
Downstaging unresectable hepatocellular carcinoma by radioembolization using 90-yttrium resin microspheres: a single center experience
Introduction
Hepatocellular carcinoma (HCC) is the most common primary liver cancer. Despite a slight but continuous decrease in incidence, HCC remains among the top five causes of cancer-related death in Italy (1). Despite surveillance recommendations, a high number of HCC are diagnosed at an advanced stage and only one third of them may benefit of curative treatments (2,3). Tumor downstaging through the use of loco-regional treatments may allow unresectable tumor to be resectable, providing the best chance of cure to these patients (4). The 5-year survival rate following tumor downstaging and salvage surgery is similar to the survival rate for patients with initially resectable HCC (5). Downstaging is the result of a treatment that makes possible a surgical procedure that would otherwise be impossible or too risky; mainly for anatomical reasons or for insufficient remnant liver or for presence of macrovascular portal or hepatic vein invasion.
Transarterial radioembolization (TARE) has some peculiarities that make it attractive for downstaging strategy. It has been reported to induce an efficient and rapid decrease in tumor size with few occurrences of post-embolization syndrome and a low number of treatments, to prolong time to progression of the tumor and to induce long survivals (6-9). TARE can induce a significant hypertrophy of the contralateral hepatic lobe (10-12) and it is to be preferred in presence of portal vein thrombosis (PVT) for its lower risk of liver ischemia (13-15). Finally, the delay from downstaging treatment to surgery (test of time) allows physicians to select those patients with a favourable biology for surgical intervention, decreasing the chance of early recurrence in the remnant liver. The goal of this study was to examine survival of patients with unresectable HCC and PVT consecutively treated with TARE with the aim to downstaging them to a curative surgical treatment. A 3-year enrollment period and a 5-year follow-up were planned in order to adequately investigate survival.
Methods
Patients
All consecutive patients who offered from June 2011 through June 2014 to our center with a first diagnosis of unresectable HCC with PVT were treated. Inclusion criteria were a well-preserved liver function (Child-Pugh A), platelet count >100,000 c/µL, international normalized ratio (INR) <1.5. Exclusion criteria was the extension of the tumor thrombus to the main trunk of the portal vein. Unresectability and downstaging were evaluated by the same surgeons at enrollment. Eligibility for surgery were re-evaluated by the same surgeons at 6 and 12 months after TARE. A second TARE was performed if indicated. The treatment protocol was approved by our Institution Ethical Committee and all patients provided informed consent.
Trans-arterial radio-embolization
A planning simulation was performed to determine the optimal tumoricide dose and avoid excessive irradiation of the surrounding liver and determine the extent of extra-hepatic shunting. Patients were subjected to an angiographic evaluation to detect collateral vessels feeding extra-hepatic organs and a subsequent infusion of technetium-99-labeled macroaggregated albumin (LyoMAA, Mallinckrodt Med, NL, USA) which mimics the vascular distribution pattern of 90Y microspheres. A total body planar image and a proton emission computed tomography (SPECT/TC) (Siemens SymbiaIntevo, IterativeFlash3D attenuation and scatter corrected) were acquired. Radioembolization was performed according to the standardized procedure (16) with resin microspheres (Sirtex Medical Europe GmbH).
Dosimetry
Activities were calculated according to the multi-compartmental (MIRD) model and the voxel-based method with the aim of delivering the highest dose to the tumor, while not exceeding 40 Gy to the surrounding liver parenchyma and 30 Gy to the lung (17,18) The MIRD model used pre-treatment 99mTc-MAA SPECT-CT images: two volume of interest (VOIs) were drawn on images to estimate 99mTc-MAA uptakes and volumes of the tumor and remnant liver parenchyma. The voxel dosimetry used the SPECT-3D corrected counts matrix. A MATLAB (Mathworks) code was developed within our department. Convolution calculations were adopted: for each targeted voxel, the mean absorbed dose from surrounding source voxels along three major axis was calculated. Twenty-four hours after Y-90 injection, a bremsstrahlung SPECT-TC was performed to verify the correct microspheres distribution comparing it with the simulation phase and the absorbed dose (Gy) was calculated.
Follow-up and safety
Patients were assessed monthly for the first 6 months following TARE and then every 6 months. Hepatic function, serum α-fetoprotein (AFP) were assessed and medical examinations were performed. During the second month after TARE, a quadriphasic CT was performed to assess the response. Response evaluation criteria in solid tumors (mRECIST) and the EASL criteria on vascular enhancement were applied to assess treatment response (19,20). Thereafter, CT scans, hepatic functions assessment and medical examination were performed at 6 month and then every six months. All adverse events (AEs) were classified for severity by using the National Cancer Institute Common Toxicity Criteria (CTCAE3.0) (21). All grade 3 or greater adverse effects occurring within 30 days were considered to be treatment-related.
Statistical analysis
Patients’ characteristics were reported using frequency and descriptive analysis when appropriate. All statistical analysis were performed using IBM SPSS Statistics for Windows, Version 2.0 Italian. The differences between continuous variables were assessed with the Kruskal-Wallis test. Follow-up was calculated from the date of treatment to the date of death or the last visit. The Kaplan-Meyer method was used to analyse survival. P<0.05 was considered as statistically significant. Data were censored at August 2018.
Results
Initially, 28 patients were enrolled, however, after the simulation session 4 patients were excluded because of the presence of excessive pulmonary shunt or intrahepatic arterio-venous fistulas. Thus, 24 patients were treated using TARE with resin microspheres. All the patients had cirrhosis. At the 6-month follow-up, 2 patients had HCC downstaged and underwent surgery. Sixteen patients had radiological response with 7 requiring a second TARE procedure and the remaining 6 patients showed progression of HCC. At the 12-month follow-up, 3 additional patients had HCC downstaged and underwent surgery, 8 maintained partial response or stable disease and 5 additional patients had HCC progression. A total of 5 patients (21%) had HCC that were successfully downstaged (group 1), 8 patients (33%) (group 2) demonstrated radiological responses (partial response or stable disease) but did not become eligible for surgery due to worsening of liver function (6 patients) or due to reduction of platelet count (2 patients). Eleven patients (46%) had progressive disease despite TARE (group 3).
Pre-treatment clinical parameters, liver function tests and tumor size were not significantly different among the three groups (Table 1). Serum AFP levels were significantly higher in patients with progressive disease (P=0.05). In contrast, high tumor absorbed doses (Gy) were significantly associated with more favourable outcomes to TARE. The median dose in downstaged patients was 454 Gy, whereas the median doses in patients who had partial response/stable disease or progression were 248 and 138 Gy respectively (P=0.001). Injected activities (GBq) were numerically higher in the downstaged patients than in the other groups, but the difference did not reach statistical significance.
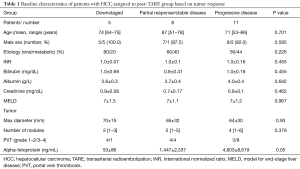
Full table
Overall median survival was 26 months for the entire cohort. Median survival for the patients in the downstaged, partial response/stable disease and progressive disease was 70, 24 and 11 months respectively. A direct significant correlation was found between the tumor absorbed dose and patients’ survivals (P<0.001) (Table 2).
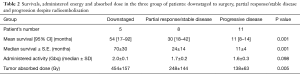
Full table
Treatment outcomes after downstaging to surgery
Patient 1 was a 72-year-old man with 3 confluent nodules and an overall tumor size of 12 cm in the right lobe with a first order branch tumor thrombus in HCV-related cirrhosis. He received a first TARE in June 2011 and a second procedure was performed 6 months later. He was revaluated for surgery and underwent a right trisectionectomy in October 2012. In November 2014 he underwent conventional TACE for HCC recurrence in the left lobe. Hepatic decompensation occurred in March 2017 and the patient died in August 2017, 75 months after the first TARE.
Patient 2 was a 64-year-old man with metabolic cirrhosis and a single 8-cm HCC in the segment 8 with a second-order branch infiltration of the portal vein and of the suprahepatic vein branch. In December 2011 he underwent TARE and experienced a complete radiologic response and a progressive reduction in the lesion size up to 3 cm. According to the study protocol, he was eligible to liver resection at both 6- and 12-month follow-up evaluation. However, he was referred for liver transplant, which was performed in 2013. As of this report, he is still alive 81 months after TARE.
Patient 3 was a 75-year-old man who had HCV-related cirrhosis and a single 8-cm HCC in the segment 4 infiltrating a second-order branch of the portal vein. He underwent TARE in November 2012 and a second procedure 6 months later. In November 2013 he underwent a right trisectionectomy (Figure 1). He is still alive, without recurrence and has a good performance status 70 months after the first TARE.
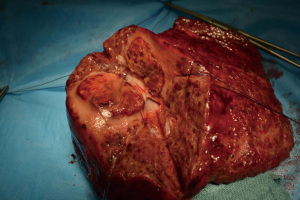
Patient 4 was a 74-year-old man who has HCV-related liver cirrhosis and two HCC nodules of 5 and 3 cm with a second-branch portal infiltration. In June 2013 TARE was performed. He received a right trisectionectomy 6 months after TARE (Figure 2). Two years later radiofrequency ablation (RFA) was performed for a 2-cm recurrence within segment 2 with complete response. He was classified as Child-Pugh B8 for liver function in march 2016, when he died from a stroke without HCC recurrence, 33 months after the first TARE.
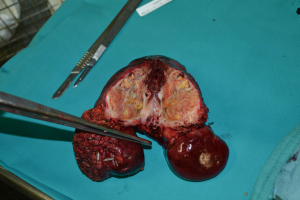
Patient 5 was a 73-year-old man who had HCV-related liver cirrhosis with a 7-cm HCC in segments 5–8 and two nodules (2 and 2.5 cm) in segments 4–5 with second-branch portal infiltration. He underwent TARE in December 2013. The major lesion was shrunken to 4 cm by 6 months after TARE but contrast enhancing tissue persisted. A second TARE was not performed because of the appearance of arteriovenous fistulas. In June 2014 he underwent a right trisectionectomy. CT was performed 3 months later and revealed bone and lymph node metastases. Patient died in December 2014, 12 months after the first TARE procedure.
Treatment-related toxicities
No radiation pneumonitis or radiation induced liver disease (RILD) or grade 3–4 adverse effects were observed after the treatment. The most common clinical side-effects were anorexia, fever and abdominal pain in 10/24 patients (41.7%), transient increase in liver enzymes and bilirubin in 9/24 patients (37.5%), worsening of the Child-Pugh score in 4/24 patients (17%) and persistent reduction of platelet count in 3/24 patients (12.5%).
Discussion
In our institutional experience, 5/24 patients (21%) or 5/28 (18% in an intention-to-treat analysis) who had unresectable, locally advanced HCC and cirrhosis were able to be downstaged to a surgical curative-intent treatment after radioembolization with 90-yttrium resin microspheres. Survivals for these patients were extended and comparable to the survival rates of well-selected patients who are eligible for surgery at the first presentation of HCC (5). This study is a further proof of concept of the potential role of TARE in this context. We do report downstaging rates that are comparable to another Spanish study that used resin microspheres (6 of 21 patients) (22). Differently, we have not found a correlation between a successful downstaging and patients’ age or larger tumor volumes, as they did, but, in our series, high tumor absorbed radiation dose and low pre-treatment AFP level were the only variables significantly associated with the probability of achieve downstaging and consequently with longer survival. The 5 patients that had HCC downstaged received a median absorbed dose of 454 Gy, much higher than the minimal tumoricidal dose reported for resin microspheres (18) and significantly higher than median absorbed dose of the other two groups. We calculated for every patient the maximum activity of yttrium-90 microspheres in order to deliver the highest dose to the tumor respecting the threshold of 40 Gy to the cirrhotic liver and 30 Gy to the lung. Therefore, the different absorbed doses depend on the two variables on which it is not possible to intervene, i.e., the arterial flow and the vessel density of the tumor. Even though variability in AFP levels existed within each group, mean pretreatment AFP values were lower in the patients with HCC that were successfully downstaged than in the patients who experienced partial response/stable disease or progressive disease. In our opinion, this is not surprising, because AFP has been identified as a predictor of biological aggressiveness of the tumor and the presence of vascular invasion (23,24).
At present, sorafenib, a multikinase inhibitor administered as continuous oral dosing, remains the standard of care for advanced HCC. Two large multicentric trials have recently compared radioembolization to sorafenib. Overall survivals were similar in both studies but adverse effects rates were significantly lower in the radioembolization group (25,26). TARE is therefore more tolerable than sorafenib but, moreover, it is able to rescue unresectable HCC to surgery.
Our study highlights that about twenty per cent of patients with locally advanced unresectable HCC who receive as first-line treatment radioembolization may be rescued to a surgical curative treatment with a good quality of life and a significant increase in their survival. Our results are encouraging but deserve to be confirmed in large studies. A high number of patients enrolled with a control arm, and a better definition of the criteria of resectability less related to the subjectivity of the surgeon are needed.
Acknowledgments
Editorial assistance was provided by Kristina Wasson-Blader, PhD, of Eubio, LLC (funded by Sirtex Medical Inc.).
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The corresponding Authors declare that the study was approved by the ethical committee of Mauriziano Hospital registration number IRB 11-368 and informed consent was taken from all patients.
References
- Bertuccio P, Turati F, Carioli G, et al. Global trend and predictions in hepatocellular carcinoma mortality. J Hepatol 2017;67:302-9. [Crossref] [PubMed]
- European Association For The Study Of The Liver. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 2012;56:908-43. [Crossref] [PubMed]
- Díaz-González Á, Reig M, Bruix J. Treatment of hepatocellular carcinoma. Dig Dis 2016;34:597-602. [Crossref] [PubMed]
- Toso C, Mentha G, Kneteman NM, et al. The place of downstaging for hepatocellular carcinoma. J Hepatol 2010;52:930-6. [Crossref] [PubMed]
- Lau WY, Ho S, Yu S, et al. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg 2004;240:299-305. [Crossref] [PubMed]
- Lewandowski RJ, Kulik LM, Riaz A, et al. A comparative analysis of transarterial downstaging for hepatocellular carcinoma: chemoembolization versus radioembolization. Am J Transplant 2009;9:1920-8. [Crossref] [PubMed]
- Sangro B, Inarrairaegui M, Bilbao JI. Radioembolization for hepatocellular carcinoma. J Hepatol 2012;56:464-73. [Crossref] [PubMed]
- Mazzaferro V, Sposito C, Bhoori S, et al. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology 2013;57:1826-37. [Crossref] [PubMed]
- Salem R, Gordon AC, Mouli S, et al. Y90 radioembolization significantly prolongs time to progression compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology 2016;151:1155-1163.e2. [Crossref] [PubMed]
- Edeline J, Lenoir L, Boudjema K, et al. Volumetric changes after (90)y radioembolization for hepatocellular carcinoma in cirrhosis: an option to portal vein embolization in a preoperative setting? Ann Surg Oncol 2013;20:2518-25. [Crossref] [PubMed]
- Fernández-Ros N, Silva N, Bilbao JI, et al. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB (Oxford) 2014;16:243-9. [Crossref] [PubMed]
- Teo JY, Allen JC Jr, Ng DC, et al. A systematic review of contralateral liver lobe hypertrophy after unilobar selective internal radiation therapy with Y90. HPB (Oxford) 2016;18:7-12. [Crossref] [PubMed]
- Kulik LM, Carr BI, Mulcahy MF, et al. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology 2008;47:71-81. [Crossref] [PubMed]
- Iñarrairaegui M, Thurston KG, Bilbao JI, et al. Radioembolization with use of yttrium-90 resin microspheres in patients with hepatocellular carcinoma and portal vein thrombosis. J Vasc Interv Radiol 2010;21:1205-12. [Crossref] [PubMed]
- Lau WY, Sangro B, Chen PJ, et al. Treatment for hepatocellular carcinoma with portal vein tumor thrombosis: the emerging role for radioembolization using yttrium-90. Oncology 2013;84:311-8. [Crossref] [PubMed]
- Kennedy A, Nag S, Salem R, et al. Recommendations for radioembolization of hepatic malignancies using yttrium-90 microsphere brachytherapy: a consensus panel report from the radioembolization brachytherapy oncology consortium. Int J Radiat Oncol Biol Phys 2007;68:13-23. [Crossref] [PubMed]
- Cremonesi M, Chiesa C, Strigari L., et al. Radioembolization of hepatic lesions of radiology and dosimetric perspective. Front Oncol 2014;4:210. [Crossref] [PubMed]
- Strigari L, Sciuto R, Rea S, et al. Efficacy and toxicity related to treatment of hepatocellular carcinoma with 90Y Sir-Spheres: radiobiologic considerations. J Nucl Med 2010;51:1377-85. [Crossref] [PubMed]
- Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 2010;30:52-60. [Crossref] [PubMed]
- Bruix J, Sherman M, Llovet JM, et al. Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona 2000 EASL conference. European association for the study of the liver. J Hepatol 2001;35:421-30. [Crossref] [PubMed]
- Trotti A, Colevas AD, Setser A, et al. CTCAE v3.0: development of of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176-81. [Crossref] [PubMed]
- Iñarrairaegui M, Pardo F, Bilbao JI, et al. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol 2012;38:594-601. [Crossref] [PubMed]
- Duvoux C, Roudot-Thoraval F, Decaens T, et al. Liver transplantation for hepatocellular carcinoma: a model including α-fetoprotein improves the performance of Milan criteria. Gastroenterology 2012;143:986-94.e3; quiz e14-5.
- Grąt M, Krasnodębski M, Patkowski W, et al. Relevance of Pre-Transplant α-fetoprotein Dynamics in Liver Transplantation for Hepatocellular Cancer. Ann Transplant 2016;21:115-24. [Crossref] [PubMed]
- Vilgrain V, Pereira H, Assenat E, et al. Efficacy and safety of selective internal radiotherapy with yttrium-90 resin microspheres compared with sorafenib in locally advanced and inoperable hepatocellular carcinoma (SARAH): an open-label randomised controlled phase 3 trial. Lancet Oncol 2017;18:1624-36. [Crossref] [PubMed]
- Chow PKH, Gandhi M, Tan SB, et al. SIRveNIB: Selective Internal Radiation Therapy versus Sorafenib in Asia-Pacific Patients with Hepatocellular Carcinoma. J Clin Oncol 2018;36:1913-21. [Crossref] [PubMed]


