset analyses looking at patients ≥70 years of age in both the NSABP C-07 and MOSAIC trials both found that there was no survival benefit with the addition of oxaliplatin to 5-FU/LV chemotherapy (47,50). However, support for the addition of oxaliplatin in elderly patients who would fit entry criteria for adjuvant clinical trials come from pooled individual patient data from patients with stage III colon cancer in NSABP-C08, XELOXA, X-ACT and AVANT showed DFS benefit for the addition of oxaliplatin to 5-FU-based chemotherapy (HR 0.77, P<0.014) (51).
Stage II colon cancer
In average risk stage II disease, the QUASAR study showed no benefit for adjuvant chemotherapy amongst patients >70 years old (23). Given the low absolute benefit in the general population, it is reasonable to omit adjuvant treatment in this group of older patients. A survival benefit for fluoropyrimidine-based adjuvant chemotherapy was not observed in an analysis from the linked SEER/Medicare database of 24,847 patients aged 65 and older who underwent colectomy for stage II colon cancer, even in those with high-risk features (52). As with the general population, older patients with dMMR are considered at low-risk for recurrence and do not benefit from adjuvant therapy.
Application in clinical practice
There is great heterogeneity in physical function among older adults and chronologic age alone cannot be relied upon to make treatment decisions. Overall, 5-FU/LV as adjuvant therapy appears to benefit all patients with stage III disease, regardless of age. However, the evidence of benefit for the addition of oxaliplatin in patients above 70 is much less consistent and should not be the rule. In terms of toxicities, it has been shown that patient age had no impact on toxicities such as nausea, diarrhea, stomatitis, but elderly patients do have an increased risk of hematological toxicities (53).
Rectal cancer
The risk of pelvic recurrence in rectal cancer is higher than those with colon cancer due to the close proximity of the rectum to pelvic structures, absence of a serosa surrounding the rectum and technical difficulties associated with obtaining wide surgical margins at resection. Due to this, locoregional therapy such as CRT is often included as neoadjuvant or adjuvant therapy in the management of stage II and III rectal cancer. Unlike colon cancer, clinical staging (using imaging and endoscopy findings) is used to direct the recommendations for pre- or post-operative therapy in rectal cancer and this remains a challenge due to the risk of under-staging or over-staging the tumor. Therefore, careful patient selection with respect to particular treatment options and the use of sequenced multi-modality therapy with chemotherapy, radiotherapy and surgery is required. Other than T1 stage rectal cancer, total mesorectal excision (TME) is routinely performed as it has been shown to decrease local seeding and subsequent recurrences (54).
Adjuvant therapy for resected rectal cancer (with no prior neoadjuvant treatment)
The role of adjuvant CRT was proven when two studies demonstrated that 5-FU-based chemotherapy plus radiation was more effective than radiation or surgery alone in preventing local and distant recurrence (55,56). It has also been shown that prolonged infusion of 5-FU was superior to bolus administration during radiation therapy, with a 3-year DFS advantage. In clinical practice, capecitabine administered twice daily at 825 mg/m2 on days of radiation has become a widely accepted substitute for continuous infusion of 5-FU after two phase 3 trials confirmed the non-inferiority of capecitabine as a radiosensitizer compared to 5-FU (57,58). The addition of oxaliplatin to neoadjuvant CRT have been studied in multiple phase 3 randomized controlled trials and have not been shown to provide additional benefit, and therefore, should not be recommended at this time (59-61).
The toxicities associated with adjuvant radiotherapy led to the German Rectal Trials group investigating the timing of radiation therapy with respect to surgery. Patients who underwent pre-operative combined-modality therapy had a lower rate of local recurrence (6% vs. 13% at 5 years), improved acute and chronic toxicities and higher rate of sphincter preservation, establishing neoadjuvant CRT as a standard of care for stage II and III rectal cancer. Long-term follow up confirmed the improvement in local control in pre-operative CRT in 10 years (62). The main disadvantage of pre-operative radiotherapy is the possibility of over-treating early-stage tumors that do not require adjuvant radiation. Given that neoadjuvant CRT is now commonplace, post-operative CRT is usually reserved for those with clinical stage I rectal cancer who were upstaged after pathologic review of the surgical specimen.
Adjuvant chemotherapy following neoadjuvant treatment
Historically, the benefit of adjuvant chemotherapy in rectal cancer has been extrapolated from adjuvant colon cancer studies (MOSAIC and NSABP C-07) (13,63). Although many studies have attempted to answer the question of benefit of the addition of adjuvant chemotherapy following standard of care neoadjuvant CRT followed by curative resection, the data has been conflicting. In a meta-analysis of 1196 patients who received 5-FU based adjuvant chemotherapy (5-FU/LV, capecitabine or XELOX) after pre-operative therapy and surgery, survival and rate of distant recurrences were not improved in stage II and III rectal cancers (64). The PETACC-06 study as well which randomized patients to capecitabine with radiotherapy before surgery, followed by capecitabine after surgery vs. XELOX and radiotherapy before surgery, followed by XELOX after surgery showed no difference in 3-year DFS (65). The ADORE and CAO/ARO/AIO-04 studies (5-FU/LV vs. FOLFOX) suggest otherwise (66,67). Both the ADORE and the CAO/ARO/AIO-04 trials showed a significant advantage in DFS of combined fluorouracil and oxaliplatin-based adjuvant chemotherapy compared with fluorouracil alone. The 3-year DFS reported in these studies were similar to those reported in the MOSAIC and NSABP C-07 adjuvant colon cancer studies. Critics argue that because neither of these two trials had an observational group, the benefit of adjuvant combination chemotherapy remains unknown. In the long-term results from the ADORE study, the 6-year OS remains similar in both 5-FU/LV and FOLFOX arms but in the subgroup analysis, patients with ypN2 and minimally regressed tumor benefited from FOLFOX over 5FU/LV (66). These results suggest that the subgroup of patients (ypN2 or minimally regressed tumors) may benefit from oxaliplatin-based adjuvant chemotherapy. Factors contributing to the difficulty of proving efficacy of adjuvant chemotherapy in rectal cancer include inaccurate baseline staging, inclusion of lower stage tumors in these trials, poor compliance to chemotherapy, varied timing of surgery in different trials, suboptimal regimens and variable control arms (68). We currently await results from clinical trials which are changing the treatment paradigm of rectal cancer with total neoadjuvant therapy and risk-adapted strategies to reduce toxicities from the current state of trimodality therapy in this disease.
Application in clinical practice
In the era of neoadjuvant CRT in the treatment of rectal cancer, adjuvant chemotherapy is generally recommended for stage II and III rectal cancers. The choice of regimen should be based on initial clinical staging, predicted circumferential resection margin (CRM) status and pathological evaluation of the surgical specimen. For higher-risk patients, an oxaliplatin-based doublet such as FOLFOX or XELOX may be considered. 5-FU/LV, or capecitabine are alternatives in other cases, especially for patients whose cancer responded to neoadjuvant treatment. The length of adjuvant chemotherapy should be for four months when pre-operative CRT is administered.
Conclusions
The benefits of adjuvant chemotherapy have been most clearly demonstrated in stage III colon cancer with oxaliplatin-based doublet preferred over single agent fluoropyrimidines in younger patients with no other competing co-morbidities. We know that shorter duration of oxaliplatin exposure reduces toxicities and the IDEA collaboration has provided evidence that in low-risk stage III patients, three months of oxaliplatin exposure with XELOX, has no detrimental effect in survival compared to six months of treatment. In stage II colon cancer, clinicopathologic risk factors and MMR status are used to guide decisions for adjuvant therapy. As the absolute benefit from adjuvant therapy in stage II disease is small, a discussion on the risks vs. benefits of adjuvant therapy and the choice of agent is warranted. In rectal cancer, adjuvant chemotherapy is recommended after neoadjuvant CRT and surgery in stage II and III disease. The choice of regimen should be based on initial clinical staging, predicted circumferential resection margin (CRM) status and pathological evaluation of the surgical specimen.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Sargent D, Shi Q, Yothers G, et al. Two or three year disease-free survival (DFS) as a primary end-point in stage III adjuvant colon cancer trials with fluoropyrimidines with or without oxaliplatin or irinotecan: data from 12,676 patients from MOSAIC, X-ACT, PETACC-3, C-06, C-07 and C89803. Eur J Cancer 2011;47:990-6. [Crossref] [PubMed]
- Sargent D, Sobrero A, Grothey A, et al. Evidence for cure by adjuvant therapy in colon cancer: observations based on individual patient data from 20,898 patients on 18 randomized trials. J Clin Oncol 2009;27:872-7. [Crossref] [PubMed]
- Biagi JJ, Raphael MJ, Mackillop WJ, et al. Association Between Time to Initiation of Adjuvant Chemotherapy and Survival in Colorectal Cancer. JAMA 2011;305:2335. [Crossref] [PubMed]
- Moertel CG, Fleming TR, Macdonald JS, et al. Levamisole and Fluorouracil for Adjuvant Therapy of Resected Colon Carcinoma. N Engl J Med 1990;322:352-8. [Crossref] [PubMed]
- Wolmark N, Rockette H, Fisher B, et al. The benefit of leucovorin-modulated fluorouracil as postoperative adjuvant therapy for primary colon cancer: results from National Surgical Adjuvant Breast and Bowel Project protocol C-03. J Clin Oncol 1993;11:1879-87. [Crossref] [PubMed]
- O’Connell MJ, Mailliard JA, Kahn MJ, et al. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J Clin Oncol 1997;15:246-50. [Crossref] [PubMed]
- Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet 1995;345:939-44. [Crossref] [PubMed]
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III Study of Fluorouracil, Leucovorin, and Levamisole in High-Risk Stage II and III Colon Cancer: Final Report of Intergroup 0089. J Clin Oncol 2005;23:8671-8. [Crossref] [PubMed]
- Saini A, Norman AR, Cunningham D, et al. Twelve weeks of protracted venous infusion of fluorouracil (5-FU) is as effective as 6 months of bolus 5-FU and folinic acid as adjuvant treatment in colorectal cancer. Br J Cancer 2003;88:1859-65. [Crossref] [PubMed]
- Twelves C, Wong A, Nowacki MP, et al. Capecitabine as Adjuvant Treatment for Stage III Colon Cancer. N Engl J Med 2005;352:2696-704. [Crossref] [PubMed]
- Haller DG, Tabernero J, Maroun J, et al. Capecitabine Plus Oxaliplatin Compared With Fluorouracil and Folinic Acid As Adjuvant Therapy for Stage III Colon Cancer. J Clin Oncol 2011;29:1465-71. [Crossref] [PubMed]
- Kuebler JP, Wieand HS, O’Connell MJ, et al. Oxaliplatin Combined With Weekly Bolus Fluorouracil and Leucovorin As Surgical Adjuvant Chemotherapy for Stage II and III Colon Cancer: Results From NSABP C-07. J Clin Oncol 2007;25:2198-204. [Crossref] [PubMed]
- André T, Boni C, Navarro M, et al. Improved Overall Survival With Oxaliplatin, Fluorouracil, and Leucovorin As Adjuvant Treatment in Stage II or III Colon Cancer in the MOSAIC Trial. J Clin Oncol 2009;27:3109-16. [Crossref] [PubMed]
- Saltz LB, Niedzwiecki D, Hollis D, et al. Irinotecan Fluorouracil Plus Leucovorin Is Not Superior to Fluorouracil Plus Leucovorin Alone As Adjuvant Treatment for Stage III Colon Cancer: Results of CALGB 89803. J Clin Oncol 2007;25:3456-61. [Crossref] [PubMed]
- Van Cutsem E, Labianca R, Bodoky G, et al. Randomized Phase III Trial Comparing Biweekly Infusional Fluorouracil/Leucovorin Alone or With Irinotecan in the Adjuvant Treatment of Stage III Colon Cancer: PETACC-3. J Clin Oncol 2009;27:3117-25. [Crossref] [PubMed]
- Ychou M, Raoul JL, Douillard JY, et al. A phase III randomised trial of LV5FU2 + irinotecan versus LV5FU2 alone in adjuvant high-risk colon cancer (FNCLCC Accord02/FFCD9802). Ann Oncol 2009;20:674-80. [Crossref] [PubMed]
- Papadimitriou CA, Papakostas P, Karina M, et al. A randomized phase III trial of adjuvant chemotherapy with irinotecan, leucovorin and fluorouracil versus leucovorin and fluorouracil for stage II and III colon cancer: A Hellenic Cooperative Oncology Group study. BMC Med 2011;9:10. [Crossref] [PubMed]
- de Gramont A, Van Cutsem E, Schmoll HJ, et al. Bevacizumab plus oxaliplatin-based chemotherapy as adjuvant treatment for colon cancer (AVANT): a phase 3 randomised controlled trial. Lancet Oncol 2012;13:1225-33. [Crossref] [PubMed]
- Taieb J, Tabernero J, Mini E, et al. Oxaliplatin, fluorouracil, and leucovorin with or without cetuximab in patients with resected stage III colon cancer (PETACC-8): an open-label, randomised phase 3 trial. Lancet Oncol 2014;15:862-73. [Crossref] [PubMed]
- Alberts SR, Sargent DJ, Nair S, et al. Effect of Oxaliplatin, Fluorouracil, and Leucovorin With or Without Cetuximab on Survival Among Patients With Resected Stage III Colon Cancer. JAMA 2012;307:1383-93. [Crossref] [PubMed]
- Grothey A, Sobrero AF, Shields AF, et al. Duration of Adjuvant Chemotherapy for Stage III Colon Cancer. N Engl J Med 2018;378:1177-88. [Crossref] [PubMed]
- Lieu C, Kennedy EB, Bergsland E, et al. Duration of Oxaliplatin-Containing Adjuvant Therapy for Stage III Colon Cancer: ASCO Clinical Practice Guideline. J Clin Oncol 2019;37:1436-47. [Crossref] [PubMed]
- QUASAR Collaborative Group, Gray R, Barnwell J, et al. Adjuvant chemotherapy versus observation in patients with colorectal cancer: a randomised study. Lancet 2007;370:2020-9. [Crossref] [PubMed]
- Amin MB, Edge S, Greene F, et al. AJCC Cancer Staging Manual (8th edition). Springer International Publishing: American Joint Commission on Cancer. 2017.
- Benson AB, Schrag D, Somerfield MR, et al. American Society of Clinical Oncology Recommendations on Adjuvant Chemotherapy for Stage II Colon Cancer. J Clin Oncol 2004;22:3408-19. [Crossref] [PubMed]
- Labianca R, Nordlinger B, Beretta GD, et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2013;24 Suppl 6:vi64-72. [Crossref] [PubMed]
- Colon Cancer NCCN.org NCCN Guidelines for Patients®. Available online: NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines ®).www.nccn.org/patients
- Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J Clin Oncol 1999;17:1356-63. [Crossref] [PubMed]
- Gill S, Loprinzi CL, Sargent DJ, et al. Pooled Analysis of Fluorouracil-Based Adjuvant Therapy for Stage II and III Colon Cancer: Who Benefits and by How Much? J Clin Oncol 2004;22:1797-806. [Crossref] [PubMed]
- Schrag D, Rifas-Shiman S, Saltz L, et al. Adjuvant Chemotherapy Use for Medicare Beneficiaries With Stage II Colon Cancer. J Clin Oncol 2002;20:3999-4005. [Crossref] [PubMed]
- Swanson RS, Compton CC, Stewart AK, et al. The prognosis of T3N0 colon cancer is dependent on the number of lymph nodes examined. Ann Surg Oncol 2003;10:65-71. [Crossref] [PubMed]
- André T, Boni C, Mounedji-Boudiaf L, et al. Oxaliplatin, Fluorouracil, and Leucovorin as Adjuvant Treatment for Colon Cancer. N Engl J Med 2004;350:2343-51. [Crossref] [PubMed]
- Markowitz SD, Bertagnolli MM. Molecular Basis of Colorectal Cancer. N Engl J Med 2009;361:2449-60. [Crossref] [PubMed]
- Sinicrope FA. Lynch Syndrome-Associated Colorectal Cancer. N Engl J Med 2018;379:764-73. [Crossref] [PubMed]
- Ionov Y, Peinado MA, Malkhosyan S, et al. Ubiquitous somatic mutations in simple repeated sequences reveal a new mechanism for colonic carcinogenesis. Nature 1993;363:558-61. [Crossref] [PubMed]
- Quah H-M, Chou JF, Gonen M, et al. Identification of patients with high-risk stage II colon cancer for adjuvant therapy. Dis Colon Rectum 2008;51:503-7. [Crossref] [PubMed]
- Bertagnolli MM, Redston M, Compton CC, et al. Microsatellite Instability and Loss of Heterozygosity at Chromosomal Location 18q: Prospective Evaluation of Biomarkers for Stages II and III Colon Cancer—A Study of CALGB 9581 and 89803. J Clin Oncol 2011;29:3153-62. [Crossref] [PubMed]
- Sargent DJ, Marsoni S, Monges G, et al. Defective Mismatch Repair As a Predictive Marker for Lack of Efficacy of Fluorouracil-Based Adjuvant Therapy in Colon Cancer. J Clin Oncol 2010;28:3219-26. [Crossref] [PubMed]
- Ribic CM, Sargent DJ, Moore MJ, et al. Tumor Microsatellite-Instability Status as a Predictor of Benefit from Fluorouracil-Based Adjuvant Chemotherapy for Colon Cancer. N Engl J Med 2003;349:247-57. [Crossref] [PubMed]
- Chee CE, Meropol NJ. Current Status of Gene Expression Profiling to Assist Decision Making in Stage II Colon Cancer. Oncologist 2014;19:704-11. [Crossref] [PubMed]
- Yothers G, O’Connell MJ, Lee M, et al. Validation of the 12-Gene Colon Cancer Recurrence Score in NSABP C-07 As a Predictor of Recurrence in Patients With Stage II and III Colon Cancer Treated With Fluorouracil and Leucovorin (FU/LV) and FU/LV Plus Oxaliplatin. J Clin Oncol 2013;31:4512-9. [Crossref] [PubMed]
- Venook AP, Niedzwiecki D, Lopatin M, et al. Biologic Determinants of Tumor Recurrence in Stage II Colon Cancer: Validation Study of the 12-Gene Recurrence Score in Cancer and Leukemia Group B (CALGB) 9581. J Clin Oncol 2013;31:1775-81. [Crossref] [PubMed]
- Yamanaka T, Oki E, Yamazaki K, et al. 12-Gene Recurrence Score Assay Stratifies the Recurrence Risk in Stage II/III Colon Cancer With Surgery Alone: The SUNRISE Study. J Clin Oncol 2016;34:2906-13. [Crossref] [PubMed]
- Tie J, Wang Y, Tomasetti C, et al. Circulating tumor DNA analysis detects minimal residual disease and predicts recurrence in patients with stage II colon cancer. Sci Transl Med 2016;8:346ra92. [Crossref] [PubMed]
- Pagès F, Mlecnik B, Marliot F, et al. International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 2018;391:2128-39. [Crossref] [PubMed]
- Hanna NN, Onukwugha E, Choti MA, et al. Comparative analysis of various prognostic nodal factors, adjuvant chemotherapy and survival among stage III colon cancer patients over 65 years: an analysis using Surveillance, Epidemiology and End Results (SEER)-Medicare data. Colorectal Dis 2012;14:48-55. [Crossref] [PubMed]
- Sanoff HK, Carpenter WR, Stürmer T, et al. Effect of Adjuvant Chemotherapy on Survival of Patients With Stage III Colon Cancer Diagnosed After Age 75 Years. J Clin Oncol 2012;30:2624-34. [Crossref] [PubMed]
- McCleary NJ, Meyerhardt JA, Green E, et al. Impact of Age on the Efficacy of Newer Adjuvant Therapies in Patients With Stage II/III Colon Cancer: Findings From the ACCENT Database. J Clin Oncol 2013;31:2600-6. [Crossref] [PubMed]
- Margalit O, Mamtani R, Yang YX, et al. A new look at the International Duration Evaluation of Adjuvant therapy (IDEA) classification—Defining novel predictive and prognostic markers in stage III colon cancer. Eur J Cancer 2018;96:105-10. [Crossref] [PubMed]
- Tournigand C, André T, Bonnetain F, et al. Adjuvant Therapy With Fluorouracil and Oxaliplatin in Stage II and Elderly Patients (between ages 70 and 75 years) With Colon Cancer: Subgroup Analyses of the Multicenter International Study of Oxaliplatin, Fluorouracil, and Leucovorin in the Adjuvant Treatment of Colon Cancer Trial. J Clin Oncol 2012;30:3353-60. [Crossref] [PubMed]
- Haller DG, O’Connell MJ, Cartwright TH, et al. Impact of age and medical comorbidity on adjuvant treatment outcomes for stage III colon cancer: a pooled analysis of individual patient data from four randomized, controlled trials. Ann Oncol 2015;26:715-24. [Crossref] [PubMed]
- O’Connor ES, Greenblatt DY, LoConte NK, et al. Adjuvant chemotherapy for stage II colon cancer with poor prognostic features. J Clin Oncol 2011;29:3381-8. [Crossref] [PubMed]
- Sargent DJ, Goldberg RM, Jacobson SD, et al. A Pooled Analysis of Adjuvant Chemotherapy for Resected Colon Cancer in Elderly Patients. N Engl J Med 2001;345:1091-7. [Crossref] [PubMed]
- Heald RJ, Ryall RD. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet 1986;1:1479-82. [Crossref] [PubMed]
- Fisher B, Wolmark N, Rockette H, et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: results from NSABP protocol R-01. J Natl Cancer Inst 1988;80:21-9. [Crossref] [PubMed]
- Krook JE, Moertel CG, Gunderson LL, et al. Effective Surgical Adjuvant Therapy for High-Risk Rectal Carcinoma. N Engl J Med 1991;324:709-715. [Crossref] [PubMed]
- Allegra CJ, Yothers G, O’Connell MJ, et al. Neoadjuvant 5-FU or Capecitabine Plus Radiation With or Without Oxaliplatin in Rectal Cancer Patients: A Phase III Randomized Clinical Trial. J Natl Cancer Inst 2015. [Crossref] [PubMed]
- Hofheinz RD, Wenz F, Post S, et al. Chemoradiotherapy with capecitabine versus fluorouracil for locally advanced rectal cancer: a randomised, multicentre, non-inferiority, phase 3 trial. Lancet Oncol 2012;13:579-88. [Crossref] [PubMed]
- Aschele C, Lonardi S, Cionini L, et al. Final results of STAR-01: A randomized phase III trial comparing preoperative chemoradiation with or without oxaliplatin in locally advanced rectal cancer. J Clin Oncol 2016;34:3521. [Crossref]
- Gérard JP, Azria D, Gourgou-Bourgade S, et al. Clinical Outcome of the ACCORD 12/0405 PRODIGE 2 Randomized Trial in Rectal Cancer. J Clin Oncol 2012;30:4558-65. [Crossref] [PubMed]
- Deng Y, Chi P, Lan P, et al. Modified FOLFOX6 with or without radiation in neoadjuvant treatment of locally advanced rectal cancer: Final results of the Chinese FOWARC multicenter randomized trial. J Clin Oncol 2018;36:3502. [Crossref]
- Sauer R, Becker H, Hohenberger W, et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med 2004;351:1731-40. [Crossref] [PubMed]
- Yothers G, O’Connell MJ, Allegra CJ, et al. Oxaliplatin As Adjuvant Therapy for Colon Cancer: Updated Results of NSABP C-07 Trial, Including Survival and Subset Analyses. J Clin Oncol 2011;29:3768-774. [Crossref] [PubMed]
- Breugom AJ, Swets M, Bosset JF, et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: a systematic review and meta-analysis of individual patient data. Lancet Oncol 2015;16:200-7. [Crossref] [PubMed]
- Schmoll H-J, Haustermans K, Price TJ, et al. Preoperative chemoradiotherapy and postoperative chemotherapy with capecitabine +/- oxaliplatin in locally advanced rectal cancer: Final results of PETACC-6. J Clin Oncol 2018;36:3500. [Crossref]
- Hong YS, Kim SY, Lee JS, et al. Long-term results of the ADORE trial: Adjuvant oxaliplatin, leucovorin, and 5-fluorouracil (FOLFOX) versus 5-fluorouracil and leucovorin (FL) after preoperative chemoradiotherapy and surgery for locally advanced rectal cancer. J Clin Oncol 2018;36:3501. [Crossref]
- Rödel C, Graeven U, Fietkau R, et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet Oncol 2015;16:979-89. [Crossref] [PubMed]
- Carvalho C, Glynne-Jones R. Challenges behind proving efficacy of adjuvant chemotherapy after preoperative chemoradiation for rectal cancer. Lancet Oncol 2017;18:e354-63. [Crossref] [PubMed]


