
Effect of KRAS mutational status on disease behavior and treatment outcome in patients with metastatic colorectal cancer: intratumor heterogeneity and mutational status
Introduction
According to the American Cancer Society, colorectal cancer (CRC) is the 3rd most common malignancy identified in men and women. In 2017, approximately 95,520 new cases of colon cancer and 39,910 new cases of rectal cancer were diagnosed in the United States. Although the incidence of CRC was equal between male [47,700] and female [47,820] patients, rectal cancer was diagnosed in a larger proportion of men [23,720] than women [16,190]. An estimated 27,150 men and 23,110 women were expected to die of CRC in 2017 (1).
Genetic alterations through a multistep process have an essential part in the development of CRC. Therefore, characterizing the genetic origin of the cancer pathway is an ongoing exploration necessary in the development of a standardized treatment guideline based on molecular studies (2).
Early diagnosis of CRC, identification of standard prognostic factors, and proper management with multimodality treatments (surgery, chemotherapy, and targeted therapy +/− radiotherapy) have contributed to the improved outcomes in these patients.
Although the TNM (tumor-node-metastasis) classification is useful for staging cancers and facilitating treatment decisions, it is not sufficient because some patients with the same disease stage may have different disease behaviors and outcomes. Hence, other prognostic factors, based on either clinical factors (obstruction or perforation) or laboratory tests (tumor grade, venous invasion, perineural invasion, 18q deletion), have to be considered to select the optimal therapy for patients with CRC. In the era of molecular-based interventions, more effort is needed to understand the underlying causes of different disease behaviors in patients with CRC, especially those with metastatic CRC (mCRC).
In 1975, Arrington et al. (3) recognized HRAS and KRAS as the first 2 RAS genes from the revisions of 2 viruses initiating malignancy (Harvey sarcoma virus and Kirsten sarcoma virus). The human isoform was then identified in 1982, leading to the establishment of the three recognized subtypes: NRAS, HRAS, and KRAS. In all human cancers, KRAS mutations had the highest incidence (21.6%), whereas NRAS and HRAS mutations had a much lower incidence at 8.0% and 3.3%, respectively (4).
RAS gene mutations have been identified in different malignancies, such as pancreatic cancer (90%), thyroid cancer (55%), lung cancer (35%), and rhabdomyosarcoma (35%). The KRAS mutant type has been recognized in 30–50% of CRC cases, and it is associated with aggressive behavior, rapid disease progression, and poor survival.
Polymerase chain reaction (PCR) is a real-time investigation used to quantitatively detect the mutational status of exon 2 (codons 12/13) and exon 3 (codon 61) of the KRAS gene. Although point alterations in codon 12 are the furthermost KRAS mutations in CRC, this test can detect up to 19 KRAS mutations (5).
Aim of the study
This study is a retrospective, multicenter chart review carried out to compare the disease behavior, therapy outcomes, as well as progression-free survival (PFS) and overall survival (OS), according to KRAS mutational status (wild or mutant) in patients with mCRC.
Methods
This multicenter retrospective study analyzed the diagnostic and monitoring workup of 360 patients with mCRC treated at three oncology hospitals (King Fahad Specialist Hospital in Saudi Arabia in collaboration with King Faisal Specialist Hospital in Riyadh and Zagazig University Hospitals in Egypt) from February 2011 to December 2015. Data were collected from the following assessments:
- Initial clinical examination: this was performed at the time of diagnosis with assessment for the presence or absence of comorbidities.
- Radiologic assessment: this included standard radiologic workup comprising chest, abdominal, and pelvic computed tomography (CT) for all patients. Magnetic resonance imaging and positron emission tomography-CT were performed for some cases, if needed.
- Laboratory assessment: This consisted of recording of pathologic characteristics and baseline carcinoembryonic antigen (CEA) levels, as well as a review of routine laboratory test results, such as complete blood counts and liver/kidney functions test, which were requested before chemotherapy.
- Staging: all cases were assessed on the basis of the 7th edition of the American Joint Committee on Cancer staging system.
- Treatment history: according to the chart review, all patients received oxaliplatin-based or irinotecan-based chemotherapy either alone or in combination with targeted therapy. Bevacizumab was administered regardless of KRAS type (wild or mutant) and to patients who did not have contraindications, whereas cetuximab was administered to wild-type KRAS cases. Only four patients in this study received regorafenib after failure of the above therapy; unfortunately, all of them showed poor tolerance even with dose reduction. Palliative surgical intervention was performed in patients with emergency obstruction or perforation, whereas palliative radiotherapy was indicated for a few patients.
- Response status: this was assessed on the basis of the RECIST (Response Evaluation Criteria in Solid Tumors) 1.0 criteria.
Interpretation of KRAS mutation assay
KRAS mutational status was recorded in this study to determine the effects of mutation on patient outcomes. KRAS mutation analysis with real-time PCR detects the wild-type sequence and seven known mutations associated with two codons (codons 12 and 13) of the KRAS oncogene.
Real-time PCR with eight primer sets was used to amplify the region of the KRAS gene containing codons 12 and 13. A set of eight probes was used to detect the KRAS type (wild type or mutant) and had the ability to identify mutations up to 1% in a wild-type background.
For statistical analysis, patients in this study were categorized into two groups on the basis of the KRAS mutational status: KRAS wild type and KRAS mutant type.
Ethical and regulatory considerations
The study protocol was approved by the Institutional Review Board of King Fahad Hospital, Saudi Arabia (ONC0310) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived.
Statistical analysis
SPSS version 17.0 was used for statistical analysis. Quantitative data are presented as means and standard deviations. Parametric and non-parametric t-tests were used for comparison of two independent groups. The Kaplan-Meier analysis was performed to assess OS. Log-rank assessment was performed to compare survival between groups and was used to calculate P values for the differences between groups.
Results
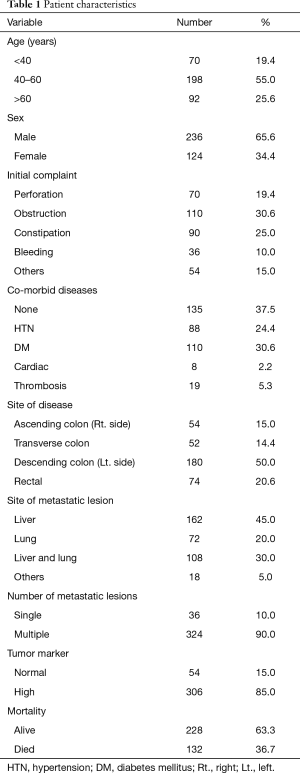
In the current study, the median patient age was 51 years (range, 35–76 years), and approximately 19.4% of patients were aged <40 years. The age of the majority of patients (55.0%) ranged from 40 to 60 years (Table 1).
Full table
Only 34.4% of patients were women. The most frequent presenting complaints were obstruction (30.6%), followed by constipation (25.0%), perforation (19.4%), and rectal bleeding (10.0%).
Among our patients, 50.0% had descending colon lesions, whereas 15.0% patients had ascending colon lesions. Lesions in the transverse colon were noted in 14.4% of patients, and rectal lesions were diagnosed in 20.6% of patients.
Isolated hepatic metastasis was observed in 45.0% of patients, and isolated pulmonary metastasis was observed in 20.0% of patients. Hepatic and pulmonary metastases, in addition to metastasis in other areas, occurred in 30.0% of patients. Single metastatic lesions occurred in 10.0% of patients.
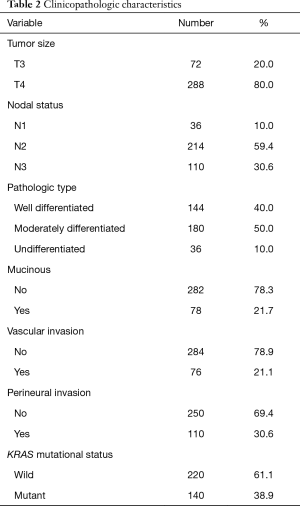
With regard to TNM stage, 80.0% of patients were classified as T4 and 59.4% were classified as N2. All patients were confirmed to have M1 disease at the time of this study. The pathologic assessment of the patients showed well-differentiated adenocarcinoma in 40.0% and isolated mucinous features in 21.7%. The majority of the patients did not exhibit perineural (69.4%) or vascular invasion (78.9%).
Wild-type KRAS was confirmed in 61.1% of patients and mutant KRAS in 38.9%. The pre-treatment evaluation showed that the serum CEA level was high in 85.0% of patients and normal in 15.0% of patients (Table 2).
Full table
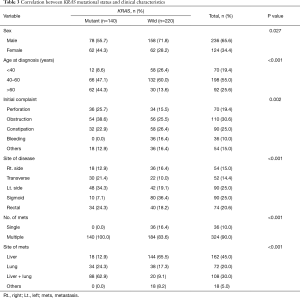
About 55.7% of KRAS mutations were observed in male patients in our study (P=0.027). For statistical analysis, the cases were categorized into three groups according to age, as follows: <40 years, 40–60 years, and >60 years (Table 3). We found that 47.1% of patients within the 40–60-year-old category had mutant KRAS, compared with 44.3% in the >60-year-old category and only 8.6% (P<0.001) in the <40-year-old category.
Full table
Although the initial symptoms varied among patients, the majority of critical presentations was observed in cases with KRAS mutation, which included intestinal obstruction (38.6% for mutant KRAS vs. 25.5% for wild type) and perforation (25.7% for mutant KRAS vs. 15.5% for wild type), whereas constipation occurred mainly in cases without KRAS mutations (26.4% for wild type vs. 22.9% for mutant KRAS) and bleeding per rectum was seen only in wild-type KRAS cases. A statistically significant relationship was established between initial presentation and both KRAS types (P=0.002) (Figure 1).
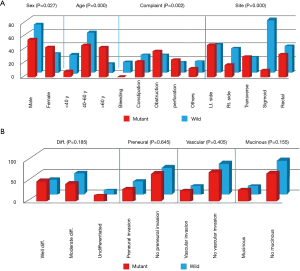
A statistically significant relationship (P<0.001) was seen between disease site and KRAS mutational status. Ascending colon lesions were mainly found in wild-type KRAS cases (16.4% wild type vs. 12.9% mutant KRAS), and descending colon lesions were found mainly in mutant KRAS cases (34.3% mutant KRAS vs. 19.1% wild type). The majority of sigmoid lesions in our study were in wild-type KRAS cases (36.4% wild type vs. 7.1% mutant KRAS). Seventy-four patients had rectal lesions; 40 of them had wild-type KRAS and 34 had mutant KRAS (Figure 1).
The liver and lung were the most common sites of metastases in mutant KRAS patients, compared with wild-type KRAS patients (62.9% mutant KRAS vs. 9.1% wild type). Only 12.9% of patients with liver metastases showed evidence of a KRAS mutation, in contrast to 65.5% for patients without a KRAS mutation. Lung metastasis was observed in 24.3% of patients with mutant KRAS, but in only 17.3% of patients with wild-type KRAS. A codon 12 mutation was found mostly in patients with liver metastases, whereas a codon 13 mutation was found in those with lung metastases (P<0.001). A statistically significant association was observed between the number of metastatic lesions and KRAS mutational status (P<0.001) (Table 3).
Although a significant correlation was observed between T-stage and KRAS mutational status (P=0.056), other pathologic parameters including pathologic type, nodal status, presence of mucinous changes, perineural invasion, and vascular invasion showed no statistically significant correlations (Table 4, Figure 1).
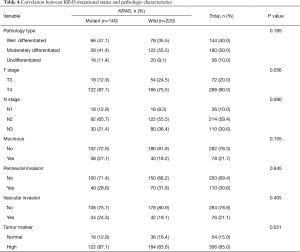
Full table
Cancer-related death occurred in 132 cases, and most of these cases had a KRAS mutation (P=0.001). A statistically significant relationship was seen between the KRAS mutation type and PFS and OS, with a P value of 0.001 and 0.002, respectively (Table 5).
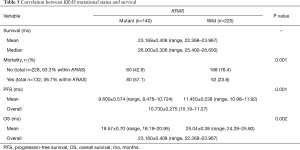
Full table
The overall PFS was 10.730±0.275 months (range, 10.19–11.27 months). Patients without a KRAS mutation showed a significantly longer PFS (11.450±0.238 months; range, 10.98–11.92 months) than those with mutant KRAS (9.600±0.574 months; range, 8.476–10.724 months; P=0.001). Since there was many censored participants and many events were there, different statistical tests were carried out, all indicated P values ≤0.05 (Figure 2).
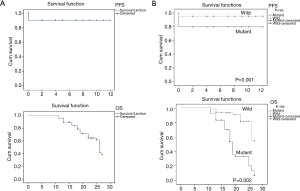
The OS was about 23 months (23.160±0.408 months; range, 22.368–23.967 months). Patients with wild-type KRAS had an OS of 25 months (25.04±0.38 months; range, 24.29–25.80 months), which was significantly longer (P=0.002) than that of patients with mutant KRAS, which was 19 months (19.57±0.70 months; range, 18.19–20.95 months) (Figure 2).
Discussion
Colorectal malignancies account for most of the malignancy-related mortality worldwide among male and female patients. In 2016, 1,344,900 new CRC cases were detected in the United States, and an estimated 491,900 persons died of CRC (6).
Since 2000, a decrease in the incidence and mortality rates of CRC was noted. This decrease is attributable to lifestyle changes including reduction in red meat consumption, increase in the use of aspirin, and decrease in smoking, as well as increased utilization of screening tests and improvements in treatment, especially targeted therapy.
A KRAS mutation is detected in approximately 40% of sporadic CRC cases. Approximately 90% of activating mutations of the KRAS gene are observed in codons 12 and 13, but very few are observed in codons 61 and 63 (7).
An interesting international study by Andreyev et al. (8) evaluated 2,721 patients with CRC from 22 research groups in 13 different countries. This study, known as the RASCAL study, clarified the relationship between KRAS mutational status and outcomes. The authors concluded that the presence of a KRAS mutation was significantly associated with a poorer prognosis.
In oncology, any improvement in survival is considered an appropriate measurement of the clinical outcome and is considered the most important endpoint. On the basis of the Food and Drug Association guidelines, OS was defined as “the time from randomization until death from any cause”, and it was measured in the intention-to-treat population, whereas PFS was identified as “the time from randomization until objective tumor progression or death”.
A statistically significant relationship was seen among patient characteristics (age and sex), cancer site, and an initial presentation with KRAS mutation status. Our findings are comparable to those of previously reported studies. Imamura et al. (9) evaluated KRAS mutation in codons 12/13 in 1,261 patients. Similar to that in our study, these mutations were detected in 36% of patients and a high mortality was noted in this group. Kim et al. (10) reported on 143 patients in whom the incidence of liver metastases was higher than that of metastases at other sites (P<0.001); however, the incidence of KRAS mutation was higher in patients with lung metastasis (P=0.003). Finally, Huang et al. (11) studied KRAS mutation in 205 patients and found the mutation in 42% of the patients. Higher OS and PFS were observed in patients with wild-type KRAS than in those with mutant KRAS (OS: 23 vs. 18.7 months; PFS: 10.2 vs. 7.9 months).
The results of our study are in contrast to those of the study by Karapetis et al. (12) which was performed in 393 patients. In this study, KRAS mutation was found in about 41% of patients, with these patients being treated with either cetuximab or best supportive care only. Zocche et al. (13) also reported similar findings in a study analyzing 149 patients with stage IV disease treated with FOLFOX-4 or a modified FOLFOX-6 regimen as a first-line treatment. The main difference between our study and these two studies was the way in which the data were analyzed. In our study, the patients’ age was analyzed in three groups, rather than in only two groups. Another contributing factor may be that we analyzed the data based on four anatomical locations (proximal colon, transverse colon, distal colon, and rectum), as opposed to only the colon and rectum. Furthermore, in our study, we classified the metastatic sites as either single or multiple sites; however, in the other studies, more metastatic subgroups were analyzed.
On the contrary, we found that KRAS mutation had no significant association with different pathologic findings except for T stage (with a strong tendency toward statistical significance, P=0.056). Birgisson et al. (14) and Huang et al. (11) also reported similar findings, except for T stage and vascular invasion. A possible explanation for this finding could be that most of the patients in these trials had stage T3 (71.7%) cancer, whereas most of the patients in the current study had stage T4 (80.0%) disease.
The main cause of mortality in cases with CRC is related to distant metastasis. In approximately 33% of the cases, the metastatic site was the liver (15,16) and the metastases may be present as synchronous metastases in about 25% of patients at the time of primary presentation. Nearly 50% of patients who underwent major dissection of CRC developed distant disease later. Santini et al. (17) revealed that KRAS C12V mutations were more frequently associated with hepatic metastasis.
Furthermore, a new trial on 143 Korean patients with metastatic or recurrent CRC showed that the lungs are the primary locations of distant metastasis in mutant KRAS cases. In this study, the metastatic site was significantly correlated with the KRAS mutational status, similar to the findings reported by Kim et al. (10).
A statistically significant relationship was found between KRAS mutational status and survival. This finding has been previously reported in the landmark RASCAL study on CRC (18), which found that cross mutations may indicate an unfavorable prognosis in CRC, especially in the advanced stages, which might lead to disease recurrence and mortality.
In conclusion, in patients with mCRC, KRAS molecular testing is a good prognostic and predictive tool. Additional molecular studies are needed to further explain the heterogeneity of the disease, in order to select the optimal treatment on the basis of molecular evaluation.
Acknowledgments
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
Ethical Statement: The study protocol was approved by the Institutional Review Board of King Fahad Hospital, Saudi Arabia (ONC0310) and was conducted in accordance with the Helsinki Declaration of 1964 (revised 2008). Due to the retrospective nature of this study, the need for informed consent was waived.
References
- Siegel RL, Miller KD, Fedewa SA, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin 2017;67:177-93. [Crossref] [PubMed]
- Kim ER, Kim YH. Clinical Application of Genetics in Management of Colorectal Cancer. Intest Res 2014;12:184-93. [Crossref] [PubMed]
- Arrington AK, Heinrich EL, Lee W, et al. Prognostic and predictive roles of KRAS mutation in colorectal cancer. Int J Mol Sci 2012;13:12153-68. [Crossref] [PubMed]
- Baines AT, Xu D, Der CJ. Inhibition of Ras for cancer treatment: the search continues. Future Med Chem 2011;3:1787-808. [Crossref] [PubMed]
- Frattini M, Saletti P, Romagnani E, et al. PTEN loss of expression predicts cetuximab efficacy in metastatic colorectal cancer patients. Br J Cancer 2007;97:1139-45. [Crossref] [PubMed]
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin 2016;66:7-30. [Crossref] [PubMed]
- Heinemann V, Stintzing S, Kirchner T, et al. Clinical relevance of EGFR- and KRAS-status in colorectal cancer patients treated with monoclonal antibodies directed against the EGFR. Cancer Treat Rev 2009;35:262-71. [Crossref] [PubMed]
- Andreyev HJ, Norman AR, Cunningham D, et al. Kirsten ras mutations in patients with colorectal cancer: the 'RASCAL II' study. Br J Cancer 2001;85:692-6. [Crossref] [PubMed]
- Imamura Y, Morikawa T, Liao X, et al. Specific mutations in KRAS codons 12 and 13, and patient prognosis in 1075 BRAF wild-type colorectal cancers. Clin Cancer Res 2012;18:4753-63. [Crossref] [PubMed]
- Kim MJ, Lee HS, Kim JH, et al. Different metastatic pattern according to the KRAS mutational status and site-specific discordance of KRAS status in patients with colorectal cancer. BMC Cancer 2012;12:347. [Crossref] [PubMed]
- Huang CW, Tsai HL, Chen YT, et al. The prognostic values of EGFR expression and KRAS mutation in patients with synchronous or metachronous metastatic colorectal cancer. BMC Cancer 2013;13:599. [Crossref] [PubMed]
- Karapetis CS, Khambata-Ford S, Jonker DJ, et al. K-ras mutations and benefit from cetuximab in advanced colorectal cancer. N Engl J Med 2008;359:1757-65. [Crossref] [PubMed]
- Zocche DM, Ramirez C, Fontao FM, et al. Global impact of KRAS mutation patterns in FOLFOX treated metastatic colorectal cancer. Front Genet 2015;6:116. [Crossref] [PubMed]
- Birgisson H, Edlund K, Wallin U, et al. Microsatellite instability and mutations in BRAF and KRAS are significant predictors of disseminated disease in colon cancer. BMC Cancer 2015;15:125. [Crossref] [PubMed]
- Van Cutsem E. Challenges in the use of epidermal growth factor receptor inhibitors in colorectal cancer. Oncologist 2006;11:1010-7. [Crossref] [PubMed]
- Cui H, Huang P, Wang Z, et al. Association of decreased mitochondrial DNA content with the progression of colorectal cancer. BMC Cancer 2013;13:110. [Crossref] [PubMed]
- Santini D, Vincenzi B, Addeo R, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol 2012;23:2313-8. [Crossref] [PubMed]
- Russo A, Bazan V, Agnese V, et al. Prognostic and predictive factors in colorectal cancer: Kirsten Ras in CRC (RASCAL) and TP53CRC collaborative studies. Ann Oncol 2005;16 Suppl 4:iv44-9. [Crossref] [PubMed]


